Abstract
Object
Whereas diffuse intrinsic pontine gliomas generally have a short symptom duration and more cranial nerve involvement, focal brainstem gliomas are commonly low grade, with fewer cranial neuropathies. Although these phenotypic distinctions are not absolute predictors of outcome, they do demonstrate correlation in most cases. Because there is a limited literature on focal brainstem gliomas in pediatric patients, the objective of this paper was to report the management and outcome of these tumors.
Methods
The authors reviewed the records of all children diagnosed with radiographically confirmed low-grade focal brainstem gliomas from 1986 to 2010. Each patient underwent biopsy or resection for tissue diagnosis. Eventfree survival (EFS) and overall survival were evaluated. Univariate analysis was conducted to identify demographic and treatment variables that may affect EFS.
Results
Fifty-two patients (20 girls, 32 boys) with follow-up data were identified. Median follow-up was 10.0 years, and the median age at diagnosis was 6.5 years (range 1–17 years). The tumor locations were midbrain (n = 22, 42%), pons (n = 15, 29%), and medulla (n = 15, 29%). Surgical extirpation was the primary treatment in 25 patients (48%). The 5- and 10-year EFS and overall survival were 59%/98% and 52%/90%, respectively. An event or treatment failure occurred in 24 patients (46%), including 5 deaths. Median time to treatment failure was 3.4 years. Disease progression in the other 19 patients transpired within 25.1 months of diagnosis. Thirteen of these patients received radiation, including 11 within 2 months of primary treatment failure. Although children with intrinsic tumors had slightly better EFS at 5 years compared with those with exophytic tumors (p = 0.054), this difference was not significant at 10 years (p = 0.147). No other variables were predictive of EFS.
Conclusions
Surgery suffices in many children with low-grade focal brainstem gliomas. Radiation treatment is often reserved for disease progression but offers comparable disease control following biopsy. In the authors’ experience, combining an assessment of clinical course, imaging, and tumor biopsy yields a reasonable model for managing children with focal brainstem tumors.
Keywords: focal brainstem glioma, low grade, radiation treatment, outcomes, oncology, event-free survival
Brainstem gliomas account for 25% of posterior fossa tumors and 10%–20% of all CNS tumors in children, totaling approximately 150–300 cases annually in the US.23 Affected children are usually younger than 10 years old, and there is no sex predilection. The advent of CT in the 1970s, followed by MRI in the 1980s, brought about recognition that brainstem tumors are a heterogeneous collection of tumor types and locations.26 Many classification systems have been proposed, incorporating features such as size, location, and imaging characteristics.1,21,23,26 Epstein and McCleary8 grouped intrinsic nonexophytic tumors as focal (circumscribed mass > 2 cm in diameter without associated edema), diffuse, or cervicomedullary. Choux et al.5 described 4 types of tumors: Type I, diffuse brainstem gliomas; Type II, focal intrinsic tumors (solid or cystic); Type III, exophytic; and Type IV, cervicomedullary. Others believe that all brainstem tumors should be categorized as simply diffuse or focal.12
As refinements in MRI revealed different brainstem tumor types, it also became clear that there were major differences in presentation and survival for affected children. With regard to prognosis and survival, brainstem tumors can be viewed dichotomously: diffuse intrinsic pontine gliomas and all others. Diffuse intrinsic pontine gliomas generally have a much shorter duration of symptoms prior to diagnosis, more cranial nerve involvement, and shorter survival.7,10–12,20,24,28 Mauffrey20 found that on average, patients with a diffuse intrinsic pontine glioma had symptoms 2.5 months before diagnosis, 77% had at least 1 cranial neuropathy, and 25% were alive after 2 years. All other patients with brainstem gliomas had a mean duration of symptoms at diagnosis of 10.6 months, 28.5% had cranial nerve involvement, and 90% were alive after 2 years. Although these phenotypic distinctions are not absolute predictors of outcome, some level of correlation is evident.
