Abstract
Recently, there is growing evidence that tight junction proteins are often abnormally regulated in human tumors. The function of tight junction proteins in the maintenance of normal epithelial physiology has been well discussed, but their role in the tumorigenesis of gastric cancer is less well defined. To explore the expression distinction of the tight junction proteins claudin-1, -3, and -4 expression in the gastric cancer, the expression of claudin-1, -3, and -4 in 92 gastric cancer tissues and the non-neoplastic tissues adjacent to the tumors were examined by immunohistochemistry. Compared with adjacent non-neoplastic tissues, the expression of claudin-1 was down regulated. However, the expression of claudin-3 and claudin-4 were up-regulated in gastric cancer tissue. In addition, the expression of claudin-3 is correlated with claudin-4 expression in gastric cancer. Our present study reveals that claudin-1, -3, and -4 protein expression altered between human gastric cancers and adjacent non-neoplastic tissues.
Keywords: Gastric cancer, tight junctions, claudin-1, claudin-3, claudin-4
Introduction
Cell-cell adhesion in epithelial cell sheets is maintained mainly through adherens junctions (AJs) and tight junctions (TJs) [1]. Tight junctions exist in the junctional complexes of epithelial and endothelial cells, where they play important roles in cell adhesion, in maintaining cell polarity and permeability [2]. The function of tight junction proteins in the maintenance of normal epithelial physiology has been well discussed, but their role in the tumorigenesis is less well defined [3,4]. The claudin family which are essential for the formation of TJs consists of approximately 27 proteins [5]. It is reported that various claudins proteins can award different properties of TJs and alterations in claudins protein expression may contribute to the destruction of tight junction permeability [6], which may lead to increased diffusion of nutrients and other factors critical for tumor growth [7]. It is possible that aberrant expression of a certain claudin may alter normal tight junction structure contributing to the neoplastic process. Recently, there is growing evidence that tight junction proteins are frequently altered in various cancers [8]. It is reported that abnormal regulation of the TJ proteins contribute to the loss of cell adhesion and polarity, enhance the invasion and metastasis of malignant tumors [9].
Claudin multigene family encoding four-transmembrane domain protein, whose N- and C-terminal ends are located in the cytoplasm [10]. The C-terminal domain of claudins can serve as a binding site for interaction with a complex set of proteins such as ZO-1, ZO-2, ZO-3, multi-PDZ domain protein 1 (MUPP1) which are potentially involved in signaling [11]. It reveals that claudins protein may be involved in neoplastic progression is through coupling of the extracellular milieu to intracellular signaling pathways [12]. For example, it is reported that claudin-1 increases the progression of colon carcinoma though β-catenin/Tcf signaling [13]. Besides, claudin-7 inhibits human lung cancer cell migration and invasion through ERK/MAPK signaling pathway [14]. These results revealed that claudin proteins may be involved in tumorigenesis through coupling of the extracellular milieu to intracellular signaling pathways [15].
Based on these studies, it is hypothesized that claudins have a causal role in the process of umorigenesis. However, the expression of claudins in gastric cancer has not been clearly defined. Thus, the aim of this study was to represent the first detailed analysis of claudin-1, -3, and -4 expression in gastric cancer and adjacent tissue and to explore the potential feasibility of using claudin-1, -3, and -4 as diagnose markers. Our data reveals that these proteins are frequently altered between human gastric cancers and adjacent non-neoplastic tissues. Whereas the functional significance of claudin expression in gastric carcinoma is unclear, these proteins obviously may become a potential target for diagnosis or therapy of gastric cancer.
Materials and methods
Patients
Paraffin blocks of gastric cancer tissues and histologically normal tissue adjacent to these neoplasms were collected from ninety-two patients being treated at the Yantai Yuhuangding Hospital during the period between March 2012 and July 2014. The patients’ medical records were reviewed to identify their age, gender, histological grade, presence or absence of regional lymph node metastasis. For the use of these clinical materials for research purposes, prior patient’s consent and approval from the Institute Research Ethics Committee was obtained. All the cancer cases were classified and graded according to the International Union Against Cancer (UICC) staging system for gastric cancer.
