Abstract
Introduction: Clear cell renal cell carcinoma (ccRCC) is the most common type of cancer in the adult kidney, and the prognosis of metastatic ccRCC remains poor with high mortality. Recent study indicated that microRNAs (miRNAs) played critical roles in tumor progression. The aim of this study was to investigate the expression, biological role and clinical significance of miR-497 in ccRCC. Methods: Quantitative real-time PCR (qRT-PCR) was performed to detect the expression of miR-497 in renal cancer cell lines and ccRCC tissues. The association between miR-497 expression and overall survival was estimated by the Kaplan-Meier method. Gain of function assays were performed in the 786-O renal cancer cell line. Results: Expression of the miR-497 was significantly decreased in renal cancer cell lines and ccRCC tissues when compared with normal human proximal tubule epithelial cells and adjacent non-tumor tissues. Decreased miR-497 expression was significantly associated with tumor stage, histological grade and lymph node metastases. Significantly shorter overall survival was observed in patients with lower expression of the miR-497. Overexpression of miR-497 significantly inhibited renal cancer cell proliferation, migration and invasion. Conclusions: Our results demonstrated that miR-497 was decreased in ccRCC tissues and may provide a potential prognostic biomarker and a potential target for therapeutic intervention.
Keywords: Clear cell renal cell carcinoma, miR-497, biomarker, prognosis
Introduction
Renal cell carcinoma (RCC) is responsible for approximately 3% of all cancers in adults and is the third most common urological cancer, with incidence rates increasing 2% per year [1]. Nowadays, there is no standard screening test for RCC, and one-third of the patients present metastasis on diagnosis and over the course of the disease, metastasis develops in another 50% of patients [2]. Radical nephrectomy is effective to cure early and local RCC, but no effective for patients at advanced stages. RCC is generally resistant to standard chemotherapy and radiotherapy, the 5 year survival rate of patients with metastatic RCC less than 10% [3,4]. Therefore, the identification of molecular markers that are predictive of RCC aggressiveness and patient outcome has the potential to improve the ability to manage patients and new molecular targets for adjuvant therapies.
MiRNAs are small 21-24 nucleotides non-coding RNAs that regulate gene expression post-transcriptionally by base pairing with the 3’-untranslated region of target messenger RNAs (mRNAs) to suppress translation or induce mRNA degradation [5]. Accumulating evidence has proved that miRNAs control various physiological processes and that the deviations from normal miRNA expression patterns play vital roles in human diseases, including cancers [6,7]. In human cancer, miRNAs can function as oncogenes or tumor suppressor genes depending on the nature of their targets [8]. Moreover, miRNAs have shown specific expression patterns in cancers, which enable microRNAs as biomarkers for cancer risk, diagnosis and prognosis, even as miRNA-based therapeutic targets [9,10]. For example, Zhang et al showed that miR-335 expression was decreased in a majority of esophageal squamous cell carcinoma (ESCC). They indicated that miR-335 expression was an independent prognostic factor for patients with esophageal cancer, which might be a potential valuable biomarker for ESCC [11]. Chu et al showed that miR-630 expression level was significantly elevated in gastric cancer in comparison to adjacent normal specimens. It is also proved that miR-630 expression was to be associated with gastric cancer invasion, lymph node metastasis, distant metastasis and TNM stage [12]. Cheng et al found that miR-152 was down-regulated in non-small cell lung cancer tissues and cell lines. Over-expression of miR-152 suppressed cell proliferation and colony formation and also limited migration and invasion [13]. Li et al showed miR-646 was down-regulated in renal cancer and low expression of miR-646 was significantly associated with distant metastatic. Furthermore, down-regulated miR-646 in renal cancer was associated with tumor metastasis through MAPK pathway by targeting NOB1 [14]. However, to our knowledge, the role of miR-497 in ccRCC remains undefined.
In the present study, we explored the expression of miR-497 in renal caner cells and tissue samples. Then, the association of miR-497 expression with the clinicopathologic features of ccRCC patients and its prognostic value were explored. Finally, experiments in vitro further confirmed that forced miR-497 expression could inhibit cell proliferation, migration, and invasion of renal cancer cells.