Focal brainstem gliomas are themselves a diverse group of tumors. They arise anywhere in the brainstem, are generally less than 2 cm in diameter, can be solid or cystic, and are commonly low grade (WHO Grade I or II). Whereas resection has no role in the treatment of diffuse tumors, surgical extirpation is a treatment option with focal tumors.16,18,27 There is limited literature on the management and outcome of pediatric focal brainstem gliomas, with no consensus on when and how to treat affected children. We review our experience with focal lowgrade brainstem neoplasms in pediatric patients to establish management methods and outcome expectations.
Methods
Study Population
A retrospective review identified all children with a low-grade focal brainstem glioma diagnosed between May 1986 and May 2010. The study was approved by the institutional review board of St. Jude Children’s Research Hospital. Eligible patients were those who underwent surgery (resection or biopsy) performed at Le Bonheur Children’s Hospital or at an outside institution with follow-up care at St. Jude. Decisions regarding treatment were typically made by consensus during a multidisciplinary brain tumor meeting. A focal tumor was defined as one in which the tumor had a well-defined border (the tumor border on a T1-weighted MRI sequence approximated the appearance on a T2-weighted sequence) contained entirely within the normal boundaries of the brainstem (intrinsic) or significantly expanding beyond the pial surface (exophytic). Patients with tectal gliomas were usually excluded because these patients rarely underwent biopsy but were included if tissue was obtained.2 We also excluded cervicomedullary and thalamopeduncular tumors because they both originate outside of the brainstem with secondary extension into it.4,29
Data Collection
We located and reviewed the initial imaging studies and confirmed the radiological diagnosis of a focal brainstem tumor as stated in the MRI report. Tissue samples were independently reviewed at initial diagnosis by a St. Jude pathologist, and retrospectively by a neuropathologist; in 6 cases the original preparations were not available for retrospective review, and the initial diagnosis was recorded for data analysis.
Demographic, clinical, radiological, pathological, surgical, and survival data were collected. Tumor-specific data included anatomical location, pattern of growth, extent of surgery, WHO grade, type of primary treatment, sequence of therapeutic modalities, survival status, and treatment failure. Gross-total resection was defined as no evidence of residual tumor on postoperative MRI; NTR was defined as excision greater than 90%, and STR as excision less than 90%.
Outcome Assessment
Outcomes assessed were EFS and overall survival both 5 and 10 years after diagnosis. Event-free survival was defined as either lack of disease progression, development of secondary malignancy, or death from any cause. Event-free survival was calculated from the date of diagnosis to the date of an event or last contact. Overall survival was determined from the date of diagnosis to the date of last follow-up or death. Overall clinical follow-up was defined as the period from diagnosis to last clinical assessment or death.
Statistical Analysis
The Kaplan-Meier method was used to estimate EFS and overall survival, and the log-rank test was performed to determine whether the following variables were predictive of EFS: sex, age (< 10 years vs ≥ 10 years), tumor location, extent of resection, treatment, WHO grade, and pattern of growth. Statistical significance was defined as p < 0.05. All statistical analysis was performed with the Stata/SE program (version 11.2, StataCorp).
Results
Table 1 shows patient characteristics for the study population. Although we located 56 patients, 4 lived remotely and underwent no follow-up after their resections, and were therefore excluded from analysis. The remaining 52 patients had a median age at diagnosis of 6.5 years (range 1–17 years) and 32 were male (62%). Seven patients (13%) were treated in the 1980s, 19 (37%) in the 1990s, 24 (46%) from 2000 through 2009, and 2 (4%) since 2010. The median follow-up was 10.0 years (range 1.2–24.6 years). There were 5 deaths. Of the remaining 47 patients, the overall follow-up was 10.6 years.
TABLE 1.