Immunohistochemistry
Immunohistochemistry was carried out as described previously [16], Rabbit anti-human claudin-1 antibody (ab15098), rabbit anti-human claudin-3 antibody (ab15102) and rabbit antihuman claudin-4 antibody (ab15104) were purchased from Abcam Company (England). The streptavidin-perosidase immunohistochemistry reagent kit were purchased from Maixin Biology (Fujian, China). The cells positively expressing claudin-1, -3, and -4 were identified by brown staining of their cell membrane after reaction with claudin-1, -3, or -4 antibody. The percentage of positive cells were serially counted in one microscopic field. The cell counting was repeated in five randomly-selected microscopic fields at × 400 magnification. The claudin-1, -3, -4 positive tissues had more than 10% positive cells.
Statistical analysis
The Chi-square test/Chi-Square Goodness-of-Fit Test was used to determine the significance value for cancer progression of each factor alone, using a P-value < 0.05 for statistically significant associations. All the data were analyzed using SPSS 13.0 statistical software.
Results
Claudin-1 expression was reduced in gastric cancer
Claudin-1 expression was evaluated in the membranes of 92 gastric cancers tissues and adjacent non-neoplastic tissues. Positive expression of claudin-1 protein was detected in 18.5% (17/92) of gastric cancer tissues and in 53.4% (49/92) of adjacent tissues (Table 1). The expression of claudin-1 in gastric cancer tissues was significantly lower than in adjacent tissues (The Chi-square test/Chi-Square Goodness-of-Fit Test, P < 0.001) (Figure 1A, 1B). The expression of claudin-1 was not correlated with age (P = 1.000), sex (P = 0.404), histological grade (P = 1.000), but correlated with lymph node metastasis (P < 0.001) (Table 1).
Table 1.
Expression of claudin-1, claudin-3, claudin-4 and clinicopathological characteristics in gastric cancer patients
| Item | n | claudin-1 (+) | claudin-1 (-) | P | n | claudin-3 (+) | claudin-3 (-) | P | n | claudin-4 (+) | claudin-4 (-) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastric cancer tissue | 92 | 17 | 75 | < 0.001 | 92 | 52 | 40 | 0.001 | 92 | 65 | 27 | 0.004 |
| Adjacent tissue | 92 | 49 | 43 | 92 | 22 | 70 | 92 | 36 | 56 | |||
| Gender | ||||||||||||
| Male | 53 | 9 | 44 | 0.404* | 53 | 27 | 24 | 0.325* | 53 | 35 | 18 | 0.245* |
| Female | 39 | 8 | 31 | 39 | 25 | 14 | 39 | 30 | 9 | |||
| Age (year) | ||||||||||||
| ≤ 60 | 40 | 7 | 33 | 1.000* | 40 | 22 | 18 | 0.385* | 40 | 32 | 8 | 0.377* |
| > 60 | 52 | 10 | 42 | 52 | 30 | 22 | 52 | 33 | 19 | |||
| Histological grade | ||||||||||||
| Well-differentiated | 38 | 6 | 32 | 1.000* | 38 | 25 | 13 | 0.473* | 38 | 28 | 10 | 1.000* |
| Moderately and poor Differentiated | 54 | 11 | 43 | 54 | 27 | 27 | 54 | 37 | 17 | |||
| Lymph node metastasis | ||||||||||||
| + | 55 | 6 | 49 | < 0.001 | 55 | 37 | 18 | < 0.001 | 55 | 42 | 13 | < 0.001 |
| - | 37 | 11 | 26 | 37 | 15 | 22 | 37 | 21 | 6 |
No statistical significance.
Figure 1.
