Abstract
Many patients who undergo general anesthesia and surgery experience cognitive dysfunction, particularly memory deficits that can persist for days to months. The mechanisms underlying this postoperative cognitive dysfunction in the adult brain remain poorly understood. Depression of brain function during anesthesia is attributed primarily to increased activity of γ-aminobutyric acid type A receptors (GABAARs), and it is assumed that once the anesthetic drug is eliminated, the activity of GABAARs rapidly returns to baseline and these receptors no longer impair memory. Here, using a murine model, we found that a single in vivo treatment with the injectable anesthetic etomidate increased a tonic inhibitory current generated by α5 subunit–containing GABAARs (α5GABAARs) and cell-surface expression of α5GABAARs for at least 1 week. The sustained increase in α5GABAAR activity impaired memory performance and synaptic plasticity in the hippocampus. Inhibition of α5GABAARs completely reversed the memory deficits after anesthesia. Similarly, the inhaled anesthetic isoflurane triggered a persistent increase in tonic current and cell-surface expression of α5GABAARs. Thus, α5GABAAR function does not return to baseline after the anesthetic is eliminated, suggesting a mechanism to account for persistent memory deficits after general anesthesia.
Introduction
Each year, more than 234 million surgical procedures are performed worldwide (1). A proportion of patients exhibit cognitive impairment, including memory deficits, after surgery and anesthesia (2). Such postoperative cognitive deficits are present in approximately 37% of young adults and 41% of elderly patients at hospital discharge and in 6% of young adults and 13% of elderly patients at 3 months after surgery (2). These deficits are associated with poor patient outcomes, including reduced quality of life, loss of independence, and increased mortality (2, 3).
The cause of postoperative cognitive dysfunction is multifactorial. For example, inflammation triggered by surgical trauma appears to contribute to cognitive deficits in both human patients and laboratory animals (4, 5). Additional factors that increase the risk of cognitive deficits include infection, opioids, stress, and sleep disturbances (6). General anesthetics may also play a causal role, given that the duration of anesthesia is positively correlated with the incidence of postoperative cognitive deficits in patients (6). In addition, a single exposure to an anesthetic can cause retrograde and anterograde memory deficits that persist for days to weeks in rodent models (7, 8). The mechanisms by which anesthetics cause persistent memory deficits in adults remain poorly understood.
Most general anesthetics act as positive allosteric modulators of inhibitory γ-aminobutyric acid type A receptors (GABAARs) (9). During anesthesia, increased activity of GABAARs contributes to the desired and profound neurodepressive properties of these drugs, including acute memory blockade (10). Once the anesthetic is eliminated, positive allosteric modulation of GABAAR function is rapidly reversed, on a time scale of seconds (11). Consequently, it has been assumed that receptor activity returns to baseline and GABAARs do not contribute to undesirable prolonged cognitive dysfunction after anesthesia. Here, we test the hypothesis that even a brief exposure to an anesthetic triggers a sustained increase in GABAAR function and that this increase causes persistent memory deficits.
Results and Discussion
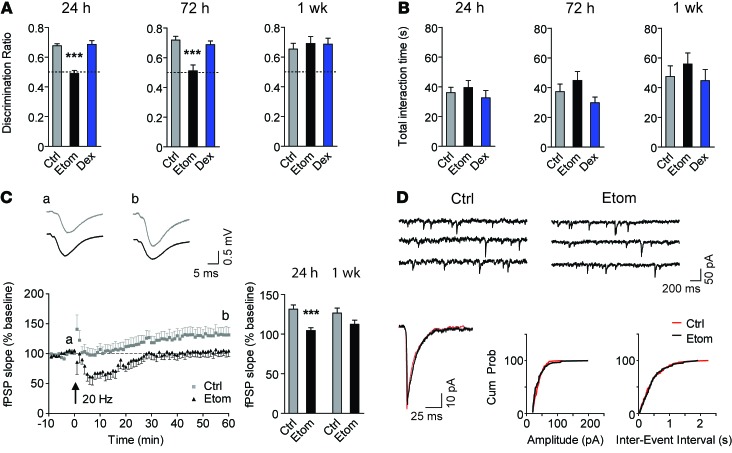
First, we investigated whether a single exposure to the injectable anesthetic etomidate causes postanesthetic memory deficits in mice using the novel object recognition assay. We selected etomidate because it preferentially binds to GABAARs and is rapidly metabolized to inactive metabolites (12, 13). Mice were treated with a low, sedative dose of etomidate (8 mg/kg, i.p.) that approximates the ED50 for loss of the righting reflex (LORR) (9, 10, 14). Memory was impaired at 24 and 72 hours but not 1 week after etomidate (Figure 1A). In contrast, no memory deficits were observed in mice treated with the active comparator dexmedetomidine (200 μg/kg, i.p.), a sedative α2-adrenergic receptor agonist that does not target GABAARs (15). Memory performance was not confounded by sedation or by reduced exploratory behavior, as the total interaction time of mice with the objects was similar in all groups (Figure 1B).
