Abstract
Asymptomatic solitary pulmonary nodules incidentally revealed by computed tomography has become a serious medical problem. Depending on their diameter, solid, part-solid, or pure ground-glass pulmonary nodules may be observed, diagnosed radiologically/invasively, or resected in accordance with international guidelines. Pure ground-glass nodules, semi-solid lesions, or solid lesions smaller than 8 mm should be monitored by serial low-dose computed tomography. In the case of solid nodules greater than 8 mm, the assessment of the risk of malignancy is recommended. Patients at high risk of lung cancer with pulmonary lesions should undergo diagnostic investigation, or the nodule should be resected. If the risk of lung cancer is low, the patients may be monitored. Needle aspiration biopsy is the most important invasive method of tumor diagnosis. Cytological or histopathological diagnosis is helpful in appropriate clinical decision making that reduces the risk of unnecessary surgery, decreasing the rate of benign nodule resections and thus reducing the costs of medical treatment.
Keywords: solitary pulmonary nodule, lung neoplasms
Abstract
Bezobjawowy guz płuca wykryty w tomografii komputerowej stanowi coraz częstszy problem kliniczny. Zmiany o charakterze litym, częściowo litym lub nielitym, w zależności od średnicy, można – zgodnie z międzynarodowymi zaleceniami – poddać obserwacji, diagnostyce radiologicznej lub inwazyjnej albo leczeniu chirurgicznemu. Zmiany o charakterze nielitym lub częściowo litym oraz zmiany lite o średnicy poniżej 8 mm powinny być obserwowane w badaniach niskodawkowej tomografii komputerowej. W przypadku zmian litych o średnicy przekraczającej 8 mm należy ocenić ryzyko wystąpienia nowotworu złośliwego. Pacjenci, u których ryzyko nowotworu jest wysokie, powinni być kwalifikowani do diagnostyki inwazyjnej lub leczenia operacyjnego. Jeżeli ryzyko zachorowania jest niskie, wskazana jest obserwacja zmian. Biopsja aspiracyjna to jedna z podstawowych inwazyjnych metod diagnostyki guza płuca. Cytologiczne lub histopatologiczne rozpoznanie pomaga w podjęciu właściwej decyzji klinicznej oraz zmniejsza ryzyko niepotrzebnych operacji, wpływając na obniżenie odsetka resekcji łagodnych guzów płuca i zmniejszenie wydatków na opiekę zdrowotną.
Introduction
Lung cancer is a serious medical, social, and economic problem. Despite the advances in the treatment of other neoplasms, achieved within recent years, the 5-year survival rate of lung cancer patients remains very low. Globally, approximately 1,448,000 individuals develop lung cancer each year. In developed countries, lung cancer is the third most frequent cause of death after coronary artery and cerebrovascular disease [1]. In the USA, lung cancer is the second most frequently developed malignant neoplasm after prostate cancer in men and breast cancer in women. In both sexes, lung cancer is responsible for the largest number of deaths resulting from neoplastic disease: in men, it surpasses the next 4 most frequent neoplasms combined; in women, it results in almost twice as many deaths as breast cancer [2]. According to global statistics, the incidence of lung cancer and the number of deaths caused by it in Poland are higher than in other highly and moderately developed countries. In 2008, the estimated number of new cases for both sexes in Poland was 71.21 per 100,000, placing our country in third place, after Hungary and Armenia [3].
Lung cancer screening have been a matter of controversy for years. It has been proven that sputum cytology and chest X-ray failed to show any benefit as a screening tools [4]. In 2011, the results of the multicenter, randomized study “National Lung Screening Trial” sponsored by NCI were published: the study demonstrated a 20% mortality reduction in the study group examined with low-dose computed tomography (LDCT) in comparison to the control group assessed with traditional X-ray examinations [5]. Low-dose computed tomography screening has been recommended by the American College of Chest Physicians (ACCP), the American Society of Clinical Oncology, and the American Association for Thoracic Surgery [6, 7]. The U.S. Preventive Services Task Force also published its positive recommendation concerning lung cancer screening in 2013 [8].
