Abstract
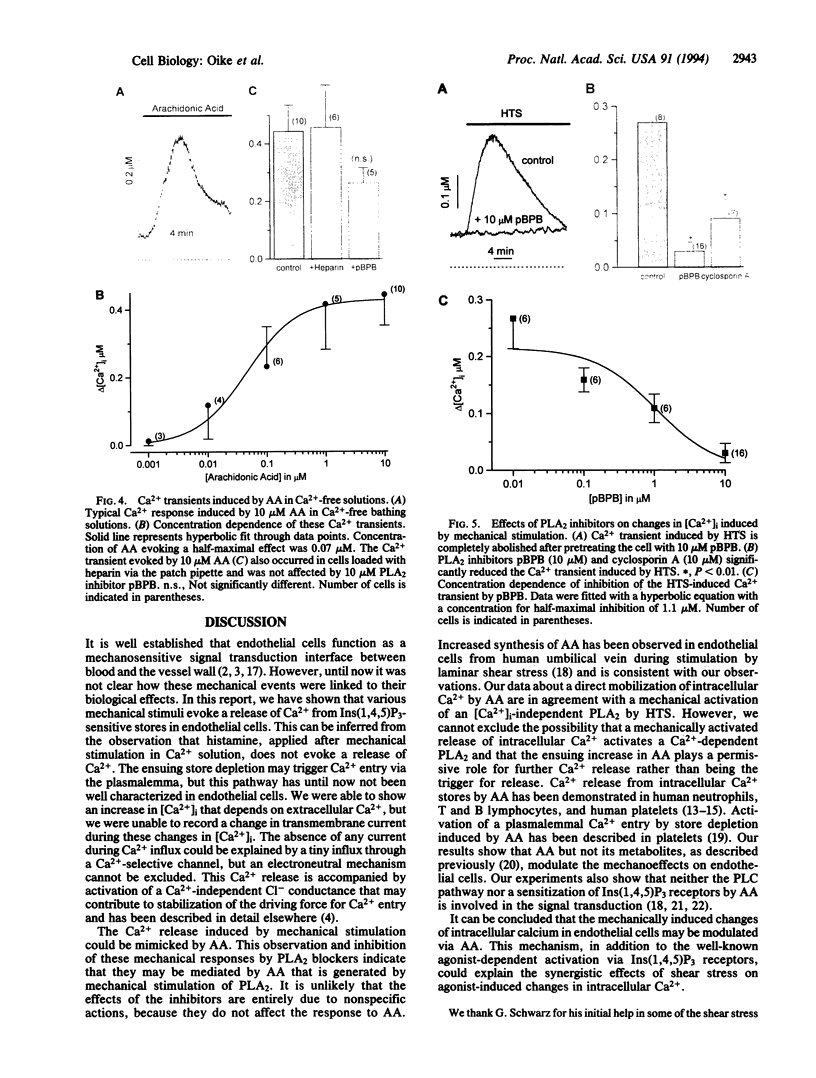
We have investigated the changes in intracellular calcium concentration ([Ca2+]i) in human endothelial cells induced by mechanical stretch due to osmotic cell swelling. Hypotonic solutions also activate a Cl- conductance that has been described elsewhere and mainly serves to clamp the membrane potential at negative values to provide a driving force for Ca2+ influx. The increase in [Ca2+]i caused by hypotonic solutions is due to release from inositol-1,4,5-trisphosphate-sensitive Ca2+ pools and a subsequent Ca2+ influx, apparently activated by store depletion. These changes in [Ca2+]i are completely abolished if the phospholipase A2 (PLA2) activity is inhibited by either 4-bromophenacyl bromide or cyclosporin A. Arachidonic acid, applied either extracellularly or intracellularly via the patch pipette, mimics the mechanosensitive response even in cells with blocked PLA2. Metabolites of the lipo- and cyclooxygenase pathways can be excluded. Phospholipase C activation and the protein kinase A pathway are not involved in this mechanical response. Although no specific pharmacological tools for probing the role of PLA2 are available, our evidence suggests that mechanosensitivity in endothelial cells may be modulated by arachidonic acid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. T., Sanchez A., Garcia-Sancho J. Arachidonic acid-induced calcium influx in human platelets. Comparison with the effect of thrombin. Biochem J. 1990 Dec 1;272(2):435–443. doi: 10.1042/bj2720435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumier L., Faucher N., Naccache P. H. Arachidonic acid-induced release of calcium in permeabilized human neutrophils. FEBS Lett. 1987 Sep 14;221(2):289–292. doi: 10.1016/0014-5793(87)80942-0. [DOI] [PubMed] [Google Scholar]
- Bhagyalakshmi A., Berthiaume F., Reich K. M., Frangos J. A. Fluid shear stress stimulates membrane phospholipid metabolism in cultured human endothelial cells. J Vasc Res. 1992 Nov-Dec;29(6):443–449. doi: 10.1159/000158963. [DOI] [PubMed] [Google Scholar]
- Corado J., Le Deist F., Griscelli C., Fischer A. Inositol 1,4,5-trisphosphate- and arachidonic acid-induced calcium mobilization in T and B lymphocytes. Cell Immunol. 1990 Apr 1;126(2):245–254. doi: 10.1016/0008-8749(90)90318-l. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Tripathi S. C. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res. 1993 Feb;72(2):239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- Force T., Hyman G., Hajjar R., Sellmayer A., Bonventre J. V. Noncyclooxygenase metabolites of arachidonic acid amplify the vasopressin-induced Ca2+ signal in glomerular mesangial cells by releasing Ca2+ from intracellular stores. J Biol Chem. 1991 Mar 5;266(7):4295–4302. [PubMed] [Google Scholar]
- Hertelendy F., Molnár M., Jamaluddin M. Dual action of arachidonic acid on calcium mobilization in avian granulosa cells. Mol Cell Endocrinol. 1992 Feb;83(2-3):173–181. doi: 10.1016/0303-7207(92)90157-2. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993 Jun;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol. 1989 Nov;94(5):789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Schwartz G., Oike M., Droogmans G. Histamine-activated, non-selective cation currents and Ca2+ transients in endothelial cells from human umbilical vein. Pflugers Arch. 1993 Aug;424(3-4):285–293. doi: 10.1007/BF00384354. [DOI] [PubMed] [Google Scholar]
- Nollert M. U., Diamond S. L., McIntire L. V. Hydrodynamic shear stress and mass transport modulation of endothelial cell metabolism. Biotechnol Bioeng. 1991 Sep;38(6):588–602. doi: 10.1002/bit.260380605. [DOI] [PubMed] [Google Scholar]
- Nozawa Y., Nakashima S., Nagata K. Phospholipid-mediated signaling in receptor activation of human platelets. Biochim Biophys Acta. 1991 Apr 3;1082(3):219–238. doi: 10.1016/0005-2760(91)90197-p. [DOI] [PubMed] [Google Scholar]
- Rustenbeck I., Lenzen S. Effect of lysophospholipids, arachidonic acid and other fatty acids on regulation of Ca2+ transport in permeabilized pancreatic islets. Cell Calcium. 1992 Apr;13(4):193–202. doi: 10.1016/0143-4160(92)90007-f. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Callewaert G., Droogmans G., Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J Physiol. 1992 Dec;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]