Figure 1. Etomidate impairs memory and synaptic plasticity but does not modify the function of postsynaptic GABAARs.
(A) Memory performance on the novel object recognition task after etomidate (8 mg/kg, i.p.) or dexmedetomidine (200 μg/kg, i.p.) and (B) total interaction time with both objects during testing (n = 9–12, 1-way ANOVA at each time point, Dunnett’s post-test). (C) Plasticity at Schaffer collateral-CA1 synapses 24 hours after treatment. Insets, representative traces recorded before (a) and 60 minutes after (b) 20-Hz stimulation. Bar graph summarizes data for the last 5 minutes of recording 24 hours (n = 7) or 1 week (n = 9–10) after etomidate (unpaired, 2-tailed Student’s t test at each time point). (D) Recordings of mIPSCs in CA1 pyramidal neurons 24 hours after etomidate. Left lower panel shows the averaged traces from control (red) and etomidate-treated (black) mice. Middle and right lower panels show the cumulative amplitude (P = 0.89) and the cumulative frequency (P = 0.25) distributions (Kolmogorov-Smirnov test, 125 events). Ctrl, vehicle control; Etom, etomidate; Dex, dexmedetomidine. Data are shown as mean ± SEM. ***P < 0.001.
Next, we sought to determine whether synaptic plasticity in ex vivo slices, a cellular correlate of memory, was impaired after etomidate (8 mg/kg). The Schaffer collateral pathway was stimulated at a threshold frequency (20 Hz) that induces synaptic potentiation (16). Under these conditions, synaptic plasticity is sensitive to changes in synaptic and extrasynaptic GABAAR activity (16, 17). Twenty-four hours after treatment, the potentiation of field postsynaptic potentials (fPSPs) was significantly lower in slices from etomidate-treated mice (60 minutes after stimulation; Figure 1C). Also, posttetanic depression (2 minutes after stimulation) and short-term depression of fPSPs (15 minutes after stimulation) occurred in slices from etomidate-treated but not vehicle-treated mice (Supplemental Figure 1, A and B; supplemental material available online with this article; doi:10.1172/JCI76669DS1). Paired-pulse facilitation, a presynaptic form of short-term plasticity (18), was similar in the 2 groups, suggesting no differences in the release of neurotransmitters from presynaptic terminals (Supplemental Figure 1C). Since memory performance recovered 1 week after etomidate, we studied plasticity at the 1-week time point. One week after etomidate (8 mg/kg), there was no significant difference in potentiation of fPSPs between groups, although there was a trend toward a reduction (control 127% vs. etomidate 113% of baseline; Figure 1C). At 1 week, posttetanic depression and short-term depression were no longer observed (Supplemental Figure 1, D and E). Paired-pulse facilitation was also similar in both groups (Supplemental Figure 1F).
