Abstract
We have previously observed that methane supplied to lake sediment microbial communities as a substrate not only causes a response by bona fide methanotrophic bacteria, but also by non-methane-oxidizing bacteria, especially by members of the family Methylophilaceae. This result suggested that methane oxidation in this environment likely involves communities composed of different functional guilds, rather than a single type of microbe. To obtain further support for this concept and to obtain further insights into the factors that may define such partnerships, we carried out microcosm incubations with sediment samples from Lake Washington at five different oxygen tensions, while methane was supplied at the same concentration in each. Community composition was determined through 16S rRNA gene amplicon sequencing after 10 and 16 weeks of incubation. We demonstrate that, in support of our prior observations, the methane-consuming communities were represented by two major types: the methanotrophs of the family Methylococcaceae and by non-methanotrophic methylotrophs of the family Methylophilaceae. However, different species persisted under different oxygen tensions. At high initial oxygen tensions (150 to 225 µM) the major players were, respectively, species of the genera Methylosarcina and Methylophilus, while at low initial oxygen tensions (15 to 75 µM) the major players were Methylobacter and Methylotenera. These data suggest that oxygen availability is at least one major factor determining specific partnerships in methane oxidation. The data also suggest that speciation within Methylococcaceae and Methylophilaceae may be driven by niche adaptation tailored toward specific placements within the oxygen gradient.
Keywords: Methylophilaceae, Methanotroph, Methylophilus, Methylobacter, Methylotenera, Methylosarcina, Methylotrophy, Lake Washington
Introduction
Methanotrophy is a well-characterized mode of microbial metabolism that supports microbial growth on methane (Trotsenko & Murrell, 2008). Methanotrophs are important players in the methane cycle, and, more generally, in the carbon cycle on Earth (Singh et al., 2010; Nisbet, Dlugokencky & Bousquet, 2014). A variety of methane-oxidizing microbes have been characterized in pure cultures (most prominently the organisms belonging to Proteobacteria) but also more recently, organisms classified as Verrucomicrobia, the latter so far only found in extreme environments (Chistoserdova & Lidstrom, 2013). While methanotrophy can be carried out by single species, it has been noted that methanotrophs in environmental samples are often associated with specific non-methanotrophic bacteria (Jensen et al., 2008; Redmond, Valentine & Sessions, 2010; He et al., 2012; Dubinsky et al., 2013; Rivers et al., 2013), suggesting some type of cooperation (Beck et al., 2013; Van der Ha et al., 2013). We have previously tested for the possibility of such cooperative behavior by analyzing the compositions of microcosms originating from Lake Washington sediment which was exposed to methane as the only carbon source and observed a prominent presence of satellite bacteria (Oshkin et al., 2014). Among the most persistent satellites, we identified members of the families Methylophilaceae and Flavobacteriaceae (Oshkin et al., 2014). These observations further suggested a novel metabolic framework for methane oxidation as carried out by communities of different metabolic guilds, rather than methanotrophs alone. However, additional experimental support was necessary in order to shift the accepted paradigms of methane oxidation (Trotsenko & Murrell, 2008). As methane oxidation in environments such as lake sediments takes place over steep counter gradients of methane and oxygen (Auman et al., 2000), the focus of this study was on investigating the effect of oxygen availability on bacterial community structure in microcosms enriched with methane as a substrate.
Material and Methods
Sample collection and experimental setup
Samples of Lake Washington sediment were collected on July 15, 2013 (Oshkin et al., 2014). A 50 ml frozen sediment sample containing 10% of dimethyl sulfoxide (a cryoprotective agent) was thawed on ice, mixed and used as an inoculum. Five ml aliquots of sediment slurry were placed into 250 ml vials and diluted with 50 ml of nitrate mineral salts (NMS) medium (Dedysh & Dunfield, 2014; 0.5 X strength), vials were sealed with rubber stoppers and flushed with N2 for 2 min (flow rate 400 ml/min), and the excess volume of N2 was removed by a syringe to equalize the pressure. Five different atmospheres were created in the headspaces by adding different volumes of ambient air, as follows: 5%, 15%, 25%, 50% or 75% of the headspace (V/V). All headspaces received 25% (V/V) of methane. Before adding the air and the methane, the respective volumes of N2 were removed from the vials. These initial oxygen tensions correspond to, respectively, approximately 15, 45, 75, 150, and 225 µM of dissolved oxygen. Three replicate microcosms for each oxygen tension were incubated in a shaker (250 RPM) at 18 °C. The headspace gas composition was recreated daily, as above. After three weeks of incubation, the microcosms were transferred into new medium, with 10-fold dilutions, similarly to the procedure described previously (Oshkin et al., 2014). Such transfers were then repeated through week 16.