Materials and methods
Patients and specimens
Following obtaining the informed consent from all patients, surgical specimens (paired tumor and non-tumor tissues) were collected from 86 patients with ccRCC in the Department of Urology, Huaihe Hospital of Henan University. Samples were flash frozen in liquid nitrogen until use. The study was approved by the Institutional Review Board of Henan University. The clinical and pathological information from patient records was gathered, and the details were listed in Table 1. The tumors were classified according to the 7th edition of the AJCC cancer staging manual and the future of TNM [15].
Table 1.
Correlation between miR-497 expression and clinicopathologic features of ccRCC
| Parameters | Group | Total | MiR-497 expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| Gender | Male | 48 | 26 | 22 | 0.730 |
| Female | 38 | 22 | 16 | ||
| Age (years) | < 65 | 47 | 27 | 20 | 0.738 |
| ≥ 65 | 39 | 21 | 18 | ||
| Tumor size (cm) | < 4 cm | 55 | 29 | 26 | 0.443 |
| ≥ 4 cm | 31 | 19 | 12 | ||
| Histological grade | I-II | 52 | 22 | 30 | 0.002 |
| III-IV | 34 | 26 | 8 | ||
| Tumor stage | T1-T2 | 47 | 16 | 31 | 0.000 |
| T3-T4 | 39 | 32 | 7 | ||
| Lymph nodes metastasis | Absence | 73 | 37 | 36 | 0.023 |
| Presence | 13 | 11 | 2 | ||
Cell culture and transfection
Immortalized normal human proximal tubule epithelial cell line HK-2 was purchased from the American Type Culture Collection (ATCC, USA). Human renal cancer cell lines 786-O, ACHN and A498 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (CCCAS, China). Renal cancer cells were cultured in RPMI-1640 medium (Gibco) with 10% fetal bovine serum (FBS, Gibco), 50 U/ml of penicillin and 50 μg/ml of streptomycin. The HK-2 cells were cultured in KSFM medium (Gibco). All cells were grown and maintained at 37°C in a 5% CO2 humidified incubator.
The miR-497 mimics, miR-negative control of mimics (miR-Ctrl) were synthesized and purified by RiboBio (Guangzhou, China), miR-497 mimics and miR-Ctrl were transfected at a final concentration of 50 nM using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instruction. Total RNA were collected 48 hours after transfection.
Quantitative realtime PCR (qRT-PCR)
Total RNA was extracted from cells or tissues using the Trizol reagent (Invitrogen) according to the manufacturer’s protocol. For the detection of miR-497, reverse transcription and qRT-PCR reactions were performed using the standard SYBR Green Assay protocol and the ABI 7900 Sequence Detection System (ABI). U6 was used as an internal control. The primers were as follows: miR-497 forward, 5’-GTGCAGGGTCCGAGGT-3’, reverse, 5’-TAGCCTGCAGCACACTGTGGT-3’. U6 forward, 5’-GCTTCGGCAGCACATATACTAAAAT-3’; reverse, 5’-CGCTTCACGAATTTGCGTGTCAT-3’. The relative expression of miR-497 was calculated and normalized using the 2-ΔΔCt method relative to U6 snRNA.
MTT assay
In vitro cell proliferation was measured by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were grown in a 96-well plate for 24 h and cultured in normal medium. Cells were then incubated in 0.1 mg/ml MTT at 37°C for 5 h and lysed in dimethyl sulfoxide (DMSO) at room temperature for 10 min at 0, 24, 48, and 72 h after transfection. The absorbance in each well was then measured at 490 nm, with a microplate reader (Bio-Rad). Each experiment was performed at least three times.