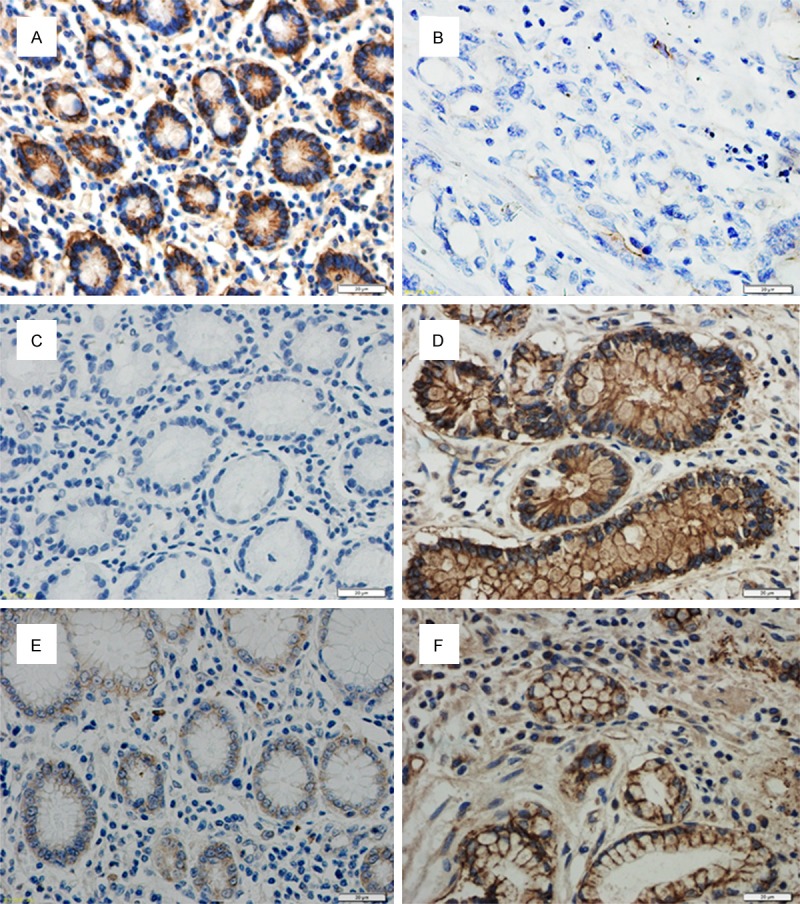
Immunohistochemical examination of claudin proteins expression in human gastric cancer and adjacent tissue. Claudins were expressed in the cell membrane. (A) high claudin-1 expression was detected in tissue adjacent to human gastric cancer compared with low claudin-1 expression in human gastric cancer tissue (F) (400 ×) (B, C) The low expression of claudin-3 in tissue adjacent to human gastric cancer compared to strong expression of claudin-3 in human gastric cancer tissue (D). Claudin-4 was expressed at low levels in epithelial cells adjacent to gastric cancer but was highly expressed in cancer tissue itself (E).
The expression of claudin-3 and claudin-4 was highly increased in gastric cancer
Positive expression of claudin-3 protein was found in 56.5% (52/92) of gastric cancer tissues and in 23.9% (22/92) of adjacent tissues (Table 1). The expression rate of claudin-3 in gastric cancer tissues was higher than the rate in adjacent tissues (The Chi-square test/Chi-Square Goodness-of-Fit Test, P = 0.001 < 0.01) (Figure 1C, 1D). The expression of claudin-3 was not correlated with age (P = 0.385), sex (P = 0.325), histological grade (P = 0.473), but correlated with lymph node metastasis (P < 0.001).
The membrane staining of claudin-4 was strong in gastric cancer tissues and weak in adjacent tissues. Claudin-4 was expressed in 70.6% (65/92) of gastric cancer tissues. Cells were positive for claudin-4 in 39.1% (36/92) of tissues adjacent to the cancer. We conclude that claudin-4 expression is significantly higher in gastric cancer samples than in histologically normal gastric tissue (Figure 1E, 1F). (The Chi-square test/Chi-Square Goodness-of-Fit Test, P = 0.004 < 0.01). The expression of claudin-4 was not correlated with age (P = 0.377), sex (P = 0.0.245), histological grade (P = 1.000), but correlated with lymph node metastasis (P < 0.001).
In addition, we investigated the correlation between claudin-1, claudin-3 and claudin-4 expression using the Chi-square test/Chi-Square Goodness-of-Fit Test, we observed that the expression of claudin-3 was positively correlated with the expression of claudin-4 (The Chi-square test/Chi-Square Goodness-of-Fit Test, φ = 0.376, P = 0.028). The detailed results of the analysis are described in Tables 2 and 3.
Table 2.