Oxygen and methane measurements
Oxygen and methane concentrations in the headspace were measured using a GC2014 gas chromatograph (Shimadzu Instruments, Pleasanton, California, USA) as described by Oshkin et al. (2014).
16S rRNA gene amplicon sequencing
Cell biomass was collected at weeks 10 and 16. DNA was isolated using the FastDNA SPIN KIT for Soil (MP Biomedicals, Burlingame, California, USA) and submitted to MR DNA service facility (www.mrdnalab.com; Shallowater, TX, USA). PCR primers 27F/519r with barcode on the forward primer were used in a 30 cycle PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, California, USA) under the following conditions: 94 °C for 3 min, followed by 28 cycles of 94 °C for 30 s, 53 °C for 40 s and 72 °C for 1 min, after which a final elongation step at 72 °C for 5 min was performed. After amplification, PCR products were checked in a 2% agarose gel to determine the success of amplification and the relative intensity of bands. Multiple individual samples were pooled together in a way that each sample was represented equally, for multiplexing. Pooled samples were purified using calibrated Ampure XP beads. Then the pooled and purified PCR products were used to prepare DNA libraries following the manufacturer’s instructions. Sequencing was performed on a MiSeq instrument following the manufacturer’s guidelines. Sequence data were processed using a proprietary MR DNA analysis pipeline in which sequences barcodes were removed, then sequences <150 bp or with ambiguous base calls were removed, sequences were denoised and chimera sequences were removed. The pairs of sequences were joined, resulting in sequences between 490 and 492 nucleotides. The data have been archived with the NCBI (Bioproject PRJNA274703, http://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA274703).
Bioinformatics
The UPARSE method was used for sequence processing and OTU clustering with USEARCH version 7.0.1001 (Edgar, 2013). Clustering was performed at 95% and chimeras were identified against the ChimeraSlayer reference database in the Broad Microbiome Utilities version r20110519 obtained from the UCHIME distribution (Edgar et al., 2011). For each OTU, a representative sequence was selected using the method of Edgar (2013), and taxonomic assignments were made using the RDP Classifier from the Ribosomal Database Project downloaded on October 22, 2013 (Wang et al., 2007). The samples were scaled so that the numbers of reads in each sample were equal. Hierarchical clustering of samples and OTUs was performed using the percentage of reads per OTU for the most abundant taxa, i.e., greater than 1.0% population in at least one sample. Bray-Curtis distances and Shannon indices were calculated and multivariate analyses were carried out using the vegan library version 2.0-10 (Oksanen et al., 2014) in R version 3.0.2 (http://www.R-project.org/). The processing and analysis code has been made available (DOI 10.5281/zenodo.13190).
Genome–genome comparisons were carried out using the Phylogenetic Profilers tool that is part of the Integrated Microbial Genomes database (IMG/JGI; https://img.jgi.doe.gov). Reciprocal searches were performed to determine all the genes present in both Methylobacter genomes but absent in the Methylosarcina genome and vice versa, and searches were performed to determine all the genes present in both Methylophilus genomes but in none of the Methylotenera genomes and vice versa, using 30% protein sequence cutoff.
Results and Discussion
Previously, we had followed short-term community dynamics in microcosms of Lake Washington sediment, under an atmosphere of methane and two oxygen tension regimens, ‘high’ and ‘low’ (Oshkin et al., 2014). However, with the experimental design utilized, the communities were limited by oxygen in both conditions for extended periods of time. Under both regimens, we observed rapid loss of community complexity and establishment of stable communities dominated by Methylobacter, a gammaproteobacterial methanotroph, and by members of Methylophilaceae (Methylotenera or Methylophilus), non-methanotrophic methylotrophs within Betaproteobacteria. We have also noted persistent presence of certain non-methylotrophic heterotrophs, such as Flavobacteriaceae (Oshkin et al., 2014).