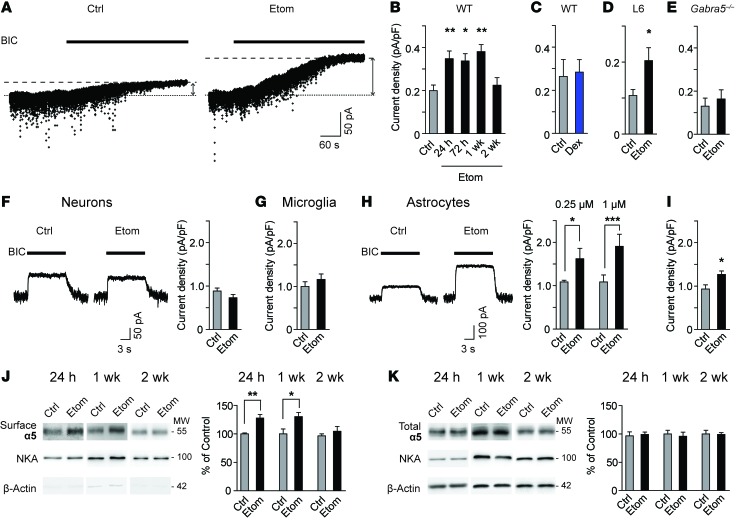
To determine whether GABAAR activity was persistently increased after etomidate (8 mg/kg), we recorded miniature inhibitory postsynaptic currents (mIPSCs) and a tonic inhibitory current in CA1 pyramidal neurons (19). Twenty-four hours after etomidate, the amplitude, frequency, and time course of mIPSCs were unchanged, suggesting no change in the activity of postsynaptic GABAARs (Figure 1D and Supplemental Table 1). In contrast, the tonic current was increased to 174% of control (Figure 2, A and B). The increase in tonic current persisted at 72 hours and 1 week, but not at 2 weeks (Figure 2B). Treatment with dexmedetomidine, the anesthetic that did not impair memory performance, caused no increase in tonic current (Figure 2C).
Figure 2. Etomidate causes a sustained increase in tonic current and cell-surface expression of α5GABAA receptors.
(A) Traces of tonic current recorded in CA1 pyramidal neurons. BIC, bicuculline (10 μM). (B–D) Tonic current in (B) WT slices 24 hours — 2 weeks after etomidate (n = 6–19, 1-way ANOVA, Dunnett’s post-test), (C) WT slices 24 hours after dexmedetomidine (n = 4–7) and (D) WT slices measured after application of L-655,708 (L6, 200 nM) 24 hours after etomidate (n = 9–12). (E) Tonic current in Gabra5–/– slices 24 hours after etomidate (n = 6–7). (F and G) Tonic current 24 hours after etomidate treatment in (F) cultured hippocampal neurons (1 μM, 1 h, n = 21), (G) neurons in microglia-neuron cocultures (1 μM, 1 h, n = 19), and (H) neurons in astrocyte-neuron cocultures (0.25 μM, 1 h, n = 6; 1 μM, 1 h, n = 10–11). Traces were obtained from astrocyte-neuron cocultures treated with 1 μM etomidate. (I) Tonic current in neurons treated with conditioned medium from etomidate-treated astrocytes (n = 19–21). (J and K) Western blots of (J) surface and (K) total expression in hippocampal slices. Separate blots for each time point (unpaired, 2-tailed Student’s t test for each time point; 24 h, n = 6; 1 wk, n = 5; 2 wk n = 3). NKA, Na+/K+ ATPase. MW is shown in kDa. Data are shown as mean ± SEM. Unpaired, 2-tailed Student’s t test unless otherwise indicated. *P < 0.05, **P < 0.01, ***P < 0.001.
Tonic current in CA1 pyramidal neurons is generated primarily by α5 subunit–containing GABAARs (α5GABAARs) (20). To determine whether α5GABAARs contributed to the increased tonic current, slices were perfused with the α5GABAAR-selective inverse agonist L-655,708 (200 nM) (21). The L-655,708–sensitive current was increased in slices from etomidate-treated mice (Figure 2D). Furthermore, tonic current was unchanged in slices from α5GABAAR null-mutant (Gabra5–/–) mice 24 hours after etomidate (Figure 2E).