Performing yearly low-dose computed tomography examinations is recommended in individuals at a high risk of lung cancer:
aged 55-74 years,
active smokers and ex-smokers who ceased smoking ≤ 15 years ago,
Apart from reducing lung cancer mortality rates in individuals undergoing yearly low-dose computed tomography examinations, another benefit of the screening procedure is that it provides an opportunity for smoking cessation interventions [6]. The combination of LDCT and smoking cessation interventions is considered to be a most cost-effective method in lung cancer screening [9]. On the other hand, the relatively large number of false positives, the potentially unnecessary invasive diagnostics in the case of benign pulmonary nodules, reduced quality of life and increased anxiety of patients, associated with the diagnosis of a lung tumor, are major drawbacks of the screening [6, 10]. With the popularization of computed tomography, finding of the new lung tumors is becoming frequent clinical problem despite the availability of many advanced examination methods and international guidelines. When selecting a diagnostic method, physicians often face various alternative opportunities that leads to difficult questions: i.e. should they be diagnose or observe? When should a lung tumor biopsy or a positron emission tomography (PET) be performed? Or should the patient be referred for surgical removal of the lesion immediately? Lately two publications of updated guidelines for lung cancer management have been issued: “Evaluation of individuals with pulmonary nodules: when is it lung cancer?” by the ACCP [11] and “Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner society” [12]. The aim of the present review is the decision making process after revealing a new pulmonary nodule up to 30 mm in diameter.
Imaging diagnostics
Radiological examination of the chest (X-ray examination)
Chest X-ray is most commonly the first examination performed for the diagnosis of lung cancer. The method is essential in diagnosing many disorders of the chest, but its efficacy as a screening examination is limited. Lung tumor detected by this method requires further examination by computed tomography. The role of X-ray examination in lung cancer diagnostics is diminished, as it is being replaced by LDCT.
Computed tomography of the chest (CT examination)
Computed tomography enables the precise assessment of the size, shape, margins, and density of lung tumors. It also facilitates the evaluation of lymph nodes, additional pulmonary changes, as well as chest wall and mediastinal infiltration. Tumor morphology features allow radiologist to estimate the risk of malignancy. Malignant tumor features include spiculated outlines, post-contrast enhancement of more than 15 Hounsfield units (HU), and vascular convergence [13]. Tumor cavitation suggests with high probability that the tumor is malignant, especially if the cavity is irregular and thick-walled [14]. The presence of spiculated outlines increases the risk of a malignant process five-fold, an image of pleural retraction increases it two-fold, and the so-called vessel sign increases it by 70% [14]. Conversely, the risk that the tumor is malignant is 30% lower if an air bronchogram is visible, and five times lower if the tumor's outlines are smooth [14].
Tumor on the CT scan – what is next?
The first step is to determine whether the nodule is a neoplasm. In patients that are potentially surgical candidates ACCP recommends to predict the probability of tumor malignancy before applying any diagnostic tools. Firstly, clinical assessment should be performed along with a quantitative evaluation by one of the validated method [15–18]. The clinical prediction models of malignancy are presented in Table I [11].
Tab. I.
Assessing the probability of lung tumor malignancy [11]
| Clinical assessment criteria | Probability of lung tumor malignancy | ||
|---|---|---|---|
| Low (< 5%) | Moderate (5-65%) | High (> 65%) | |
| Clinical examination | Young age, lower number of pack-years, smaller tumor size, smooth tumor outlines, location outside the upper lobe | A combination of high- and low-risk features | Elderly age, higher number of pack-years, history of neoplastic disease, larger tumor size, irregular, spiculated outlines, location in the upper lobe |
| PET/CT | Low metabolic activity in PET/CT | Slight, moderate metabolic activity in PET/CT | High metabolic activity in PET/CT |
| Biopsy or bronchoscopy | Benign tumor diagnosis | Non-diagnostic examinations | Suspicion of malignancy |
| CT follow-up | Lesion regression or disappearance, tumor size reduction. Consistent tumor image (solid: for 2 years, non-solid: for 3-5 years of follow-up) | Tumor progression | |
PET – positron emission tomography, CT – computed tomography
Quantitative assessment of the probability of tumors’ malignancy
Several quantitative methods of assessing the probability of pulmonary nodule malignancy have been proposed [16–22]. These models are based on basic probability theory and Bayes’ theorem. Table II presents the variables that are applied in these models.