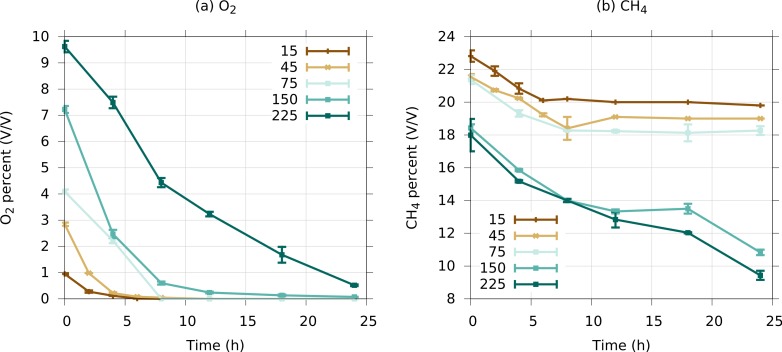
In the research described here, one of our goals was to test for the reproducibility of microcosm trajectories, adding a few modifications to the experimental design (such as a slightly modified medium, slightly higher temperature, and most notably a different sequencing technology) and to confirm the persistence of specific bacterial taxa in such microcosms, under the selective pressure of methane. Our second goal was to test a broader range of oxygen tensions, with a stricter control over the oxygen concentration in the headspace. We employed five discrete initial oxygen tensions, calculated to correspond to approximately 225, 150, 75, 45 and 15 µM dissolved oxygen, mimicking the oxygen gradient between 0 and 5 mm in the native sediment of Lake Washington where most of the methane oxidizing activity takes place (Auman et al., 2000). With the headspace compositions recreated daily, only the communities exposed to 150 and 225 µM initial dissolved oxygen remained oxygenated (starting with week 7 for the 225 µM oxygen community and week 8 for the 150 µM community; Fig. S1). Communities exposed to lower initial oxygen tensions depleted oxygen before the next addition (Fig. S1). Typical rates of methane and oxygen consumption in the established communities are shown in Fig. 1.
Figure 1. Typical dynamics of oxygen (A) and methane (B) consumption in low complexity microcosms, over the course of 24 h.
For this experiment, six additional replicates were prepared for each microcosm at week 16, and these were allowed to incubate for 48 h, with the atmospheres recreated at the 24-h point. At the 48-h point, the atmospheres were recreated again, and measurements were taken every two (15 and 45 µM treatments) or four (75 to 225 mM treatments) hours. Bars indicate standard error across the replicates.
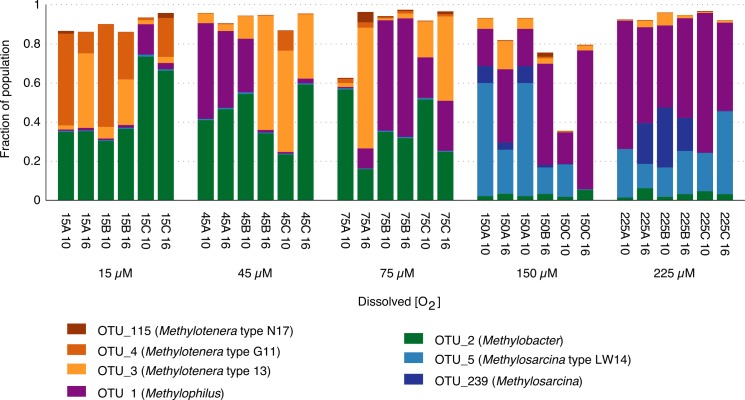
The community composition was measured after ten and sixteen weeks of incubation, in three replicated microcosms for each oxygen tension (Table S1). Illumina-based analysis of microcosm communities uncovered that they were simple communities (20 to 40 operational taxonomic units (OTUs) per microcosm; Fig. S2), and they contained two dominant bacterial guilds, methanotrophs of the family Methylococaceae and methylotrophs of the family Methylophilaceae. In most of the microcosms (66.6%), sequence reads ascribed to these two functional guilds made up over 90% of all reads (Fig. 2). These data are in agreement with the data from our prior study, in which similar community structures were observed after approximately four weeks of incubation under methane (Oshkin et al., 2014). Only one microcosm (microcosm 150C 10) was dominated by non-methylotroph species. Specifically, a Janthinobacterium and a Flavobacterium species were present at highest relative abundances in this microcosm. These species were also noted as highly abundant in some of the previously characterized samples, likely a result of a stochastic event of ‘community crash’ resulting in death and lysis of the dominant species (Oshkin et al., 2014).
Figure 2. Relative abundance of Methylococcaceae and Methylophilaceae in methane-fed microcosms.
