Abstract
Objective:
To determine the effects of 12 weeks of neuromuscular electrical stimulation (NMES) training with ankle weights on intermuscular fascial length and patellar tendon cross-sectional area (CSA) in persons with spinal cord injury (SCI).
Methods:
This study was a pre-post intervention. Seven men with motor complete SCI were randomly assigned to a resistance training plus diet (RT + diet) group (n = 4) or a diet control group (n = 3). Participants in the RT + diet group were enrolled in a 12-week leg extension weight-lifting program via surface NMES of the knee extensor muscle group. The length of mid-thigh intermuscular fascia and the patellar tendon CSA were measured using MRI.
Results:
In the RT + diet group, a nonsignificant 8% increase in the CSA of the patellar tendon (P = .14) was noted. The length of the mid-thigh intermuscular fascia increased by 19% and 23% in the right (P = .029) and left (P = .015) legs, respectively, with no changes in the diet control group. Positive relationships were noted between skeletal muscle CSAs of the whole thigh (r = 0.77, P = .041) and knee extensors (r = 0.76, P = .048) and intermuscular fascial length.
Conclusion:
The preliminary results suggest that noncontractile connective tissue structures of the knee extensors respond differently to NMES training after SCI. Skeletal muscle hypertrophy is associated with an increase in the intermuscular fascial length.
Keywords: fascia length, MRI, neuromuscular electrical stimulation, rehabilitation, spinal cord injury, tendon
Surface neuromuscular electrical stimulation (NMES) training has been shown to evoke skeletal muscle hypertrophy, decrease ectopic adipose tissue, and enhance mitochondrial function in individuals with chronic spinal cord injury (SCI).1–3 During NMES training, the force developed is transmitted to the bone via noncontractile collagenous structures such as fascia and tendon. The interaction between skeletal muscle and the surrounding connective tissues is pivotal to maintain structural integrity and facilitate functional activities such as gliding and sliding movements of skeletal muscle during contraction, securing the anatomical locations of skeletal muscles and other connective tissue structures, and allowing the transmission of force to bone during exercise.
Little is known about the effects of SCI on these dense connective tissue structures, and little attention has been paid to the effects of training on musculotendinous or fascial structures. A decline in the patellar tendon cross-sectional area (CSA) by 17% was previously noted in persons with SCI compared with able-bodied control subjects,4 suggesting the need for appropriate rehabilitation strategies to restore structural properties of the tendon. Another study showed no major adaptations in the patellar tendon CSA between the trained and untrained limbs in individuals with SCI.5 To the best of our knowledge, no studies have examined fascial adaptations in response to SCI or training. The fascial network remodels based on the loading activity as indicated by the fibroblast activity. The resilience and elasticity of this network are important for injury prevention during exercise.6
It is unclear whether a training paradigm that evokes 35% hypertrophy of the extensor muscle group can influence the noncontractile structures in persons with SCI.1 The purpose of this brief report is to investigate the effects of 12 weeks of evoked resistance training (RT) via surface NMES on suprapatellar tendon CSA and intramuscular fascial length.
Methods
Seven individuals with clinically motor complete SCI were randomly assigned to an RT + diet group (n = 4; age, 35 ± 10 years; weight, 73 ± 20 kg; height, 1.87 ± 0.04 m; body mass index [BMI], 21 ± 6 kg/m2; level of injury, C5-C7; time since injury, 15 ± 9 years) or a diet control group (n = 3; age, 29 ± 4 years; weight, 74.5 ± 4.5 kg; height, 1.84 ± 0.05 m; BMI, 22 ± 2 kg/m2; level of injury, T4-T7; time since injury, 4 ± 2 years).1,7 Participants in the RT + diet group were enrolled in a 12-week leg extension weight-lifting program via surface NMES of the knee extensor muscle group.1,7 Participants in both the RT + diet and diet control groups were encouraged to follow a standard diet. The recommended dietary program was set to include 45% carbohydrate, 30% fat, and 25% protein.1 The inclusion and exclusion criteria have been listed previously in detail.1,7 All participants signed an informed consent statement that was approved by the local ethics committee.