We asked whether etomidate acts directly on neurons to increase tonic current. Etomidate did not change tonic current in cultured hippocampal neurons 24 hours after treatment (1 μM, 1 hour; Figure 2F). Since glial–neuron interactions might contribute to the increase in tonic current observed in ex vivo slices, neuron-glia cocultures were treated with etomidate. Etomidate did not change the tonic current in microglia-neuron cocultures 24 hours after treatment (1 μM, 1 hour; Figure 2G). However, 24 hours after etomidate treatment (0.25 μM or 1 μM, 1 hour), the tonic current was increased in neurons cocultured with astrocytes (Figure 2H). To determine whether etomidate acting on astrocytes was sufficient to increase the tonic current in neurons, conditioned medium was collected from astrocytes cultured alone and treated with etomidate (1 μM, 1 hour). The conditioned medium was then applied to hippocampal neurons for 24 hours. Under these conditions, the tonic current in neurons was increased to 136% of control (Figure 2I). Thus, treatment of astrocytes with etomidate was necessary and sufficient to trigger an increase in tonic current in hippocampal neurons.
We postulated that etomidate enhanced the tonic current by increasing cell-surface expression of α5GABAARs in the hippocampus. The cell-surface expression of α5 subunits was indeed increased to 128% of control at 24 hours and to 130% of control at 1 week; however, levels returned to baseline by 2 weeks (Figure 2J). The total expression of α5 subunits was unchanged at all time points (Figure 2K). Cell-surface expression of β3 subunits, which partner with α5 subunits to form GABAARs (19), was also increased at 24 hours but not at 1 or 2 weeks after etomidate (Supplemental Figure 2A). In contrast, the expression of δ subunits and α1 subunits, which contribute to hippocampal extrasynaptic and synaptic receptors, respectively (19), was unchanged after etomidate (Supplemental Figure 2, B and C). Thus, etomidate selectively increased the cell-surface expression of α5GABAARs in the hippocampus.
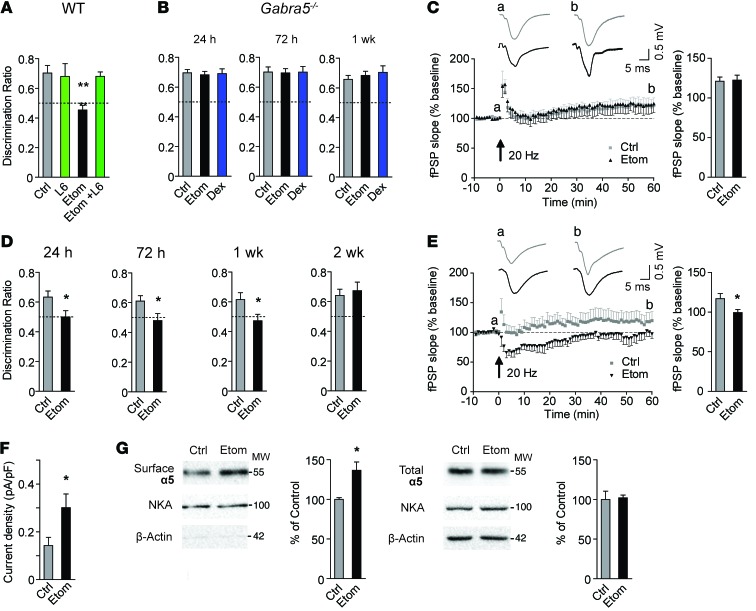
We next explored whether pharmacological or genetic inhibition of α5GABAARs reverses memory deficits after etomidate. Treatment with L-655,708 (0.5 mg/kg, i.p.) 30 minutes before training on the novel object recognition task completely reversed the memory deficits after etomidate, whereas L-655,708 alone did not alter performance (Figure 3A). Also, no memory deficits were observed in Gabra5–/– mice treated with etomidate or dexmedetomidine (Figure 3B). The total interaction time with the objects was similar in all groups (Supplemental Figure 3, A and B). Consistent with these behavioral results, postsynaptic and presynaptic plasticity at Schaffer collateral-CA1 synapses was not impaired in slices from Gabra5–/– mice treated with etomidate (Figure 3C and Supplemental Figure 3, C–E).
Figure 3. Reversal of impairment after a sedative dose of etomidate and the effects of an anesthetizing dose of etomidate.