Samples were ordered from the lowest to the highest concentration of oxygen. Sample designations include oxygen tension, followed by the alphabetical name of a replicate and by the week of sampling.
Of the methanotroph types, a total of three OTUs were recognized: OTU_2 was classified as Methylobacter, and OTU_5 and OTU_239 were classified as Methylosarcina (Table 1). Of the Methylophilaceae types, a total of four OTUs were recognized, one classified as Methylophilus (OTU_1) and three classified as Methylotenera (OTU_3, OTU_4 and OTU_115). These were most closely related to, respectively, Methylotenera mobilis 13, Methylotenera sp. G11, and Methylotenera sp. N17 (Table 1), all isolated from Lake Washington. Most of the remaining persistent OTUs (more that 1% of total sequences in at least one sample) belonged to Flavobacteriaceae (Fig. 3 and Table S1).
Table 1. Methylotroph OTUs, cultivated proxy organisms, and nitrate metabolism functions.
Methylobacter isolates from Lake Washington have not been formally described. Phenotypically and genomically they are similar to each other and to the described strain of Methylobacter tundripaludum (Wartiainen et al., 2006).
| OTU | Cultivated proxy organism |
% 16S rRNA identity |
Respiratory nitrate reductase |
Respiratory nitrite reductase |
Nitric oxide reductase |
Nitrous oxide reductase |
N2 fixation machinery |
Rnf complex |
Hydrogenase |
|---|---|---|---|---|---|---|---|---|---|
| OTU_2 | Methylobacter 21/22a | 99.4 | + | + | − | − | + | + | + |
| OTU_2 | Methylobacter 31/32a | 99.4 | + | + | − | − | + | + | + |
| OTU_5 | Methylosarcina lacus LW14b | 99.8 | − | − | +e | − | − | − | − |
| OTU_3 | Methylotenera 13c | 99.8 | + | + | + | + | − | + | − |
| OTU_4 | Methylotenera G11d | 98.6 | − | + | + | − | − | + | − |
| OTU_115 | Methylotenera N17d | 99.6 | − | − | − | − | − | + | − |
| OTU_1 | Methylophilus 1c | 99.8 | − | − | +e | − | − | − | − |
| OTU_1 | Methylophilus Q8d | 99.8 | − | − | − | − | − | − | − |
Notes.
Data from the IMG/JGI public database.
Data from Kalyuzhnaya et al. (2005).
Data from Beck et al. (2014).
Data from McTaggart et al. (2015).
Gene product is likely nonfunctional.
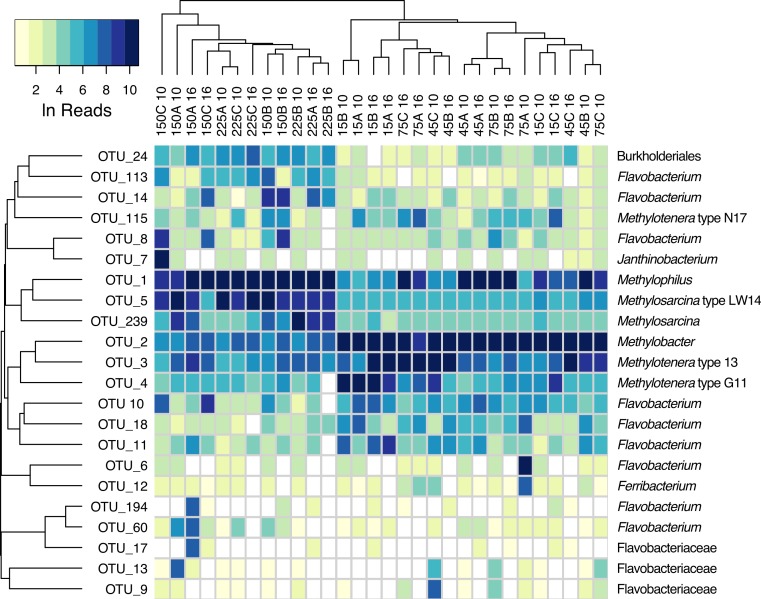
Figure 3. Heatmap of major OTU relative abundances across samples.
Abundances were measured as Ln of reads. Sample designations are the same as in Fig. 2. Samples and OTUs were clustered hierarchically (average linkage), based on Bray-Curtis dissimilarity index of relative abundance profiles.
