Abstract
General anesthetics induce loss of consciousness by inhibiting ascending arousal pathways, and they interfere with gap junction electrical coupling. The present study aimed to determine whether inhibition of gap junction-mediated signaling could influence general anesthetic-induced loss of consciousness. The general anesthetics sevoflurane and propofol were used. Intracerebroventricular administration of carbenoxolone, a gap junction blocker, significantly decreased the time to loss of the righting reflex (P < 0.05), but prolonged the time to recovery of the reflex (P < 0.05). Moreover, intracerebroventricular administration of carbenoxolone increased the sensitivity to sevoflurane, with a leftward shift of the loss of righting reflex dose-response curve, and decreased the 50% effective concentration of sevoflurane. These results suggest that the gap junction blocker carbenoxolone enhances propofol and sevoflurane-mediated general anesthesia.
Keywords: gap junction, blocker, propofol, sevoflurane, general anesthesia, nerve block, neuropharmacology
INTRODUCTION
Gap junctions are composed of proteins that form a channel connecting the cytoplasm of adjacent cells, which allows various molecules and ions to pass freely between them[1,2]. There are numerous gap junction protein families connecting different types of cells such as neurons and oligodendrocytes. In the central nervous system, gap junctions play an important role in brain physiology. They synchronize neuronal activity and connect glial cells participating in the regulation of brain metabolism and homeostasis[3,4]. The behavioral and clinical effects of gap junction blockers suggest that gap junctions are involved in the regulation of locomotor activity, arousal, memory, breathing and consciousness[5]. General anesthetics act on multiple receptors, ion channels, and cell signaling systems in the central nervous system to produce anesthesia[6]. However, it remains poorly understood how general anesthetics induce loss of consciousness. Recent studies have suggested that general anesthetics induce loss of consciousness by inhibiting ascending arousal pathways or by potentiating sleep promoting pathways[7,8]. Recent studies suggest that certain anesthetic agents decrease gap junctional electrical coupling in vitro [9]. We therefore hypothesized that inhibiting gap junction activity would enhance general anesthetic-induced loss of consciousness. The present study was designed to explore the influence of gap junction blockade on the hypnotic effects of the general anesthetics propofol and sevoflurane in rats.
RESULTS
Grouping and treatment of experimental animals
Eighty male adult Sprague-Dawley rats were randomly assigned to eight groups (n = 10): sham-surgery (normal saline injection via the lateral ventricle); carbenoxolone (CA); propofol (propofol injection via the lateral ventricle); propofol + CA (further divided into propofol + 200 μg CA, propofol + 300 μg CA and propofol + 400 μg CA); sevoflurane; sevoflurane + CA. In total, 80 rats were included in the final analysis.
Effects of different doses of CA on propofol-mediated anesthesia
To study the effects of gap junction channel blockade on intravenous anesthesia, animals first received intracerebroventricular administration of CA at different doses. CA alone did not produce any observable change in behavior. However, intracerebroventricular pretreatment with CA, in a dose-dependent manner, decreased the time to loss of the righting reflex in rats receiving intraperitoneal injection of 5 mg/100 g propofol (propofol = 12.4 ± 3.32 minutes, propofol + 200 μg CA = 6.27 ± 1.26 minutes, propofol + 300 μg CA = 3.47 ± 1.31 minutes, propofol + 400 μg CA = 3.52 ± 0.58 minutes; P < 0.05). Furthermore, intracerebroventricular pretreatment with CA significantly increased the time it took for the righting reflex to recover compared with the sham-surgery group (propofol = 24.25 ± 4.57 minutes, propofol + 200 μg CA = 74.73 ± 13.1 minutes, propofol + 300 μg CA = 76.48 ± 7.46 minutes, propofol + 400 μg CA = 73.67 ± 8.87 minutes; P < 0.05; Figures 1, 2).
Figure 1.
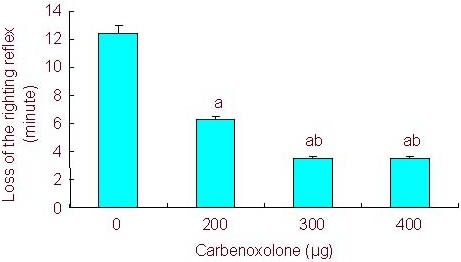
Effect of different doses of carbenoxolone on the time to loss of the righting reflex.
Rats were pretreated with different doses of carbenoxolone followed by propofol (5 mg/100 g).
All values are expressed as mean ± SEM of 10 rats in each group.a P < 0.05, vs. 0 μg, bP < 0.05, vs. 200 μg (one-way analysis of variance and Student-Newman- Keuls test).