Tab. II.
Models for quantitative assessment of the probability of lung lesion malignancy
| Variable | Swensen [19] | Dewan [21], Gurney [17, 22] | Gould [16] | Mc Williams [20] |
|---|---|---|---|---|
| Age | (+) | (+) | (+) | (+) |
| Sex | (–) | (–) | (–) | (+) |
| Tobacco smoking | (+) | (+) | (+) | (–) |
| Time since quitting smoking | (–) | (–) | (+) | (–) |
| History of neoplastic disease | (+) | (+) | (–) | (–) |
| Lung cancer within the family | (–) | (–) | (–) | (+) |
| Pulmonary emphysema | (–) | (–) | (–) | (+) |
| Tumor outline characteristic | (–) | (+) | (–) | (–) |
| Presence of spiculated outlines | (+) | (–) | (–) | (+) |
| Location | (–) | (+) | (–) | (–) |
| Upper lobe location | (+) | (–) | (–) | (+) |
| Tumor diameter | (–) | (+) | (+) | (+) |
| Lesion type (solid, part-solid, non-solid) | (–) | (–) | (–) | (+) |
| Hemoptysis | (–) | (+) | (–) | (–) |
| Lesion increase | (–) | (+) | (–) | (–) |
| Number of nodules | (–) | (–) | (–) | (+) |
| Wall thickness of cavitary lesions | (–) | (+) | (–) | (–) |
| Calcifications | (–) | (+) | (–) | (–) |
| Contrast enhancement > 15 HU | (–) | (+) | (–) | (–) |
| Metabolism in PET | (–) | (+) | (–) | (–) |
PET – positron emission tomography
Volumetric assessment
CT examinations and in particular LDCT screening, often reveal nodules in the lung parenchyma [10, 23]. In order to reduce the proportion of false positive results, a volumetric evaluation of pulmonary nodule in ml or mm3 is used. Tumor doubling time is also assessed, in accordance with Schwartz's theory of exponential neoplasm growth [24]. The Dutch-Belgian randomized NELSON study successfully employed a method of volumetric tumor growth evaluation. Tumor growth by 25% and tumor doubling time < 400 days were determined as criteria for malignancy [25].
Management of the solid lung tumor 8-30 mm
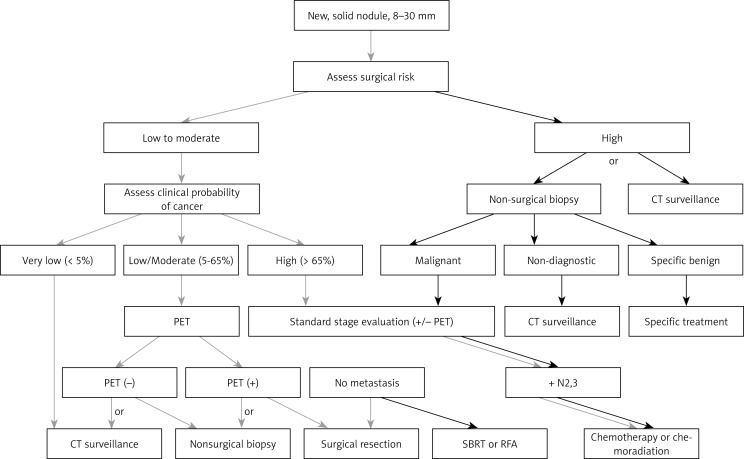
The physician should inform the patient about the health problem, presenting the alternative management methods – diagnostics, observation, and surgical removal – and explain to him the risk of potential complications. Figure 1 presents a managing algorithm of the newly detected solid lung tumors. As the diagram above indicates, there is a certain amount of freedom in deciding how to proceed with a solid lung tumor larger than 8 mm in diameter. Intuitively, the management of the round lesions with smooth outlines found in young non-smoker should be different from the management of a large spiculated lesion that are found in regular smoker.