A Theratouch NMES unit (Richmar, Inola, OK) with large conductive adhesive gel electrodes (8 cm × 10 cm) was used in the training. The electrodes were placed on the skin 2 to 3 cm above the superior aspect of the patella over the vastus medialis muscle and 30 cm above the patella over the vastus lateralis muscle. The parameters were set at a frequency of 30 Hz, pulse duration of 450 µs, and amplitude of the current that was gradually adjusted to evoke dynamic leg extension (0-200 mA). The current was delivered in a pattern of 5 seconds on/5 seconds off to attain full leg extension and to limit muscle fatigue. Briefly, the current was manually controlled via the stimulator dial-up knob to deliver the current to the knee extensors. The leg was lifted for 3 seconds, and the current was then manually reduced to bring the leg down into a resting position. After 3 seconds of rest, the cycle was then repeated for 10 repetitions per leg for 4 sets and alternated between the right and left legs. RT protocol was implemented twice weekly for 12 weeks, and leg extensions were performed using surface NMES with ankle weights. The weights were progressively increased by 2 lb on a weekly basis after 40 repetitions of full knee extension had been achieved in a session.1,2,7
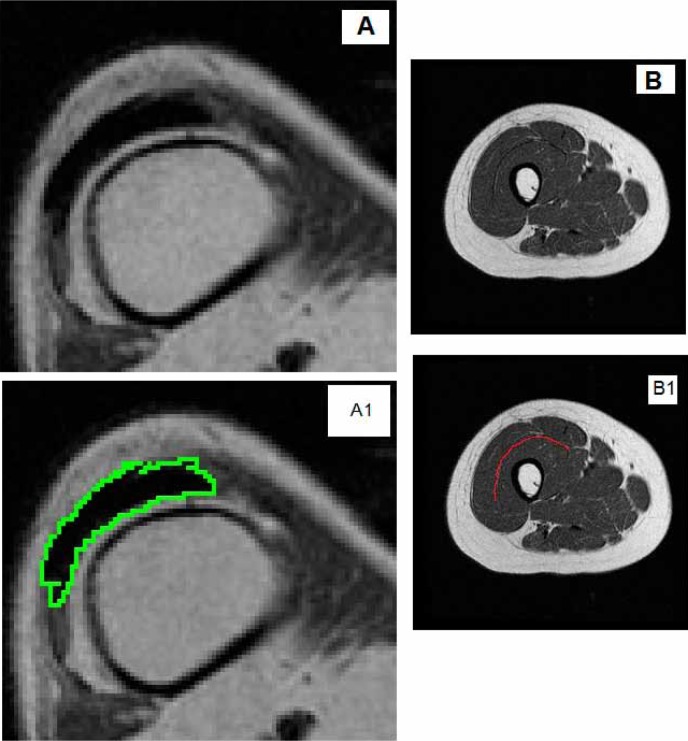
Transaxial magnetic resonance images were obtained for both thighs before and after interventions (repetition time, 550 ms; echo time, 14 ms; field of view, 20 cm; matrix size, 256 × 256, 10 mm thick and 5 mm apart). Skeletal muscle CSAs of the whole thigh and knee extensors have been previously reported in detail.1,2 The last 3 axial MRI slices proximal to the knee joint and supracondylar were used to measure patellar tendon CSA using Win Vessels program (Ronald Meyer, Michigan State University) (Figure 1A). Every effort was made to match the pre- and postimages using bony landmarks similar to the distal end of the shaft or supracondylar regions of the shaft of the femur. A second investigator who was originally blinded to the match of the images ensured the accuracy of matching between pre- and post-intervention images.
Figure 1. A representative MRI showing the tracing of (A) suprapatellar tendon cross-sectional area, (A1) following tracing the region of interest, and (B) intermuscular fascicle length, (B1) following tracing the region of interest, in a person with motor complete spinal cord injury.