Characteristics of 52 patients included in the study
| Variable | Value |
|---|---|
| age (yrs) | |
| median | 6.5 |
| mean | 7.7 |
| range | 1–17 |
| sex (%) | |
| male | 32 (62) |
| female | 20 (38) |
| follow-up | |
| mean yrs | 10.02 |
| no. w/ <5 yrs | 11 |
| no. w/ 5–10 yrs | 15 |
| no. w/ 10–15 yrs | 12 |
| no. w/ >15 yrs | 14 |
| race (%) | |
| white | 41 (79) |
| black | 8 (15) |
| other | 3 (6) |
| neurofibromatosis (%) | 2 (4) |
| presentation (%)* | |
| increased intracranial pressure | 27 (52) |
| unilateral hemiparesis | 13 (25) |
| unilateral cranial neuropathy | 9 (17) |
| ataxia | 15 (29) |
| hydrocephalus | 19 (37) |
| tumor location (%) | |
| midbrain | 22 (42) |
| pons | 15 (29) |
| medulla | 15 (29) |
| tumor appearance (%) | |
| intrinsic | 26 (50) |
| exophytic | 26 (50) |
| tumor grade (%) | |
| I | 42 (81) |
| II | 10 (19) |
| extent of surgery (%) | |
| biopsy | 17 (33) |
| GTR | 9 (17) |
| NTR | 17 (33) |
| STR | 9 (17) |
| mode of treatment (%) | |
| surgery only | 25 (48) |
| radiation only | 14 (27) |
| surgery + radiation | 7 (13) |
| surgery + chemotherapy | 2 (4) |
| surgery + radiation + chemotherapy | 1 (2) |
| chemotherapy only | 2 (4) |
| chemotherapy + radiation | 1 (2) |
Numbers do not sum to 52 because patients may have had more than 1 presenting element.
Fifty-two percent of patients presented with increased intracranial pressure secondary to tumoral mass effect. Clinical findings were mostly unilateral, including hemiparesis in 25% and unilateral cranial nerve dysfunction in 17%, which was always opposite the hemiparesis. Almost half of the tumors were located in the midbrain, equally distributed as intrinsic and exophytic. Only 2 patients (4%) had tectal gliomas. Tissue samples were available for review in 46 (88%) of 52 patients. The original diagnosis was used for 6 patients for whom preparations were not available. Forty-two patients (81%) had a WHO Grade I tumor, while 10 had a Grade II tumor. The following pathologies were recorded: pilocytic astrocytoma (n = 41, 79%), fibrillary astrocytoma (n = 9, 17%), pilomyxoid glioma (n = 1, 2%), and ganglioglioma (n = 1, 2%).
All patients underwent surgery: biopsy only in 17 (33%), GTR in 9 (17%), NTR in 17 (33%), and STR in 9 (17%). Surgery was the sole treatment in 25 patients (48%), of which 8 were GTR (32%), 14 were NTR (56%), and 3 were STR (12%). Conversely, biopsy followed by radiotherapy was the primary treatment in 14 patients (27%). Maximal safe resection followed by radiation therapy was the treatment in 7 patients (13%), of whom 5 underwent STR and 2 underwent NTR. The remaining 6 patients received postbiopsy chemotherapy (n = 2) or chemotherapy in combination with surgery or radiation (n = 4).