Migration assay
Cell migration potential was evaluated using a wound-healing assay. Cells were seeded in 12-well plates and cultured for 24 h to form confluent monolayers. A wound was created by dragging a 10 μl pipette tip through the monolayer and the plates were washed with phosphate-buffered saline (PBS) to remove cellular debris. Photographic images were taken at 0 and 48 h along the scrape line by microscope. Results were expressed as relative scratch width, based the distance migrated relative to the original scratched distance. Each experiment was performed in three times independently.
Transwell invasion assay
Transwell invasion experiments were performed with 24-well Matrigel-coated chambers from BD Biosciences. Briefly, the cells were seeded into inserts at 4×103/insert in serum-free medium and then transferred to wells filled with the culture medium containing 10% FBS as a chemoattractant. After 24 h of incubation, non-invading cells on the top of the membrane were removed by scraping. Invaded cells on the bottom of the membrane were fixed, followed by staining with 0.05% crystal violet. The number of invaded cells on the membrane was counted under a microscope. The results represented the average of three independent experiments.
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software. A paired-samples t-test was used to analyze differences in miR-497 expression between ccRCC tissues and matched non-tumor tissues. The relationships between miR-497 and clinicopathologic features were evaluated by a chi-square test. The survival rates for miR-497 expression were estimated by using the Kaplan-Meier method and the difference in survival curves were tested by log-rank test. Univariate and multivariate Cox regression analyses were performed to analyze the survival data. The data are shown as the mean ± SD from at least three independent experiments. Values of P less than 0.05 were considered statistically significant.
Results
miR-497 was significantly decreased in renal cancer cell lines and ccRCC tissues
The expression of miR-497 was examined in three renal cancer cell lines (786-O, ACHN and A498) and Immortalized normal human proximal tubule epithelial cell line (HK-2) by qRT-PCR. Our results showed that renal cancer cells expressed significantly lower levels of miR-497 than HK-2 cells (Figure 1A). To explore whether miR-497 was down-regulated in ccRCC tissues, the expression of miR-497 was determined in 86 pairs of ccRCC tissues and adjacent non-tumor tissues by qRT-PCR. We found that the average expression of miR-497 was significantly down-regulated in ccRCC tissues compared with adjacent normal tissues (Figure 1B). The data indicated that abnormal miR-497 expression may be related to renal cancer pathogenesis.
Figure 1.
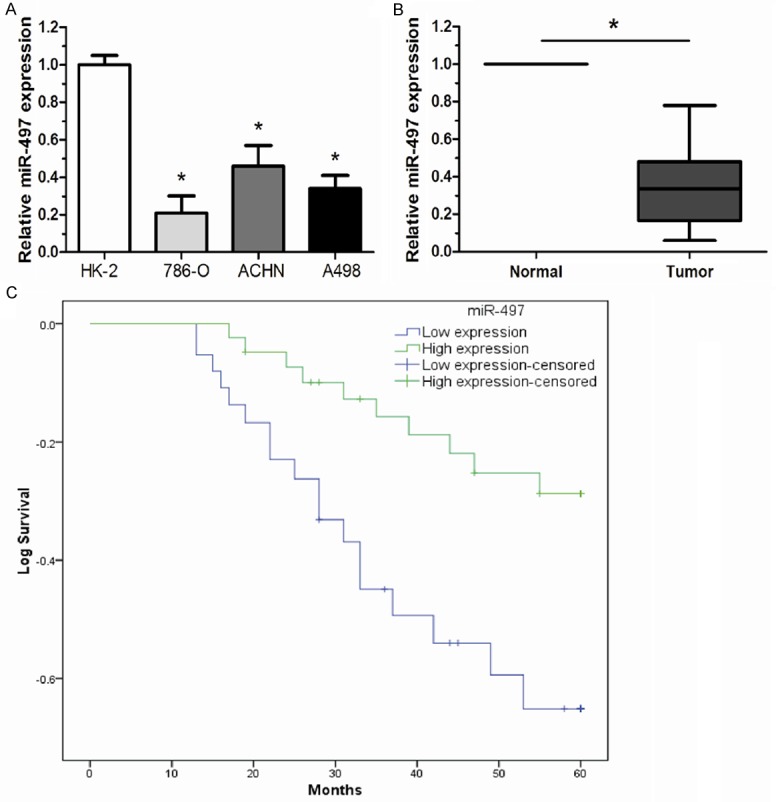
Low expression level of miR-497 was correlated with poor prognosis of ccRCC patients. A. The relative miR-497 expression in renal cancer cell lines was much lower than that of the HK-2 cells. B. The mean expression level of miR-497 in ccRCC tissues was significantly lower than that in adjacent non-tumor tissues. C. The Kaplan-Meier analysis revealed that low-level expression of miR-497 was associated with shorter overall survival of patients with ccRCC (log-rank test). The relative miR-497 expression levels were determined using qRT-PCR. Results were expressed as mean ± SD for three replicate determination. *P < 0.05.