Fig. 1.
ACCP 2013 guidelines. Management of solid pulmonary nodules 8-30 mm in diameter [11]
Several studies have demonstrated validated models of quantitative malignancy risk assessment in solid lung nodules. The range of quantitative methods for assessing the probability of malignancy in lung nodule may additionally facilitate the selection of a proper method of further procedure [15, 16, 18]. Communication with the patient, is one of the most important element, that always should be a part of decision making; the patient should be presented with the available options in a straightforward manner. The decision concerning management course should be taken by an informed patient.
Monitoring
Repeated control LDCT examination is the less invasive option that can be suggested. This course of action may be applied in the case of a fully informed patient, especially if the probability that the lesion is malignant is very low (< 5%). This option may also be selected if the risk is moderate (5-65%), PET/CT scans reveal low metabolic levels, or contrast-enhanced CT does not reveal an enhancement of more than 15 HU. It may also be chosen if the lung tumor biopsy is negative and PET/CT shows low metabolic levels. Further monitoring should be conducted based on LDCT without contrast after 3-6 months, and, subsequently, after 9-12 and 18-24 months. Solid lung tumors whose dimensions do not increase within 2 years of observation do not require further examinations. However, if the dimensions of the pulmonary nodules increase during the follow-up period, biopsy or excision is recommended. If the nodule's size is reduced, observation should be continued until complete lesion regression or up to 2 years [11].
Positron emission tomography/computed tomography examination
In patients with moderate risk of lung cancer development (5-65%), PET examinations are recommended in order to evaluate the metabolic activity of the tumor. If the risk is high (> 65%), PET examination is not required for the assessment of tumor metabolism. In this situation, PET may be indicated for staging the neoplasm [11].
Computed tomography guided tumor biopsy
Tumor biopsy should be performed in the following cases:
if the results of the imaging and the malignancy risk evaluation are inconsistent;
if the probability of malignancy is moderate (5-65%);
if benign disease requiring specific treatment is suspected [11].
Surgical resection
Surgical resection is recommended if the malignancy is confirmed. If malignant or definitive benign diagnosis has not been proven, surgical excision can be considered in the following situations:
the clinical probability of a malignant lesion is high (> 65%),
the tumor shows increased metabolic activity in PET/CT,
the cytological results from the biopsy is suspicious for malignancy,
the fully informed patient demands a definitive diagnostic procedure.
When possible, it is recommended to use minimally invasive operative techniques and tools for perioperative tumor localization [11].
Managing solid nodules smaller than 8 mm in diameter in patients at low risk for developing lung cancer
Pulmonary nodules smaller than ≤ 4 mm do not require CT surveillance, but the patient should be informed about the potential consequences. Nodules measuring 4-6 mm in diameter should be evaluated by control LDCT after 12 months; if their size does not change, no further monitoring is typically required. Nodules with 6-8 mm in diameter should undergo LDCT surveillance after 6-12 months and, if they do not increase, 18-24 months after the first examination. Patients with multiple pulmonary nodules should be monitored based on the diameter of the largest nodule. Nodules smaller than 8 mm should be monitored using low-dose computed tomography without contrast [11].
Managing solid nodules smaller than 8 mm in diameter in patients at high risk for developing lung cancer
Pulmonary nodules smaller than ≤ 4 mm require reevaluation with LDCT after 12 months; if their size does not change, no further monitoring is typically required. Nodules measuring 4-6 mm in diameter should be monitored after 6-12 months, and, if no increase is revealed, 18-24 months after the first examination.