Figure 2.
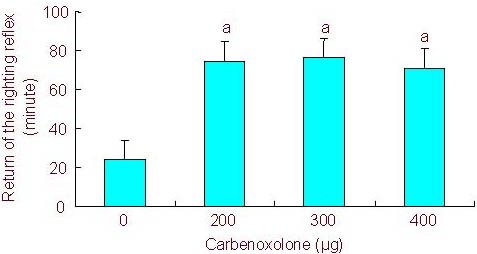
Effect of different doses of carbenoxolone on return of the righting reflex.
Rats were pretreated with different doses of carbenoxolone followed by propofol (5 mg/100 g).
All values are expressed as mean ± SEM of 10 rats in each group.aP < 0.05, vs. 0 μg (one-way analysis of variance and Student-Newman-Keuls test).
Effects of different doses of CA on sevoflurane-mediated anesthesia
We also assessed the effects of gap junction channel blockade on inhalational anesthesia. Exposure to sevoflurane produced anesthetic effects or hypnosis in a dose-dependent manner in both groups of rats. However, rats that received CA exhibited increased sensitivity to sevoflurane compared with rats that received normal saline. The concentration at which one half of the rats showed loss of the righting reflex was 1.5% in the sham-surgery group (n = 10) and 1.3% in the CA group (n = 10). The 50% effective concentration (EC50) decreased (sevoflurane = 1.5%, sevoflurane + CA = 1.3%, P < 0.05; Figure 3).
Figure 3.
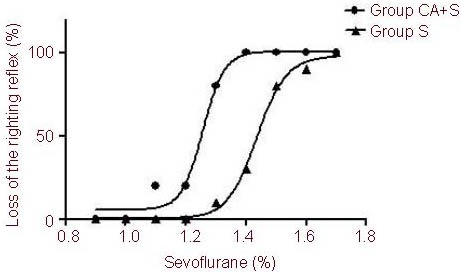
Dose-response curve of the loss of righting reflex versus sevoflurane concentration.
Rats were pretreated with sevoflurane (S group) or 200 μg carbenoxolone through the lateral ventricle followed by different doses of sevoflurane (CA + S group).
All values were expressed as mean ± SEM of 10 rats in each group. aP < 0.05, vs. sevoflurane group (F test for nonlinear regressions).
DISCUSSION
Gap junctions are mainly composed of the connexin protein, and they allow for the movement of important inorganic ions, second messengers and other small water-soluble molecules between cells[10,11,12]. The present study demonstrates for the first time that intracerebroventricular administration of the gap junction blocker CA decreases the time to onset of the propofol-induced loss of the righting reflex and increases the duration of this loss in a dose-dependent manner. CA pretreatment also induced a leftward shift in the loss of the righting reflex dose-response curve. Therefore, these results suggest that gap junction blockade facilitates general anesthetic-induced loss of consciousness. CA is a central nervous system gap junction inhibitor, which can change the structure of the gap junction by modulating the phosphorylation and dephosphorylation of the connexin subunits[13]. CA is a general gap junction blocker, not specific for a particular type of gap junction[14]. In addition to its ability to block gap junctions, CA is used as a blocker of the enzyme 11β-hydroxysteroid dehydrogenase. Long-term administration of CA (100 mg three times per day for 4 weeks) was found to improve verbal fluency and verbal memory in healthy elderly men[14,15]. Since CA was acutely infused in the present study, the effects of CA observed here are likely due to inhibition of all central nervous system gap junction channels.
A number of studies have reported that gap junctions can be regulated by certain general anesthetics. Intravenous anesthetic propofol (15 μM) at clinically relevant concentrations attenuates gap junction permeability in P19 cells and in cultured organotypic hippocampal slices[16,17]. In the present study, the gap junction blocker CA alone had no effect on loss of the righting reflex, suggesting that inhibition of gap junction function is not sufficient to induce loss of consciousness. However, administration of CA shortened the time to loss of the righting reflex mediated by propofol, and it prolonged the duration of this loss as well. CA also decreased the EC50 value for sevoflurane-mediated loss of the righting reflex. A potential role of gap junctions in modulating the anesthesia response was also demonstrated by a recent study showing that connexin36 knockout mice were more sensitive to the hypnotic effects of isoflurane compared with wild-type animals[18]. While these knockout mice have a lack of functional gap junctions from birth, and thus may have acquired compensatory mechanisms. The present study demonstrated that acute gap junction blockade strongly enhanced the anesthetic response.