In most of the microcosms (73.3%), the methanotroph types made up less than 50% of the total population, and in most (63.3%), the Methylophilaceae types were most relatively abundant (Fig. 2). These data support our prior observations on a strong response by Methylophilaceae to the methane stimulus, and on a successful carbon transfer between the methanotrophs and non-methanotrophs (Oshkin et al., 2014).
We observed a dramatic difference between community responses to high (150 to 225 µM) versus low (15 to 75 µM) initial oxygen tensions, especially in terms of the major methane-oxidizing types. While the Methylosarcina types were dominant in high-oxygen microcosms, they were almost absent from the low-oxygen microcosms. Conversely, the Methylobacter types were dominant in the low-oxygen microcosms while constituting only a minor population in the high-oxygen microcosms (Figs. 2 and 3). We have not identified Methylosarcina species in our prior experiments, except for the native lake sediment communities, at low relative abundances (Oshkin et al., 2014). This is likely due to the fact that, in the previous study, the ‘high’ oxygen microcosms were only fed oxygen weekly, thus becoming hypoxic for a significant duration of time (Oshkin et al., 2014). This suggests that the Methylosarcina species are only competitive when oxygen is present, and that they become outcompeted by the Methylobacter types during hypoxia. Although it is unlikely, this behavior could simply be explained by the differences in oxygen affinity, as all of the oxygen concentrations used in this study were well above the reported Km values for methanotrophs (Joergensen, 1985; Dunfield et al., 1999). More likely, the differences are due to the different metabolic strategies employed during hypoxia (see below).
The occurrence of specific Methylophilaceae types was also oxygen-dependent. The Methylophilus types prevailed at higher oxygen tensions, and the Methylotenera types prevailed at lower oxygen tensions. Of the latter, OTU_3 was the most relatively abundant among the samples, and OTU_115 was the least relatively abundant (Figs. 2 and 3). However, the transition between the Methylophilus and Methylotenera types was more gradual. While the Methylophilus types were dominant at high oxygen tensions, they were present at variable levels at the intermediate oxygen tensions. The Methylotenera types were more represented in the samples with the lowest oxygen, suggesting competitive advantage for these species during hypoxia. These data are in agreement with our prior data on Methylophilus being more competitive in the conditions of higher oxygen and stable in the conditions of lower oxygen when no competitor is present (Oshkin et al., 2014).
The distribution of the non-methylotrophic heterotrophic species among the communities investigated was also nonrandom. As with the methylotrophs, a clear switch was observed between some of the prevailing satellite species between the high- and the low oxygen tension conditions. While the more prominent satellites in the high oxygen samples were OTU_14 (Flavobacterium), OTU_24 (Burkholderiales) and OTU_113 (Flavobacterium), the most prominent satellites in the low oxygen conditions were OTU_10, OTU_11 and OTU_18, all Flavobacterium species.
At this moment, we have no mechanistic knowledge of interactions within these methane-oxidizing communities, beyond the observation that carbon from methane does get transferred to Methylophilaceae and potentially to a broader range of microbes, based on stable isotope analysis (Kalyuzhnaya et al., 2008; Beck et al., 2013) and based on rapid population growth of Methylophilaceae and of certain non-methylotroph heterotrophs in the microcosms. However, the associations of methanotrophs with non-methanotrophs are persistent, and they do select for specific types. The communities are roughly stable over time, with the methanotroph population typically oscillating between one third and two thirds of the community.
We carried out comparative genomics in order to obtain hints regarding which metabolic features might be responsible for oxygen level adaptation, including survival and/or growth during the periods of hypoxia. The genomes of two cultivated Methylobacter strains were compared to the genome of a Methylosarcina strain, and the genomes of Methylotenera strains were compared to the genomes of Methylophilus (see Table 1 for the list of organisms). Only a few metabolic features were uncovered that differentiated the functional counterparts, the most notable being nitrogen metabolism functions. The Methylosarcina genome only encoded functions for nitrate conversion into ammonium (assimilatory denitrification) and for a single, likely nonfunctional subunit of nitric oxide reductase. On the contrary, the Methylobacter genomes encoded, in addition, respiratory nitrate and nitrite reductases (Table 1). The Methylobacter genomes also contained genes predicted to encode functions essential to dinitrogen fixation, including the subunits of the Rnf complex that is essential for this metabolism, at least in some species (Schmehl et al., 1993). These genomes also encoded multiple hydrogenases and accessory functions. While at this moment the potential role of dinitrogen fixation in the fitness of Methylobacter is not obvious, its ability to denitrify presents a mechanism by which it may be able to outcompete Methylosarcina during hypoxia. Methanotrophy has been recently demonstrated during hypoxia, linked to nitrate reduction, in a related methanotroph (Kits, Klotz & Stein, 2015). Interestingly, one other difference between Methylobacter and Methylosarcina genomes was the presence of the pxmABC gene cluster (in the former but not the latter), encoding homologs of the subunits of methane monooxygenase (Tavormina et al., 2011). While the function of these genes remains unknown, they were found overexpressed during hypoxia in a denitrifying methanotroph (Kits, Klotz & Stein, 2015).