Nodules with 6-8 mm in diameter should be monitored after 6-12 months, and, if they do not increase, 18-24 months after the first examination. Patients with multiple pulmonary nodules should be monitored based on the diameter of the largest nodule. Nodules smaller than 8 mm should be monitored using low-dose computed tomography without contrast [11].
Managing pure ground-glass nodules
Individuals diagnosed with pure ground-glass nodules (GGNs) smaller than 5 mm do not require further surveillance. According to the Fleischner Society, pure GGNs > 5 mm should be followed up after 3 months in order to determine whether they have regressed; subsequently, they should be followed up annually for at least 3 years [12].
According to the ACCP, pure ground-glass nodules with diameters exceeding 10 mm should be followed up after 3 months, and, if no regression is observed, they should undergo biopsy or surgical removal [11]. Table III presents the recommendations of the Fleischner Society with regard to pure GGNs [12].
Tab. III.
Recommendations of the Fleischner Society for management of non-solid lung tumors [12]
| Solitary non-solid tumor | |
| < 5 mm | Surveillance not required |
| > 5 mm | LDCT follow-up at 3 months. If the tumor persists, yearly surveillance for at least 3 years is recommended. |
| Solitary part-solid lung tumor | LDCT follow-up at 3 months. If the tumor persists, and the solid component's diameter does not exceed 5 mm, yearly surveillance for at least 3 years is recommended. If the solid component's diameter exceeds 5 mm, biopsy or surgical excision is recommended. |
| Numerous non-solid lesions up to 5 mm | CT follow-up after 2 and 4 years is recommended. |
| Non-solid lesions > 5 mm without a dominant lesion | LDCT follow-up at 3 months. If the tumor persists, yearly surveillance for at least 3 years is recommended. |
| The dominant nodule has a solid or part-solid component | LDCT follow-up at 3 months. If the tumor persists, biopsy or surgical excision is recommended, especially if the solid component exceeds 5 mm. |
LDCT – low-dose computed tomography, CT – computed tomography
Managing part-solid ground-glass nodules
Part-solid GGNs with diameter ≤ 8 mm should be monitored after 3, 12, and 24 months, and then annually for a period of up to 3 years. It is recommended to use low-dose computed tomography, without contrast with thin layer techniques. If part-solid and non-solid nodules are shown to grow, consolidate are potentially malignant, special evaluation is required, and surgical resection may be considered. In the case of part-solid tumors with diameters > 8 mm, reevaluation in LDCT is recommended after 3 months. If the lesion does not regress, biopsy and/or surgical excision should be considered. Ground-glass nodules that have changed their state from non-solid during the observation or have direct contact with the pleura deserve special attention, as the probability that the lesion is malignant is very high in such cases [12]. Positron emission tomography scans are not recommended for part-solid nodules in which the solid component is smaller than 8 mm. A non-diagnostic biopsy does not allow one to exclude tumor malignancy. During the biopsy, the use of a radioactive dye marker or wire can be considered in order to facilitate the performance of a surgical resection [11, 12].
Is a cytological diagnosis from lung tumor biopsy indispensable?
No
Fine-needle aspiration biopsy or core-needle biopsy of lung tumor is an invasive test with some risk of complications. The most common complication are pneumothorax, bleeding, air embolism, and implantation of the cancer cells into the chest wall. The risk of death associated with biopsy remains below 1% [11]. Surgical excision can be considered regardless of biopsy result. Omitting biopsy may allow the patient to avoid potentially painful procedure carried considerable risk of complications. The ACCP recommends that qualifying the patient for a surgical procedure (wedge resection of the tumor with intraoperative frozen section) can be considered if, according to clinical or quantitative evaluation, patient is at high-risk of malignancy (above 65%). Table IV presents an overview of the available reports concerning complications.