Recent evidence suggests that general anesthetics induce loss of consciousness by inhibiting ascending arousal pathways or by potentiating sleep promoting pathways[7,8]. Gap junction electrical coupling is present in certain nuclei within the reticular activating system, including the parafascicular nucleus, the pedunculopontine nucleus and the subcoeruleus nucleus[19,20]. Blockade of gap junctions in these nuclei may modulate neurotransmitter signaling and the effect of general anesthetics, producing changes in the loss of the righting reflex.
In summary, CA, a gap junction blocker, decreased the time to loss of the righting reflex mediated by propofol. It also prolonged the duration of this loss, and decreased the EC50 of sevoflurane-mediated loss of the righting reflex. We propose that the inhibition of gap junction dependent activities in the central nervous system partly underlies the anesthetic-induced loss of consciousness, and gap junction-mediated interneuronal communication could be a site of action of anesthetics. Future studies should examine changes in neuronal activity and neurotransmitter signaling to better understand the mechanisms responsible for the observed behavior.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Experimental Center of Renmin Hospital of Wuhan University, China, from March 2009 to October 2010.
Materials
A total of 80 male adult Sprague-Dawley rats, aged 6–8 weeks, weighing 210–260 g, were purchased from the Experimental Animals Centre for Disease Control and Prevention of Hubei Province, China (Certificate No. 00012298). Protocols were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[21].
Methods
Model establishment by intracerebroventricular cannula implantation
Rats were anesthetized with chloral hydrate (10 g/100 mL of distilled water; 4 mg/kg, intraperitoneally) and placed in a stereotaxic frame (Huaibei Zhenghua, Anhui, China). A guide cannula for chronic drug infusion was implanted in the lateral cerebral ventricle[22] (posterior −0.8 mm; lateral 1.4 mm; dorsal 3.0 mm) and fixed to the cranium with the use of dental acrylic and small screws, as described previously[22]. On the experiment day, CA or a saline control solution was infused into the lateral cerebral ventricle via a 30-gauge injection needle connected to a 25-μL microsyringe with polyethylene (PE-20) tubing. The injection needle protruded 1 mm beyond the tip of the cannula to reach the lateral cerebral ventricle[23]. At the end of testing, rats were anesthetized and perfused transcardially with normal saline (0.9%) and then formalin (4%). Cannula placement was verified under a light microscope.
Detection of the loss of the righting reflex
The loss of the righting reflex was used to evaluate hypnotic action in rats. For the propofol study, the time to loss of the righting reflex and the time to recovery of the righting reflex after intraperitoneal injection of propofol were recorded. For the sevoflurane study, the animals were exposed to increasing concentrations of sevoflurane and the concentration at which 50% of rats lost the righting reflex was calculated. After adjusting the vaporizer to achieve a new concentration, a 3-minute equilibration period was allowed before testing. The percentage of animals with a loss of the righting reflex at concentrations of sevoflurane 0.9% and above (using increments of 0.1%) was used to establish the dose-response curve. Dose-response data were fitted to a formula: Y = Ymin + (Ymax−lYmin)/[1 + 10log(ED50 −X)*m],
where Y is the percentage of rats with a loss of the righting reflex; Ymin and Ymax are the minimal and maximal values of Y, respectively; ED50 is the drug dose for a half (Ymax−Ymin); X is the logarithmic drug dose; and m is the Hill’s slope constant.
Statistical analyses
All data were expressed as mean ± SEM and analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). The results of different groups were compared by one-way analysis of variance and Student-Newman-Keuls test. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Hubei Province, No. 2010CHB01001.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Renmin Hospital of Wuhan University, China.