Likewise, while the Methylophilus genomes only encoded assimilatory denitrification reactions, the Methylotenera genomes varied in terms of their denitrification potential, from assimilatory in strain N17 to partial dissimilatory in strain G11 to complete dissimilatory in strain 13 (Beck et al., 2014). The denitrification capability has been experimentally demonstrated in at least one Methylotenera species (Mustakhimov et al., 2013). The Methylotenera genomes also encoded the Rnf complex, in the absence of any dinitrogen fixation genes.
It is tempting to speculate that nitrogen metabolism functions, and especially the denitrification capability, confer competitive advantage at low oxygen to both Methylobacter and Methylotenera. It is also possible that these organisms may exchange nitrogen species such as nitrite, nitric or nitrous oxide. However, as yet we do not have information regarding how a methanotroph can provide carbon to a community of non-methanotrophs and as to what advantage the methanotroph may be gaining from the satellite community.
Overall, the experiments described here provide further support to our observations on a special relationship between the Methylococcaceae and the Methylophilaceae, and also provide further support to the observation that non-methylotrophic species, especially Flavobacteriaceae, may also play a role in this proposed mutualistic relationship. Moreover, we now conclude that oxygen availability is a major factor determining what species engage in cooperative behavior. At high oxygen tensions, Methylosarcina appear to have advantage over Methylobacter, and Methylophilus appears to have advantage over Methylotenera. At intermediate oxygen tensions, Methylobacter appears to cooperate with either Methylophilus or Methylotenera. At low oxygen tensions, including extended periods of hypoxia, Methylobacter and Methylotenera outcompete, respectively, Methylosarcina and Methylophilus. However, different types within each genus are also identifiable (see also Oshkin et al., 2014), and these may be selected by more discrete factors. These details will be addressed in future studies.
Supplemental Information
It is evident that as the communities simplify, oxygen consumption is reduced. By the time of the first sampling, the 150–225 µM (50–75% air) treatments remained constantly oxygenated while 15–75 µM (5–25% air) treatments went hypoxic before the next oxygen addition. Oxygen and methane were measured immediately after recreating the atmosphere and after 24 h. Blue columns, red columns, oxygen and methane, respectively, measured immediately after the atmosphere was created. Green columns, purple columns, oxygen and methane, respectively, measured after 24 h. Error bars indicate standard error across the replicates.
The diversity indices were computed for each of the three biological replicates and are shown with error bars indicating standard deviation across the replicates.
OTU abundance by read counts
Funding Statement
This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-SC-0010556. The Mexican National Council for Science and Technology (CONACYT) provided Scholarship No. 208120 for Maria E. Hernandez. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Ludmila Chistoserdova is an Academic Editor for Peer J.
Author Contributions
Maria E. Hernandez conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
David A.C. Beck analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Mary E. Lidstrom wrote the paper, reviewed drafts of the paper.
Ludmila Chistoserdova conceived and designed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
NCBI Bioproject number PRJNA274703.