Tab. IV.
Complications after lung tumor biopsy and predisposing factors
| Complication type | % | Source |
|---|---|---|
| Pneumothorax | ||
| Frequency of occurrence | 17-26.6 | [28-30] |
| Percentage of patients requiring drainage | 1-14.2 | [28-30] |
| Factors predisposing to pneumothorax COPD No previous surgery on the side of the performed biopsy Lesions located deep within the lung Increased number of pleural taps |
[28-30] | |
| Hemorrhage | ||
| Intraparenchymal hemorrhage | 4-27 | [29, 30] |
| Hematoma in the pleural cavity | 0.092 | [29, 30] |
| Factors predisposing to bleeding: Small tumor size Deep lesion location Pulmonary emphysema Pulmonary hypertension |
[29, 30] | |
| Air embolism | ||
| A very rare complication | 0.061 | [30] |
| May cause life-threatening cardiac dysrhythmia and cardiac or cerebral ischemia | [31] | |
Yes, biopsy is indicated in lung tumor diagnostic work-ups
Transthoracic needle biopsy can confirm diagnosis of malignancy with significant probability – 70-90%. In 30% of patients, it can also confirm definitive benign diagnosis. Preoperative cytological diagnostic work-up may help reduce the resection rate for benign lesions, which is approximately 9-15% [26]. Preoperative neoplasm diagnostic may change the methods of managing the disease and result in reducing health care costs. It also allows patients to make more informed decisions [27]. In experienced centers, the diagnostic accuracy of performed biopsies, regardless of nodule size, is nearly 100%.
Conclusions
Cytological diagnosis of lung cancer is helpful in making correct clinical decisions and reduces the risk of unnecessary interventions. Transthoracic needle biopsy is a relatively safe and reliable diagnostic method. This minimally invasive procedure may help reduce the percentage of futile thoracotomies and result in lowering healthcare costs.
Biography

Disclosure
Authors report no conflict of interest.
References
- 1.The global burden of disease: 2004 update; Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]; Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 4.Pastorino U. Lung cancer screening. Br J Cancer. 2010;102:1681–1686. doi: 10.1038/sj.bjc.6605660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, Sabichi AL, Smith-Bindman R, Wood DE, Qaseem A, Detterbeck FC. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaklitsch MT, Jacobson FL, Austin JHM, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, Strauss GM, Swanson SJ, Travis WD, Sugarbaker DJ. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 8.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force Recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 9.McMahon PM, Kong CY, Bouzan C, Weinstein MC, Cipriano LE, Tramontano AC, Johnson BE, Weeks JC, Gazelle GS. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rzyman W, Jelitto-Gorska M, Dziedzic R, Biadacz I, Ksiazek J, Chwirot P, Marjanski T. Diagnostic work-up and surgery in participants of the Gdansk lung cancer screening programme: the incidence of surgery for non-malignant conditions. Interact CardioVasc Thorac Surg. 2013;17:969–973. doi: 10.1093/icvts/ivt388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, Macchiarini P, Crapo JD, Herold CJ, Austin JH, Travis WD. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304–317. doi: 10.1148/radiol.12120628. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Z, Zhou H, Hu CP, Liu JK, Chen H, Chen W, He XY, Zhou ML, Zhu ZM. Correlation between computed tomographic vascular convergence sign and enhancement value in patients with pulmonary nodules. Zhonghua Yi Xue Za Zhi. 2013;93:3015–3018. [PubMed] [Google Scholar]
- 14.Siegelman SS, Khouri NF, Leo FP, Fishman EK, Braverman RM, Zerhouni EA. Solitary pulmonary nodules: CT assessment. Radiology. 1986;160:307–312. doi: 10.1148/radiology.160.2.3726105. [DOI] [PubMed] [Google Scholar]