(A–C) Effects of etomidate (8 mg/kg i.p.) on (A) memory performance in WT mice treated with L-655,708 (L6, 0.5 mg/kg, n = 6–11, 1-way ANOVA, Dunnett’s post-test), (B) memory performance in Gabra5–/– mice (n = 8–13, 1-way ANOVA, Dunnett’s post-test at each time point), and (C) plasticity at Schaffer collateral-CA1 synapses 24 hours after etomidate in Gabra5–/– slices. For all fPSP data, insets: traces recorded before (a) and 60 minutes after (b) 20-Hz stimulation. Bar graph shows summarized data for the last 5 minutes of recording (n = 7–8). (D–G) Effects of an anesthetizing dose of etomidate (20 mg/kg i.p.) in WT mice on (D) memory performance (n = 9–10), (E) plasticity (n = 6–7), (F) tonic current (n = 7–8), and (G) surface and total expression of α5 subunits (n = 4) in hippocampal slices 24 hours after etomidate. MW is shown as kDa. Data are shown as mean ± SEM. Unpaired, 2-tailed Student’s t test unless otherwise indicated. *P < 0.05; **P < 0.01.
We next studied a higher, anesthetizing dose of etomidate. Mice were treated with etomidate at 20 mg/kg, i.p., which is the ED100 dose for LORR (10). Object recognition memory was impaired for 1 week but recovered by 2 weeks after treatment (Figure 3D). This dose did not alter total interaction time with the objects (Supplemental Figure 4A). The longer duration of memory impairment after the higher (20 mg/kg) versus the lower (8 mg/kg) dose of etomidate suggests a dose-dependent effect. Similar to the sedative dose, treatment with etomidate (20 mg/kg) reduced potentiation of fPSPs (Figure 3E) and induced posttetanic depression and short-term depression of fPSPs, but did not change paired-pulse facilitation 24 hours after treatment (Supplemental Figure 4, B–D). Furthermore, etomidate (20 mg/kg) increased the tonic current and cell-surface expression of α5 subunits in the hippocampus but did not alter the expression of α1 subunits 24 hours after treatment (Figure 3, F and G, and Supplemental Figure 4E).
Finally, we investigated whether an inhaled anesthetic caused a similar increase in tonic current and cell-surface expression of α5GABAARs. For these experiments, we selected isoflurane because it is widely used in clinical practice, it acts on GABAARs, and it is at undetectable or trace levels in the brain 24 hours after treatment (22). A low, sedative dose of isoflurane (0.7%, 20 minutes) (23) caused no change in the cell-surface expression of α5 subunits or δ subunits 24 hours after treatment (Supplemental Figure 5, A and B). In contrast, a higher, anesthetizing dose of isoflurane (24) (1.3%, 1 hour) increased the tonic current to 237% of control and the cell-surface expression of α5 to 134% of control, but the expression of δ subunits was unchanged (Supplemental Figure 5, C–E). Notably, the sedative dose of etomidate but not isoflurane increased cell-surface expression of α5GABAARs, possibly because of the shorter duration of action and more rapid elimination of isoflurane (13, 25).
Our findings present the first evidence, to our knowledge, that even a brief exposure to a GABAergic general anesthetic triggers a sustained increase in tonic current and cell-surface expression of α5GABAARs in the hippocampus. This increase in α5GABAAR activity in turn causes deficits in anterograde memory. In contrast, the anesthetic dexmedetomidine, which targets adrenergic receptors rather than GABAARs, causes no change in the amplitude of the tonic current or memory performance. These results refute the assumption that the activity of primary target receptors for GABAergic anesthetics returns to baseline after the drugs are eliminated.
Interestingly, at 1 week after etomidate (8 mg/kg), memory performance recovered, yet the tonic current remained elevated. The sustained increase in tonic current may trigger compensatory changes that contribute to the recovery of memory performance, given that homeostatic plasticity has been widely demonstrated in the hippocampus (26). Indeed, our results suggest such compensatory changes do occur. One week after etomidate, tonic current and cell-surface expression remained elevated, whereas synaptic plasticity partially recovered and memory performance returned to baseline.
In conclusion, we have presented evidence for what we believe is a previously unrecognized long-term effect of general anesthetics on α5GABAARs. Additional studies are required to determine whether the sustained increase in α5GABAAR activity is triggered by the initial direct allosteric actions of anesthetics on GABAARs or by other mechanisms.