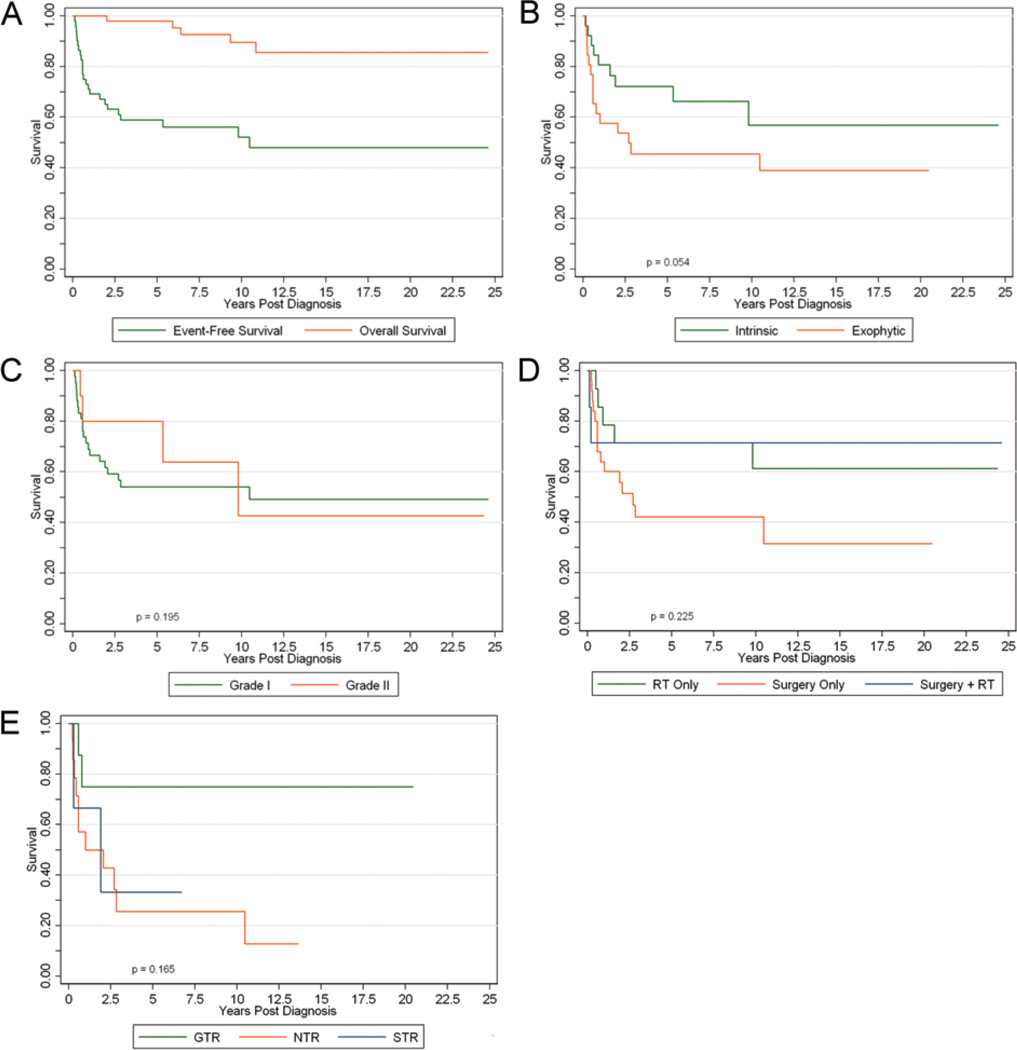
The 5-year EFS and overall survival were 58.9% (95% CI 44.2%–71.0%) and 98% (95% CI 86.6%–99.7%), respectively (Fig. 1A). At 10 years, the EFS and overall survival were 52.1% (95% CI 36.3%–65.8%) and 89.6% (95% CI 74.3%–96.0%), respectively. An event or treatment failure occurred in 24 patients (46%) after a median of 3.4 years. This included 5 deaths, of which only 2 were directly related to tumor progression. One patient died of presumed ventricular shunt failure without imaging evidence of disease progression, and 2 patients (3.5%) who were initially diagnosed as having Grade 2 fibrillary astrocytoma had glioblastoma documented on repeat biopsy within the confines of the original tumor bed (see Discussion). Of the 19 patients with disease progression who were still alive after an event, notable imaging or clinical events reflective of tumor progression occurred within 25.1 months of diagnosis. Only 4 of the 24 patients with treatment failures had received radiation therapy before the recorded first failure. Three of these 4 patients experienced postirradiation therapy failure between 5 and 10 months after radiation therapy, and 1 patient experienced therapy failure 78.4 months after radiation therapy. Of the remaining 20 patients with treatment failure, 6 have yet to receive radiation therapy, and radiation therapy was initiated within 2 months in 11 children, and between 11.2 and 45.6 months in the remaining 3 children.