References
- Auman et al. (2000).Auman AJ, Stolyar S, Costello AM, Lidstrom ME. Molecular characterization of methanotrophic isolates from freshwater lake sediment. Applied and Environmental Microbiology. 2000;66:5259–5266. doi: 10.1128/AEM.66.12.5259-5266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck et al. (2013).Beck DAC, Kalyuzhnaya MG, Malfatti S, Tringe SG, Glavina del Rio T, Ivanova N, Lidstrom ME, Chistoserdova L. A metagenomic insight into freshwater methane-utilizing communities and evidence for cooperation between the Methylococcaceae and the Methylophilaceae. PeerJ. 2013;1:e801. doi: 10.7717/peerj.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck et al. (2014).Beck DAC, McTaggart TL, Setboonsarng U, Vorobev A, Kalyuzhnaya MG, Ivanova N, Goodwin L, Woyke T, Lidstrom ME, Chistoserdova L. The expanded diversity of Methylophilaceae from Lake Washington through cultivation and genomic sequencing of novel ecotypes. PLoS ONE. 2014;9:e801. doi: 10.1371/journal.pone.0102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistoserdova & Lidstrom (2013).Chistoserdova L, Lidstrom ME. Aerobic methylotrophic prokaryotes. In: Rosenberg E, DeLong EF, Thompson F, Lory S, Stackebrandt E, editors. The prokaryotes. Fourth edition. Berlin: Springer-Verlag Berlin Heidelberg; 2013. pp. 267–285. [Google Scholar]
- Dedysh & Dunfield (2014).Dedysh SN, Dunfield PF. Cultivation of methanotrophs. In: McGenity T, Timmis K, Nogales B, editors. Hydrocarbon and lipid microbiology protocols. Berlin: Springer-Ferlag; 2014. (Springer protocols handbooks). [Google Scholar]
- Dubinsky et al. (2013).Dubinsky EA, Conrad ME, Chakraborty R, Bill M, Borglin SE, Hollibaugh JT, Mason OU, Piceno MY, Reid FC, Stringfellow WT, Tom LM, Hazen TC, Andersen GL. Succession of hydrocarbon-degrading bacteria in the aftermath of the deepwater horizon oil spill in the Gulf of Mexico. Environmental Science Technology. 2013;47:10860–10867. doi: 10.1021/es401676y. [DOI] [PubMed] [Google Scholar]
- Dunfield et al. (1999).Dunfield PF, Liesack W, Henckel T, Knowles R, Conrad R. High-affinity methane oxidation by a soil enrichment culture containing a type II methanotroph. Applied Environmental Microbiology. 1999;65:1009–1014. doi: 10.1128/aem.65.3.1009-1014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar (2013).Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Edgar et al. (2011).Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2012).He R, Wooller MJ, Pohlman JW, Catranis C, Quensen J, Tiedje JM, Leigh MB. Identification of functionally active aerobic methanotrophs in sediments from an arctic lake using stable isotope probing. Environmental Microbiology. 2012;14:1403–1419. doi: 10.1111/j.1462-2920.2012.02725.x. [DOI] [PubMed] [Google Scholar]
- Jensen et al. (2008).Jensen S, Neufeld JD, Birkeland NK, Hovland M, Murrell JC. High diversity of microplancton surrounds deep-water coral reef sediment off the coast of Norway. FEMS Microbiology Ecology. 2008;66:320–330. doi: 10.1111/j.1574-6941.2008.00575.x. [DOI] [PubMed] [Google Scholar]
- Joergensen (1985).Joergensen L. The methane mono-oxygenase reaction system studied in vivo by membrane-inlet mass spectrometry. Biochemical Journal. 1985;225:441–448. doi: 10.1042/bj2250441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhnaya et al. (2008).Kalyuzhnaya MG, Lapidus A, Ivanova N, Copeland AC, McHardy AC, Szeto E, Levine SR, Barry K, Green-Tringe S, Grigoriev I, Markowitz V, Rigoutsos I, Richardson PM, Lidstrom ME, Chistoserdova L. High resolution metagenomics targets major functional types in complex microbial communities. Nature Biotechnology. 2008;26:1029–1034. doi: 10.1038/nbt.1488. [DOI] [PubMed] [Google Scholar]
- Kalyuzhnaya et al. (2005).Kalyuzhnaya MG, Stolyar SM, Auman AJ, Lara JC, Lidstrom ME, Chistoserdova L. Methylosarcina lacus sp. nov., a methanotroph from Lake Washington, Seattle, USA, and emended description of the genus Methylosarcina. International Journal of Systematic and Evolutionary Microbiology. 2005;55:2345–2350. doi: 10.1099/ijs.0.63405-0. [DOI] [PubMed] [Google Scholar]
- Kits, Klotz & Stein (2015).Kits KD, Klotz MG, Stein LY. Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. Type Strain FJG1. Environmental Microbiology. 2015 doi: 10.1111/1462-2920.12772. [DOI] [PubMed] [Google Scholar]
- McTaggart et al. (2015).McTaggart TL, Benuska G, Shapiro N, Woyke T, Chistoserdova L. Draft genomes of five new strains of Methylophilaceae isolated from Lake Washington sediment. Genome Announcements. 2015;3:e801. doi: 10.1128/genomeA.01511-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustakhimov et al. (2013).Mustakhimov I, Kalyuzhnaya MG, Lidstrom MW, Chistoserdova L. Insights into denitrification in Methylotenera mobilis from denitrification pathway and methanol metabolism mutants. Journal of Bacteriology. 2013;195:2207–2211. doi: 10.1128/JB.00069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet, Dlugokencky & Bousquet (2014).Nisbet EG, Dlugokencky EJ, Bousquet P. Atmospheric science. Methane on the rise-again. Science. 2014;343:493–495. doi: 10.1126/science.1247828. [DOI] [PubMed] [Google Scholar]
- Oksanen et al. (2014).Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2014. Vegan: community ecology package.