Clinicopathologic significance of miR-497 in renal cancer
The correlation between miR-497 expression level and clinicopathological features was further analyzed in 86 ccRCC patients. Patients were divided into high and low expression group by the mean expression level of miR-497. The statistical analysis revealed that the low miR-497 level was significantly correlated with higher tumor stage, histological grade and lymph node metastases while no significant correlation was observed in other features (Table 1). The potential of miR-497 as prognostic marker was further explored. Kaplan-Meier survival analysis showed that the patients with a low miR-497 expression had shorter overall survival times than those with a high miR-497 expression (Figure 1C). Next, Univariate analysis indicated that the overall survival of patients with ccRCC was associated with tumor stage, histological grade, lymph node metastases and miR-497 expression. Furthermore, multivariate Cox proportional hazard regression analysis demonstrated that miR-497 expression, tumor stage, histological grade, and lymph node metastases were significantly associated with overall survival of ccRCC patients as independent prognostic factors (Table 2).
Table 2.
Univariate and multivariate analysis of factors associated with overall survival in ccRCC patients
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk ratio | 95% CI | P | Risk ratio | 95% CI | P | |
| Gender | ||||||
| Male vs Female | 0.913 | 0.617-1.593 | 0.378 | |||
| Age (years) | ||||||
| ≥ 65 vs < 65 | 1.764 | 0.712-2.349 | 0.447 | |||
| Tumor size | ||||||
| ≥ 4 cm vs < 4 cm | 2.182 | 0.593-3.718 | 0.133 | |||
| Tumor stage | ||||||
| T3-4 vs T1-2 | 2.826 | 1.712-4.936 | 0.0291 | 2.315 | 1.527-4.024 | 0.019 |
| Histological grade | 3.251 | |||||
| III-IV vs I-II | 2.148-5.376 | < 0.001 | 2.837 | 1.893-4.775 | 0.008 | |
| Lymph node | ||||||
| Presence vs Absence | 3.752 | 2.214-7.165 | 0.008 | 3.017 | 2.019-6.833 | 0.005 |
| MiR-497 | ||||||
| Low vs High | 2.716 | 1.933-6.704 | 0.009 | 2.583 | 1.691-6.361 | < 0.001 |
Overexpression of miR-497 inhibited cell proliferation, migration and invasion of renal cancer cells
To investigate the biological function of miR-497 in renal cancer, we up-regulated the expression of miR-497 in 786-O cells with a commercially available miR-497 mimics, which increased the level of miR-497 in 786-O as compared to the negative control (P < 0.05, Figure 2A). MTT assay showed that miR-497 mimics could markedly promote the proliferation of 786-O cells compared with the negative control (P < 0.05, Figure 2B). To further explore miR-497 function on the progression and metastasis of renal cancer, we determined the effect of miR-497 overexpression on migratory and invasive capacity in cultured renal cancer cells using the wound healing and matrigel chamber assays. The in vitro wound healing assay showed that miR-497 overexpression in 786-O cells impaired the ability to close the wound compared with the negative control-treated cells (P < 0.05, Figure 2C). Moreover, the cell invasive capacity was significantly impaired by overexpression of miR-497 expression in 786-O cells (P < 0.05, Figure 2D). Taken together, our results demonstrated that miR-497 acts as a tumor suppressor on malignant growth, invasion and metastasis in renal cancer cells.