Correlation between the expressions of claudin-1 and claudin-3, claudin-4 in gastric cancer
| Item | claudin-3 (+) | claudin-3 (-) | φ* | P | claudin-4 (+) | claudin-4 (-) | φ* | P |
|---|---|---|---|---|---|---|---|---|
| claudin-1 (+) | 10 | 7 | 0.168 | 0.405* | 11 | 6 | 0.176 | 0.430 |
| claudin-1 (-) | 42 | 33 | 54 | 21 |
φPhi coefficient.
Table 3.
Correlation between the expression of claudin-3 and claudin-4 in gastric cancer
φPhi coefficient.
Discussion
TJs appear to be an obstacle to the unlimited growth and metastasis phenotype in several huaman cancer [17]. For instance, disruption of TJ structure was observed in hepatocellular carcinoma, colon cancer and thyroid cancer [18]. Abnormal TJ proteins expression has been reported as a mechanism for disruption of TJ structure [19]. To date, the majority of studies have focused in the roles of claudins protein in forming TJs functioning as a barrier, only few study have examined the expression pattern of claudins in the human cancer [20]. Claudin-1 was the first reported claudin protein which described as component of TJs, and so a lot of researches are related to this protein. Claudin-1 which is structurally related with claudin-2 were identified as the second components of TJ strands [21]. In addition, data revealed that different members of the claudin family are involved in the formation of TJ in different tissues [22], which means that claudin family members are involved in the formation of TJ strands in a tissue-dependent manner.
Claudins expression has been reported to be altered in several cancers [23], for instance, claudin-1 was often down-regulated in breast cancer and colon cancer, parallelly, claudin-7 has also been found to be reduced in invasive breast cancer [24]. These studies of reduced tight junction protein expression in cancer are according with the traditionally accepted thought that oncogenesis is coupled with loss of tight junctions [19], which contribute to the loss of polarity and deficiency of cohesion observed in cancer cells [25]. Paradoxically, a variety of recent studies have revealed that several claudin proteins are often up-regulated in certain cancer. A serial analysis of gene expression (SAGE) study revealed that claudin-3 and claudin-4 was highly up-regulated in ovarian cancer [26]. Besides, Several additional reports have also verified the up-regulation of claudin-3 and claudin-4 in breast, prostate, and pancreatic cancers [27]. To date, It is widely accepted claudins are differentially expressed in a number of human neoplasms.
As mentioned above, it is hypothesized that the abnormal expression of claudins in cancer has been interpreted as a mechanism for the progression of cancer. Consistent with this hypothesis, Claudin-1 and claudin-7 which were undetectable in normal cervical squamous epithelium may play a significant role in tumor progression of cervical neoplasia [28]. In addition, increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer [29]. It is also reported that loss of claudin-7 protein expression correlated with histological grade in breast invasive ductal carcinoma [30]. In the present study we examined the expression of the claudin family, namely claudin-1, claudin-3 and claudin-4 in gastric cancer and adjacent non-neoplastic tissues, levels of claudin-1 have been showed to be down-regualted while claudin-3 and claudin-4 have been shown to be upregulated in gastric cancer and correlated significantly with lymphatic metastasis. In conclusion, these finding suggests that alteration expression of certain claudins may participate in gastric cancer aggression.
In consideration of claudins locates at the cell membrane surface [11], they may represent potential target for drug therapeutic. Claudin-3 and claudin-4 are the natural receptors for the Clostridium perfringens enterotoxin (CPE) which is a potent cytolytic toxin. Binding of the CPE to claudin-3 and claudin-4 contributes to a rapid cytolysis of the cancer cells [31]. Recent preclinical studied revealed that CPE can inhibit the malignancies of claudin-3 and -4 expressing cells [32]. CPE which targets cancer cells expressing claudin-3 or claudin-4 specifically, should be deserving of attention in gastric cancer. Our data reveals that claudin-3 and claudin-4 are highly up regulated in gastric cancer, therefore, CPE treatment is certainly a possibility.
Our research is to examine the expression pattern of certain claudin proteins in gastric neoplasms and to explore the relevance between their expression and pathological indices of gastric cancer patients. Claudin-1, -3 and -4 proteins may prove to be a useful diagnose marker for gastric cancer. In addition, our data reveals that down-regulation of claudin-1 and up-regulation of claudin-3 and claudin-4 were obviously associated with lymphatic metastasis of gastric neoplasms. In summary, it can be inferred that reduction of claudin-1 expression may contribute to the progression of gastric tumors. Meanwhile, increased claudin-3 and claudin-4 expression may play a postive role in the progression and metastasis of gastric cancer.