- Oshkin et al. (2014).Oshkin I, Beck DACB, Lamb AE, Tchesnokova V, Benuska G, McTaggart TL, Kalyuzhnaya MG, Dedysh S, Lidstrom ME, Chistoserdova L. Methane fed microcosms show differential community dynamics and pinpoint specific taxa involved in communal response. The ISME Journal. 2014 doi: 10.1038/ismej.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond, Valentine & Sessions (2010).Redmond MC, Valentine DL, Sessions AL. Identification of novel methane-, ethane-, and propane-oxidizing bacteria at marine hydrocarbon seeps by stable isotope probing. Applied Environmental Microbiology. 2010;76:6412–6422. doi: 10.1128/AEM.00271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers et al. (2013).Rivers AR, Sharma S, Tringe SG, Martin J, Joye SB, Moran MA. Transcriptional response of bathypelagic marine bacterioplankton to the Deepwater Horizon oil spill. The ISME Journal. 2013;7:2315–2329. doi: 10.1038/ismej.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmehl et al. (1993).Schmehl M, Jahn A, Meyer zu Vilsendorf A, Hennecke S, Masepohl B, Schuppler M, Marxer M, Oelze J, Klipp W. Identification of a new class of nitrogen fixation genes in Rhodobacter capsulatus: a putative membrane complex involved in electron transport to nitrogenase. Molecular and General Genetics. 1993;241:602–615. doi: 10.1007/BF00279903. [DOI] [PubMed] [Google Scholar]
- Singh et al. (2010).Singh BK, Bardgett RD, Smith P, Reay DS. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nature Reviews Microbiology. 2010;8:779–790. doi: 10.1038/nrmicro2439. [DOI] [PubMed] [Google Scholar]
- Tavormina et al. (2011).Tavormina PL, Orphan VJ, Kalyuzhnaya MG, Jetten MS, Klotz MG. A novel family of functional operons encoding methane/ammonia monooxygenase-related proteins in gammaproteobacterial methanotrophs. Environmental Microbiology Reports. 2011;3:91–100. doi: 10.1111/j.1758-2229.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- Trotsenko & Murrell (2008).Trotsenko YA, Murrell JC. Metabolic aspects of aerobic obligate methanotrophy. Advancements in Applied Microbiology. 2008;6:183–229. doi: 10.1016/S0065-2164(07)00005-6. [DOI] [PubMed] [Google Scholar]
- Van der Ha et al. (2013).Van der Ha D, Vanwonterghem I, Hoefman S, De Vos P, Boon N. Selection of associated heterotrophs by methane-oxidizing bacteria at different copper concentrations. Antonie Van Leeuwenhoek. 2013;103:527–537. doi: 10.1007/s10482-012-9835-7. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2007).Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartiainen et al. (2006).Wartiainen I, Hestnes AG, McDonald IR, Svenning MM. Methylobacter tundripaludum sp. nov., a methane-oxidizing bacterium from Arctic wetland soil on the Svalbard islands, Norway (78 degrees N) International Journal of Systematic and Evolutionary Microbiology. 2006;56:109–113. doi: 10.1099/ijs.0.63728-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
It is evident that as the communities simplify, oxygen consumption is reduced. By the time of the first sampling, the 150–225 µM (50–75% air) treatments remained constantly oxygenated while 15–75 µM (5–25% air) treatments went hypoxic before the next oxygen addition. Oxygen and methane were measured immediately after recreating the atmosphere and after 24 h. Blue columns, red columns, oxygen and methane, respectively, measured immediately after the atmosphere was created. Green columns, purple columns, oxygen and methane, respectively, measured after 24 h. Error bars indicate standard error across the replicates.
The diversity indices were computed for each of the three biological replicates and are shown with error bars indicating standard deviation across the replicates.
OTU abundance by read counts