Figure 2.
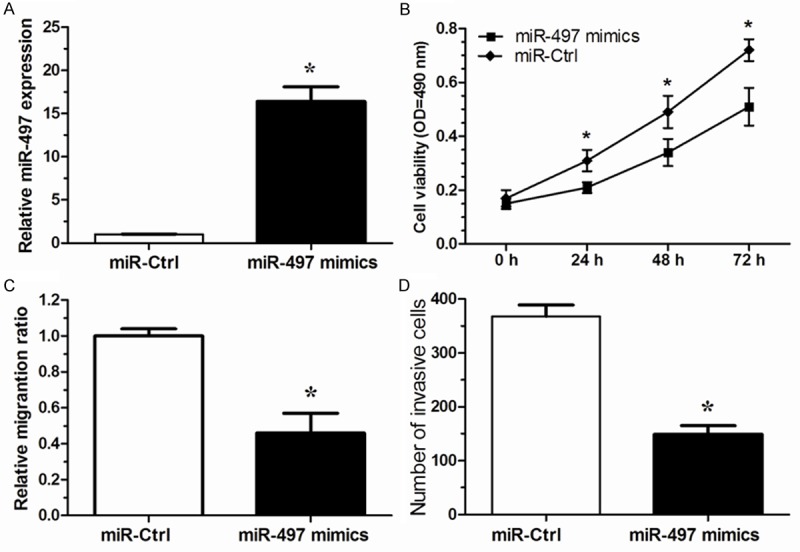
Ectopic miR-497 expression inhibited cell proliferation, migration and invasion in renal cancer cells. A. The relative expression level of miR-497 in 786-O cells are significantly increased by miR-497 mimics compared with the miR-Ctrl. B. Cell proliferation of 786-O cells was detected by MTT assay after transfected with miR-497 mimics or miR-Ctrl. C. Migration assay showed over-expression of miR-497 in 786-O cells produced a lower migration capacity than observed in controls tansfected with miR-Ctrl. D. Invasion assay showed 786-O cells tansfected with miR-497 mimics displayed a lower invasion capacity compared with those tansfected with miR-Ctrl. Results are expressed as means ± SD for three replicate determination. *P < 0.05.
Discussion
Renal cell carcinoma remains to be one of the leading causes of death, so finding new molecular targets for its diagnosis, prognosis and treatment has the potential to improve the clinical strategies and outcomes of this disease [16]. In recent years, more and more studies showed that dysregulation in microRNAs are proved to contribute in tumor development in many cancer types and can be used to develop as biomarkers and prognosis factors [17]. In the present study, our attention focused on the miR-497.
miR-497, identified from the microRNA cluster site at chromosome 17p13.1, has been reported to function as a tumor suppressor in a variety of human cancers. For example, Wang et al found that down-regulation of miR-497 was correlated with breast cancer progression, and miR-497 might be a potential molecular biomarker for predicting the prognosis of patients [18]. Luo et al showed that miR-497 was down-regulated in cervical cancer and that the decreased expression of miR-497 expression was correlated with advanced clinical stage. miR-497 might be a potential prognostic marker and function as a tumor suppressor in human cervical cancer by post-transcriptionally targeting IGF-1R [19]. Xu et al revealed that miR-497 expression was significantly down-regulated in pancreatic cancer and low expression of miR-497 was an independent adverse prognostic factor of pancreatic cancer, in vivo assay, they showed over-expression of miR-497 could inhibit tumor growth [20]. Guo et al indicated that miR-497 targeted IGF1-R and was frequently down-regulated by gene copy number reduction in colorectal cancer. In addition, they demonstrated that down-regulation of miR-497 contributes to elevated activation of PI3K/Akt signaling and malignant behavior in colorectal cancer cells [21]. However, miR-497 expression in ccRCC and underlying mechanism remains unclear.