- 15.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 16.Gould MK, Ananth L, Barnett PG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383–388. doi: 10.1378/chest.06-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurney JW, Lyddon DM, McKay JA. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application. Radiology. 1993;186:415–422. doi: 10.1148/radiology.186.2.8421744. [DOI] [PubMed] [Google Scholar]
- 18.Tammemagi MC, Freedman MT, Pinsky PF, Oken MM, Hu P, Riley TL, Ragard LR, Berg CD, Prorok PC. Prediction of true positive lung cancers in individuals with abnormal suspicious chest radiographs – a prostate, lung, colorectal, and ovarian cancer screening trial study. J Thorac Oncol. 2009;4:710–721. doi: 10.1097/JTO.0b013e31819e77ce. [DOI] [PubMed] [Google Scholar]
- 19.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 20.McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, Yasufuku K, Martel S, Laberge F, Gingras M, Atkar-Khattra S, Berg CD, Evans K, Finley R, Yee J, English J, Nasute P, Goffin J, Puksa S, Stewart L, Tsai S, Johnston MR, Manos D, Nicholas G, Goss GD, Seely JM, Amjadi K, Tremblay A, Burrowes P, MacEachern P, Bhatia R, Tsao MS, Lam S. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewan NA, Shehan CJ, Reeb SD, Gobar LS, Scott WJ, Ryschon K. Likelihood of malignancy in a solitary pulmonary nodule: comparison of Bayesian analysis and results of FDG-PET scan. Chest. 1997;112:416–422. doi: 10.1378/chest.112.2.416. [DOI] [PubMed] [Google Scholar]
- 22.Gurney JW. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part I. Theory. Radiology. 1993;186:405–413. doi: 10.1148/radiology.186.2.8421743. [DOI] [PubMed] [Google Scholar]
- 23.Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gatsonis CA, Gierada DS, Jain A, Jones GC, Mahon I, Marcus PM, Rathmell JM, Sicks J. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369:920–931. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz M. A biomathematical approach to clinical tumor growth. Cancer. 1961;14:1272–1294. doi: 10.1002/1097-0142(196111/12)14:6<1272::aid-cncr2820140618>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, van Iersel CA, van den Bergh KAM, van ‘t Westeinde S, van der Aalst C, Thunnissen E, Xu DM, Wang Y, Zhao Y, Gietema HA, de Hoop B-J, Groen HJM, de Bock GH, van Ooijen P, Weenink C, Verschakelen J, Lammers J-WJ, Timens W, Willebrand D, Vink A, Mali W, de Koning HJ. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–2229. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 26.Kuo E, Bharat A, Bontumasi N, Sanchez C, Zoole JB, Patterson GA, Meyers BF. Impact of video-assisted thoracoscopic surgery on benign resections for solitary pulmonary nodules. Ann Thorac Surg. 2012;93:266–273. doi: 10.1016/j.athoracsur.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert S, Zhang H, Villeneuve PJ, Seely AJ, Maziak DE, Shamji FM, Cadaval A, Sundaresan S. Optimizing health care resource utilization in the surgical management of patients with suspected lung cancer. Ann Thorac Surg. 2012;94:1667–1672. doi: 10.1016/j.athoracsur.2012.04.096. [DOI] [PubMed] [Google Scholar]
- 28.Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15:479–483. doi: 10.1097/01.rvi.0000124951.24134.50. [DOI] [PubMed] [Google Scholar]
- 29.Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, Chou AS. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 30.Khan MF, Straub R, Moghaddam SR, Maataoui A, Gurung J, Wagner TO, Ackermann H, Thalhammer A, Vogl TJ, Jacobi V. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008;18:1356–1363. doi: 10.1007/s00330-008-0893-1. [DOI] [PubMed] [Google Scholar]
- 31.Hiraki T, Fujiwara H, Sakurai J, Iguchi T, Gobara H, Tajiri N, Mimura H, Kanazawa S. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest. 2007;132:684–690. doi: 10.1378/chest.06-3030. [DOI] [PubMed] [Google Scholar]