The length of mid-thigh intermuscular fascia separating the vastus lateralis and rectus femoris muscles proximally and vastus intermedius muscle distally was measured. The intermuscular fascia boundaries were visually determined, and a curved line was drawn to trace the fascia line from the medial to the lateral end of the thigh. The average of 3 mid-thigh images was measured for each participant using Image J software (National Institutes of Health) (Figure 1B). Images were matched to ensure the exact anatomical locations before and after interventions.
Statistical analyses
All values are presented as mean ± SD. Because of the small sample size (n = 7), data were checked for normality. Because the patellar tendon CSA data departed from normality, nonparametric Kruskal-Wallis tests were used for analysis. Paired t tests were used for analysis of intermuscular fascial length data. Pearson correlations were used to test for the relationships between percentage changes in muscle CSAs of the whole thigh and knee extensor and percentage changes in intermuscular fascial length. The percentage changes were calculated using the following equation [(Postintervention – Preintervention)/Preintervention)×100] for both the RT + diet and diet control groups. Statistical analyses were performed using IBM SPSS version 21.0 (IBM, Armonk, NY).
Results
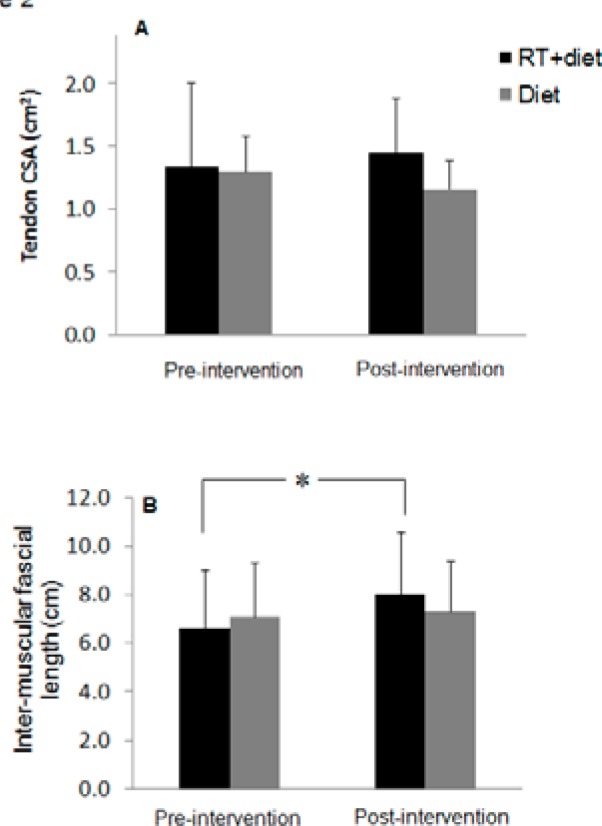
In the RT + diet group, 12 weeks of surface NMES training demonstrated, on average, a nonsignificant 8% increase in the CSA of right and left supracondylar patellar tendons (P =.14) (Figure 2A). However, there was a nonsignificant 10% decline in the CSA of the patellar tendons in the diet control group (P = .18). Kruskal-Wallis analysis indicated that there was no difference between the RT + diet and diet control groups in the CSAs of the patellar tendons of the right (P = .16) and left (P = .64) legs. The mid-thigh intermuscular fascial length increased by 19% in the right thigh (6.68 ± 2.7 to 7.94 ± 3.1 cm; P = .029) and 23% in the left thigh (6.60 ± 2.0 to 8.1 ± 2.1 cm; P = .015) (Figure 2B). However, there were no changes noted in the diet control group for the right (7.13 ± 2.4 to 7.75 ± 2.5 cm; P = .27) and left (7 ± 2.2 to 6.9 ± 1.7 cm; P = .70) thighs after intervention. There was no difference between the RT + diet and diet control groups in the fascial length of the right (P = .95) and left (P = .81) thighs.
Figure 2. The average of both right and left (A) cross-sectional areas (CSAs) of suprapatellar tendon and (B) lengths of intermuscular fascicles in resistance training + diet and diet control groups before and after interventions.