(Edited by Zhang DS, Gong QH/Su LL/Song LP)
REFERENCES
- [1].Retamal MA, Froger N, Palacios-Prado N, et al. Cx43 hemichannels and gap junction channels in astroeytes are regulated oppositely by Proinflammatory cytokines released from aetivated microglia. J Neurosci. 2007;27(50):13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bennett MV, Barrio LC, Bargiello TA, et al. Gap junction: new tool, new answers, new questions. Neuron. 1991;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- [3].Orthmann-Murphy JL, Freidin M, Fischer E, et al. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendroeyte connexins. J Neurosci. 2007;27(51):13949–13957. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mantz J, Cordier J, Giaume C. Effects of general anesthetics on intracellular communications mediated by gap junctions between astrocytes in primary culture. Anesthesiology. 1993;78(5):892–901. doi: 10.1097/00000542-199305000-00014. [DOI] [PubMed] [Google Scholar]
- [5].Wentlandt K, Samoilova M, Carlen PL, et al. General anesthetics inhibit gap junction communication in cultured organotypic hippocampal slices. Anesth Analg. 2006;102(6):1692–1698. doi: 10.1213/01.ane.0000202472.41103.78. [DOI] [PubMed] [Google Scholar]
- [6].Masaki E, Kawamura M, Kato F. Attenuation of gap-junction-mediated signaling facilitated anesthetic effect of sevoflurane in the central nervous system of rats. Anesth Analg. 2004;98(3):3647–3652. doi: 10.1213/01.ane.0000103259.72635.72. [DOI] [PubMed] [Google Scholar]
- [7].Zecharia AY, Nelson LE, Gent TC, et al. The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J Neurosci. 2009;29(7):2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zecharia AY, Franks NP. General anesthesia and ascending arousal pathways. Anesthesiology. 2009;111(4):695–696. doi: 10.1097/ALN.0b013e3181b061bc. [DOI] [PubMed] [Google Scholar]
- [9].Jäderstad J, Brismar H, Herlenius E. Hypoxic preconditioning increases gap-junctional graft and host communication. Neuroreport. 2010;21(17):1126–1132. doi: 10.1097/WNR.0b013e328340a77b. [DOI] [PubMed] [Google Scholar]
- [10].Masaki E, Yamazaki K, Ohno Y, et al. The anesthetic interaction between adenosine triphosphate and N-methyl-d-aspartate receptor antagonists in the rat. Anesth Analg. 2001;92(1):134–139. doi: 10.1097/00000539-200101000-00026. [DOI] [PubMed] [Google Scholar]
- [11].Tomas MA, Zosso N, Scerri I, et al. A tyrosine-based sorting singnal is involved in connexin43 stability and gap junction turnover. J Cell Sci. 2003;116(Pt 11):2213–2222. doi: 10.1242/jcs.00440. [DOI] [PubMed] [Google Scholar]
- [12].Lin JH, Lou N, Kang N, et al. A central role of connexin 43 in hypoxic preconditioning. Neuroscience. 2008;28(3):681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol. 2001;85(4):1543–1551. doi: 10.1152/jn.2001.85.4.1543. [DOI] [PubMed] [Google Scholar]
- [14].Pietro G, Daniele C, Natale B, et al. Antiabsence effects of carbenoxolone in two genetic animal models of absence epilepsy. Neuropharmacology. 2005;49(4):551–563. doi: 10.1016/j.neuropharm.2005.04.012. [DOI] [PubMed] [Google Scholar]
- [15].Nuotio-Antar AM, Hachey DL, Hasty AH. Carbenoxolone treatment attenuates symptoms of metabolic syndrome and atherogenesis in obese, hyperlipidemic mice. Am J Physiol Endocrinol Metab. 2007;293(6):1517–1528. doi: 10.1152/ajpendo.00522.2007. [DOI] [PubMed] [Google Scholar]
- [16].Wentlandt K, Caden PL, Kushnir M, et al. General anesthetics attenuate gap junction coupling in P19 cell line. J Neurosci Res. 2005;81(5):746–752. doi: 10.1002/jnr.20577. [DOI] [PubMed] [Google Scholar]
- [17].Rozental R, Andrade-Rozental AF, Zheng X, et al. Gap junction-mediated bidirectional signaling between human fetal hippocampal neurons and astrocytes. Dev Neurosci. 2001;23(6):420–431. doi: 10.1159/000048729. [DOI] [PubMed] [Google Scholar]
- [18].Jacobson GM, Voss LJ, Melin SM, et al. The role of connexin36 gap junctions in modulating the hypnotic effects of isoflurane and propofol in mice. Anaesthesia. 2011;66(5):361–367. doi: 10.1111/j.1365-2044.2011.06658.x. [DOI] [PubMed] [Google Scholar]
- [19].Guan BC, Si JQ, Jiang ZG. Blockade of gap junction coupling by glycyrrhetinic acids in guinea pig cochlear artery: a whole-cell voltage- and current-clamp study. Br J Pharmacol. 2007;151(7):1049–1060. doi: 10.1038/sj.bjp.0707244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beck P, Odle A, Wallace-Huitt T, et al. Modafinil increases arousal determined by P13 potential amplitude: an effect blocked by gap junction antagonists. Sleep. 2008;31(12):1647–1654. doi: 10.1093/sleep/31.12.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [22].Methippara MM, Bashir T, Kumar S, et al. Salubrinal, an inhibitor of protein synthesis, promotes deep slow wave sleep. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R178–184. doi: 10.1152/ajpregu.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luo T, Leung LS. Involvement of tuberomamillary histaminergic neurons in isoflurane anesthesia. Anesthesiology. 2011;115(1):36–43. doi: 10.1097/ALN.0b013e3182207655. [DOI] [PubMed] [Google Scholar]