In the present study, we first detected the expression of miR-497 in renal cancer cell lines and ccRCC tissues, our results showed that the relative expression level of miR-497 in renal cancer cell lines and ccRCC tissues was significantly lower than that in HK-2 cells and adjacent non-tumor tissues. By clinicopathologic analysis, we found that status of miR-497 expression in tissues was significantly correlated with tumor stage, histological grade and lymph node metastases but not with other clinicopathologic factors. Then, we analyzed the correlation of miR-497 expression with prognosis of ccRCC patients, Our data indicated that patients with low miR-497 expression showed shorter overall survival than those with high miR-497 expression. More importantly, Multivariate Cox proportional hazard regression analysis showed that low miR-497 expression was an unfavorable prognostic factor for patients with ccRCC. These results indicated that the decrease in miR-497 might play a critical role in the progression and development of renal cancer.
To further understand the mechanism of miR-497 in renal cancer cell process, in vitro experiments were conducted. We showed that ectopic expression of miR-497 could inhibit proliferation, migration and invasion capability of 786-O cells compared with control group, which suggesting that increased miR-497 expression decreased the growth and metastasis capability of renal cancer cells. Our results indicated that miR-497 may play a tumor suppressor role in ccRCC.
In conclusion, the current study demonstrated that miR-497 was decreased in ccRCC tissues compared to that in the adjacent non-tumor tissues and was significantly associated with the clinical pathological stages and overall survival rate of the disease. The down-regulation of miR-497 played critical roles in ccRCC progression. These results suggested that miR-497 could prove to be useful prognostic diagnosis and therapeutic strategies for patients with ccRCC.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:2353–2358. doi: 10.1016/j.juro.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. The Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 6.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 10.Nana-Sinkam SP, Croce CM. MicroRNAs as therapeutic targets in cancer. Translational Research. 2011;157:216–225. doi: 10.1016/j.trsl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Zhang BJ, Gong HY, Zheng F, Liu DJ, Liu HX. Up-regulation of miR-335 predicts a favorable prognosis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:6213. [PMC free article] [PubMed] [Google Scholar]
- 12.Chu D, Zhao Z, Li Y, Li J, Zheng J, Wang W, Zhao Q, Ji G. Increased MicroRNA-630 Expression in Gastric Cancer Is Associated with Poor Overall Survival. PLoS One. 2014;9:e90526. doi: 10.1371/journal.pone.0090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Z, Ma R, Tan W, Zhang L. MiR-152 suppresses the proliferation and invasion of NSCLC cells by inhibiting FGF2. Exp Mol Med. 2014;46:e112. doi: 10.1038/emm.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Liu M, Feng Y, Xu YF, Huang YF, Che JP, Wang GC, Yao XD, Zheng JH. Downregulated miR-646 in clear cell renal carcinoma correlated with tumour metastasis by targeting the nin one binding protein (NOB1) Br J Cancer. 2014;111:1188–1200. doi: 10.1038/bjc.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 16.Lam JS, Shvarts O, Leppert JT, Figlin RA, Belldegrun AS. Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173:1853–1862. doi: 10.1097/01.ju.0000165693.68449.c3. [DOI] [PubMed] [Google Scholar]
- 17.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Li H, Wang J, Wang D. Expression of microRNA-497 and its prognostic significance in human breast cancer. Diagn Pathol. 2013;8:172. doi: 10.1186/1746-1596-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Luo M, Shen D, Zhou X, Chen X, Wang W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery. 2013;153:836–847. doi: 10.1016/j.surg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Wang T, Cao Z, Huang H, Li J, Liu W, Liu S, You L, Zhou L, Zhang T, Zhao Y. MiR-497 downregulation contributes to the malignancy of pancreatic cancer and associates with a poor prognosis. Oncotarget. 2014;5:6983–6993. doi: 10.18632/oncotarget.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S, Jiang C, Wang G, Li Y, Wang C, Guo X, Yang R, Feng Y, Wang F, Tseng H. MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene. 2012;32:1910–1920. doi: 10.1038/onc.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]