Methods
Further information is available in Supplemental Methods.
Statistics.
Results are presented as mean ± SEM. An unpaired Student’s t test was used to compare 2 groups. For 3 or more groups, 1-way ANOVA followed by Dunnett’s test was applied. The Kolmogorov-Smirnov test and Shapiro-Wilk test were used to validate the assumption of normality. When the assumption was not met, the Mann-Whitney U test was employed. Statistical Package for the Social Sciences (IBM Corp.) and GraphPad Prism software, version 4.0, were used. A P value of less than 0.05 was considered statistically significant.
Study approval.
All experimental procedures were approved by the Animal Care Committee of the University of Toronto.
Supplementary Material
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (grant numbers: 38028 and 79428) and a Canada Research Chair in Anesthesia to B.A. Orser. A.A. Zurek was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Heart and Stroke Foundation of Canada. J. Yu was supported by NSERC. S.C. Haffey was supported by the Kirk Weber Award in Anesthesia and the Ontario Student Opportunities Trust Fund. A. Penna was supported by the Sleep and Biological Rhythms Toronto Program and Becas Chile. I. Lecker was supported by the Savoy Foundation and CIHR. We thank Ella Czerwinska for preparation of cell cultures.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2014;124(12):5437–5441. doi:10.1172/JCI76669.
References
- 1.Weiser TG, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Monk TG, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 3.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108(1):8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): A meta-analysis. PLoS One. 2013;8(11):e79624. doi: 10.1371/journal.pone.0079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cibelli M, et al. Role of interleukin-1β in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moller JT, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. doi: 10.1016/S0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 7.Crosby C, Culley DJ, Baxter MG, Yukhananov R, Crosby G. Spatial memory performance 2 weeks after general anesthesia in adult rats. Anesth Analg. 2005;101(5):1389–1392. doi: 10.1213/01.ANE.0000180835.72669.AD. [DOI] [PubMed] [Google Scholar]
- 8.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96(4):1004–1009. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5(9):709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 10.Cheng VY, et al. α5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26(14):3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belelli D, Callachan H, Hill-Venning C, Peters JA, Lambert JJ. Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors expressed in Xenopus laevis oocytes. Br J Pharmacol. 1996;118(3):563–576. doi: 10.1111/j.1476-5381.1996.tb15439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the γ-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci U S A. 1997;94(20):11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology. 2011;114(3):695–707. doi: 10.1097/ALN.0b013e3181ff72b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarren HS, Moore JT, Kelz MB. Assessing changes in volatile general anesthetic sensitivity of mice after local or systemic pharmacological intervention. J Vis Exp. 2013;(80):e51079. doi: 10.3791/51079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders RD, Maze M. α2-adrenoceptor agonists. Curr Opin Investig Drugs. 2007;8(1):25–33. [PubMed] [Google Scholar]
- 16.Martin LJ, et al. α5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci. 2010;30(15):5269–5282. doi: 10.1523/JNEUROSCI.4209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steele PM, Mauk MD. Inhibitory control of LTP and LTD: Stability of synapse strength. J Neurophysiol. 1999;81(4):1559–1566. doi: 10.1152/jn.1999.81.4.1559. [DOI] [PubMed] [Google Scholar]
- 18.Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14(9):5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 20.Caraiscos VB, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101(10):3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quirk K, et al. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit. Neuropharmacology. 1996;35(9–10):1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 22.Saab BJ, et al. Short-term memory impairment after isoflurane in mice is prevented by the α5 γ-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology. 2010;113(5):1061–1071. doi: 10.1097/ALN.0b013e3181f56228. [DOI] [PubMed] [Google Scholar]
- 23.Sonner JM, et al. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express α1 γ-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology. 2007;106(1):107–113. doi: 10.1097/00000542-200701000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Sonner JM, Gong D, Eger EI. Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg. 2000;91(3):720–726. doi: 10.1213/00000539-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 25.Holaday DA, Fiserova-Bergerova V, Latto IP, Zumbiel MA. Resistance of isoflurane to biotransformation in man. Anesthesiology. 1975;43(3):325–332. doi: 10.1097/00000542-197509000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.