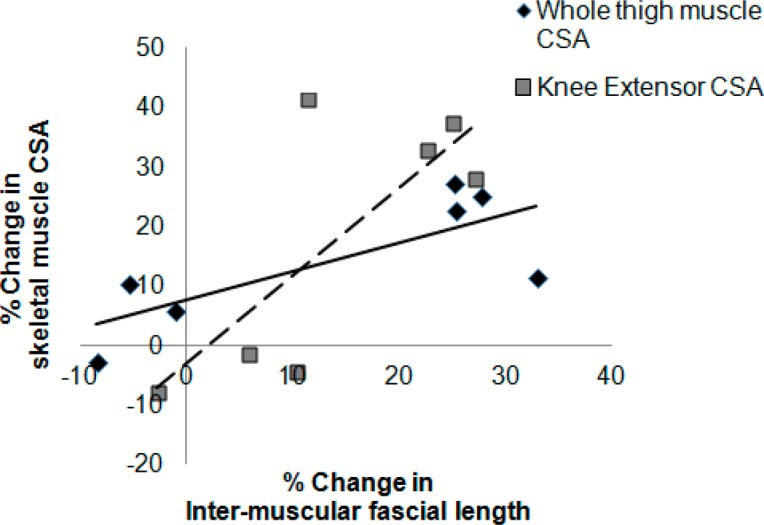
Pearson correlation showed positive relationships between percentage change in intermuscular fascial length and percentage changes in the whole thigh muscle (r = 0.77, P = .041) and knee extensor (r = 0.76, P = .048) CSAs (Figure 3).
Figure 3. Relationships between percentage changes in intermuscular fascial length and whole thigh muscle (solid line) (r = 0.77, P = .041) and knee extensor muscle (dashed line) (r = 0.76, P = .048) cross-sectional areas (CSAs) as measured by MRI in a previous study.1 The negative values refer to the decline in muscle CSAs of 2 individuals in the diet group after 12 weeks of intervention.
Discussion
Skeletal muscle hypertrophy was associated with an increase in the intramuscular fascial length and a small, but nonsignificant, increase in the suprapatellar tendon CSA after 12 weeks of RT in persons with SCI. The results indicate positive relationships between whole thigh and knee extensor muscle hypertrophy and intermuscular fascial length, suggesting that the increase in muscle CSA is associated with an increase in fascial length.
Today, the effects of SCI on connective tissue structures are not well established. A previous study demonstrated a decline in tendon CSA by 17% in persons with SCI compared with able-bodied control subjects.4 These preliminary results support previous findings that indicated no changes in patellar tendon CSA after training in persons with SCI.5 Collagenous materials of the tendon may require a longer training duration (ie, greater than 12 weeks) to stimulate hypertrophy. For example, in habitually elite athletes, the lead lower extremity had approximately 19% to 22% larger patellar tendon CSA compared with the nonlead lower extremity.8 Another possible explanation is that tendon force during NMES is 75% lower in persons with SCI compared with able-bodied control subjects,9 and this low force may be inadequate to evoke tendon hypertrophy.
The increase in intermuscular fascial length may be explained by the expansion in the connective tissue structures to accommodate the increase in fibers’ diameters following muscle hypertrophy as a result of RT in persons with SCI.1,2,7 This increase in fascial length may improve the interplay between intermuscular fascia and different muscle actions and provide resilience to the fascial network in this region to accommodate the increase in muscle size.6 It may also provide an energy dissipation mechanism of the contractile force to protect against muscle injury during RT. The positive adaptation in fascial length after RT may eventually suggest a delayed adaptation in tendon CSA, because intermuscular fascia are bundled into the whole patellar tendon.