Conclusion
The present work reveals that the expression of claudin-1, claudin-3, and claudin-4 varied between human gastric cancers and adjacent non-neoplastic tissues and correlate with their lymphatic metastasis. However, the specific mechanism responsible for these observations needs to be addressed in the future.
Disclosure of conflict of interest
None.
References
- 1.Dejana E. Endothelial cell-cell junctions: happy together. Nature Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 2.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 3.Tobioka H, Isomura H, Kokai Y, Tokunaga Y, Yamaguchi J, Sawada N. Occludin expression decreases with the progression of human endometrial carcinoma. Hum Pathol. 2004;35:159–164. doi: 10.1016/j.humpath.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev. 2005;57:919–928. doi: 10.1016/j.addr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 6.Peppi M, Ghabriel MN. Tissue - specific expression of the tight junction proteins claudins and occludin in the rat salivary glands. J Anat. 2004;205:257–266. doi: 10.1111/j.0021-8782.2004.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo B. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 8.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 11.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Ding L, Hong H, Hoggard J, Lu Q, Chen YH. Claudin-7 inhibits human lung cancer cell migration and invasion through ERK/MAPK signaling pathway. Exp Cell Res. 2011;317:1935–1946. doi: 10.1016/j.yexcr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Xu X, Liu Z, Zhang T, Zhang X, Wang L, Wang M, Liu Y, Lu Y, Liu Y. Apoptosis signal-regulating kinase 1 is associated with the effect of claudin-6 in breast cancer. Diagn Pathol. 2012;7:1–6. doi: 10.1186/1746-1596-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Mariscal L, Bautista P. Tight junctions. Springer; 2006. [Google Scholar]
- 18.Kojima T, Sawada N. Regulation of tight junctions in human normal pancreatic duct epithelial cells and cancer cells. Ann N Y Acad Sci. 2012;1257:85–92. doi: 10.1111/j.1749-6632.2012.06579.x. [DOI] [PubMed] [Google Scholar]
- 19.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and-2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira S, Morgado-Diaz J. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 2007;64:17–28. doi: 10.1007/s00018-006-6314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morohashi S, Kusumi T, Sato F, Odagiri H, Chiba H, Yoshihara S, Hakamada K, Sasaki M, Kijima H. Decreased expression of claudin-1 correlates with recurrence status in breast cancer. Int J Mol Med. 2007;20:139–143. [PubMed] [Google Scholar]
- 25.Mitic L, Anderson J. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 26.Rangel LB, Agarwal R, D’Souza T, Pizer ES, Alò PL, Lancaster WD, Gregoire L, Schwartz DR, Cho KR, Morin PJ. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res. 2003;9:2567–2575. [PubMed] [Google Scholar]
- 27.Shang X, Lin X, Alvarez E, Manorek G, Howell SB. Tight junction proteins claudin-3 and claudin-4 control tumor growth and metastases. Neoplasia. 2012;14:974–985. doi: 10.1593/neo.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JW, Lee SJ, Seo J, Song SY, Ahn G, Park CS, Lee JH, Kim BG, Bae DS. Increased expressions of claudin-1 and claudin-7 during the progression of cervical neoplasia. Gynecol Oncol. 2005;97:53–59. doi: 10.1016/j.ygyno.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 29.Lanigan F, McKiernan E, Brennan DJ, Hegarty S, Millikan RC, McBryan J, Jirstrom K, Landberg G, Martin F, Duffy MJ. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int J Cancer. 2009;124:2088–2097. doi: 10.1002/ijc.24159. [DOI] [PubMed] [Google Scholar]
- 30.Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 31.Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001;61:7878–7881. [PubMed] [Google Scholar]
- 32.English DP, Santin AD. Claudins overexpression in ovarian cancer: potential targets for Clostridium perfringens enterotoxin (CPE) based diagnosis and therapy. Int J Mol Sci. 2013;14:10412–10437. doi: 10.3390/ijms140510412. [DOI] [PMC free article] [PubMed] [Google Scholar]