Clinically, there is a growing interest in surgical restoration of arm and hand function or correction of foot drop by means of tendon transfer. However, there is no established consensus on the appropriate rehabilitation strategies that should be considered before surgical restoration, especially in persons with SCI. A recent study documented that tendon rupture may interfere with functional restorations and have devastating effects on the patients.10 Therefore, understanding the mechanism by which the noncontractile structures adapt to training can provide beneficial insights on the appropriate training stimulus necessary to evoke bone adaptations after SCI. It is unclear whether spasticity or muscle spasms may influence the outcomes of training on noncontractile connective tissue structures. We have previously shown a positive relationship between spasticity as determined by a modified Ashworth scale and muscle size.11,12 This may warrant further investigation to determine the effects of spasticity on fascial length and tendon CSA.
Limitations
This study is limited by the small size and failure to measure the tendon CSA distal to the patella. Another limitation is that only structural changes were assessed. However, these structural changes may or may not translate into functional changes. Future studies should include more functional assessments (ie, tendon stiffness/elasticity, modulus, stress, and strain) of noncontractile tissue.
Conclusions
The results of this preliminary report suggest that muscle hypertrophy after RT is accompanied by an increase in facial length, but not in the CSA of the patellar tendon. These differential outcomes may be time dependent because RT twice weekly for 12 weeks does not evoke a reasonable stimulus to induce tendon hypertrophy. The measurements of the adaptations of noncontractile structures after SCI are considered a novel aspect of this report. Finally, the increase in facial length may accommodate the contractile properties of the muscles during concentric/eccentric actions. Further studies are warranted to investigate the effects of NMES training on noncontractile structures after SCI.
Acknowledgments
This work was supported by research fund and grant support from Indiana University. The authors declare no conflicts of interest.
References
- 1.Gorgey AS, Mather KJ, Cupp HR, Gater DR.Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165–174. [DOI] [PubMed] [Google Scholar]
- 2.Gorgey AS, Shepherd C.Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: Case report. J Spinal Cord Med. 2010;33(1):90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan TE, Brizendine JT, Backus D, McCully KK.Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil. 2013;94(11):2166–2173. [DOI] [PubMed] [Google Scholar]
- 4.Maganaris CN, Reeves ND, Rittweger J, et al. Adaptive response of human tendon to paralysis. Muscle Nerve. 2006;33(1):85–92. [DOI] [PubMed] [Google Scholar]
- 5.Dudley-Javoroski S, McMullen T, Borgwardt MR, Peranich LM, Shields RK.Reliability and responsiveness of musculoskeletal ultrasound in subjects with and without spinal cord injury. Ultrasound Med Biol. 2010;36(10):1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schleip R, Müller DG.Training principles for fascial connective tissues: Scientific foundation and suggested practical applications. J Bodyw Mov Ther. 2013;17(1):103–115. [DOI] [PubMed] [Google Scholar]
- 7.Gorgey AS, Dolbow DR, Cifu DX, Gater DR.Neuromuscular electrical stimulation attenuates thigh skeletal muscles atrophy but not trunk muscles after spinal cord injury. J Electromyogr Kinesiol. 2013;23(4):977–984. [DOI] [PubMed] [Google Scholar]
- 8.Couppé C, Kongsgaard M, Aagaard P, et al. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol. 2008;105(3):805–810. [DOI] [PubMed] [Google Scholar]
- 9.Rittweger J, Gerrits K, Altenburg T, Reeves N, Maganaris CN, de Haan A.Bone adaptation to altered loading after spinal cord injury: A study of bone and muscle strength. J Musculoskelet Neuronal Interact. 2006;6(3):269–276. [PubMed] [Google Scholar]
- 10.Merenda LA, Rutter L, Curran K, Kozin SH.Rupture following biceps-to-triceps tendon transfer in adolescents and young adults with spinal cord injury: An analysis of potential causes. Top Spinal Cord Inj Rehabil. 2012;18(3):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorgey AS, Dudley GA.Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord. 2008;46(2):96–102. [DOI] [PubMed] [Google Scholar]
- 12.Gorgey AS, Gater DR.Insulin growth factors may explain relationship between spasticity and skeletal muscle size in men with spinal cord injury. J Rehabil Res Dev. 2012;49(3):373–380. [DOI] [PubMed] [Google Scholar]