Figure 1.
Survival curves for EFS and overall survival for the entire study population (A), and EFS curves for intrinsic versus exophytic tumors (B), WHO Grade I versus Grade II tumors (C), mode of treatment (D), and extent of resection (E). RT = radiation therapy.
Table 2 shows the 5- and 10-year event-free outcomes and the corresponding p values for the various subgroups. Among the univariate tests, there was a trend (p = 0.054) for patients with intrinsic tumors to have better 5-year EFS than patients with exophytic tumors (Fig. 1B), but at 10 years there was no significant difference (p = 0.147). There was no significant difference in the 5- and 10-year EFS based on sex, age, WHO grade (Fig. 1C), location of tumor, mode of treatment (Fig. 1D), or extent of resection (Fig. 1E). Among those undergoing surgery only (n = 25), the 5-year EFS rates for GTR, NTR, and STR were 75%, 25.7%, and 33.3%, respectively. Although there does appear to be a trend in favor of GTR as noted by the separation of curves between GTR and NTR/STR, this did not prove to be significant at 5 or 10 years (p = 0.165).
TABLE 2.
Univariate analysis of 5- and 10-year EFS
| Comparison | 5-year EFS* | 10-year EFS |
|---|---|---|
| sex | ||
| % male | 55 | 48.9 |
| % female | 65 | 57.8 |
| p value | 0.619 | 0.702 |
| age | ||
| % <10 yrs | 57.1 | 53.1 |
| % ≥10 yrs | 61.8 | 49.5 |
| p value | 0.742 | 0.856 |
| location | ||
| % midbrain | 67.6 | 58 |
| % pons | 66.7 | 60 |
| % medulla | 36.6 | 36.6 |
| p value | 0.091 | 0.141 |
| WHO grade | ||
| % I | 54.1 | 54.1 |
| % II | 80 | 42.7 |
| p value | 0.195 | 0.656 |
| intrinsic vs exophytic | ||
| % intrinsic | 72.3 | 56.8 |
| % exophytic | 45.6 | 45.6 |
| p value | 0.054 | 0.147 |
| extent of resection | ||
| % GTR | 75 | 75 |
| % NTR | 25.7 | 25.7 |
| % STR | 33.3 | none† |
| p value | 0.165 | 0.165 |
| mode of treatment | ||
| % radiation | 71.4 | 61.2 |
| % surgery only | 42.1 | 42.1 |
| % surgery + radiation | 71.4 | 71.4 |
| p value | 0.225 | 0.304 |
Probability values obtained using the log-rank test.
Ten-year estimate for STR cannot be given because all patients undergoing STR either had treatment failure or were censored before reaching 10 years.
Discussion
Our study showed that patients with low-grade focal brainstem gliomas have 5- and 10-year survival rates of 98% and 90%, respectively. Teo and Siu27 found a 100% 5-year survival rate in their group of 23 patients with lowgrade focal tumors, Mauffrey20 reported a 90% 2-year and 60% 5-year survival rate in patients with nondiffuse brainstem tumors, and Sandri et al.24 found an 87% 5-year survival rate in patients with focal brainstem tumors. Despite these survival rates, disease progression (symptomatic or radiographic) is not uncommon, as evidenced by our 5-year EFS of 58.9%. Similarly, Lesniak et al.18 reported a 5-year progression-free survival of 45.6%, but 12 of their 89 patients had high-grade gliomas. The study of Sandri et al.24 showed a 60% 5-year EFS rate. Recently, the group at The Hospital for Sick Children in Toronto reported 5-year overall and progression-free survival rates of 89% and 57%, respectively, in children with low-grade brainstem tumors.13
In our patients, unilateral hemiparesis and cranial neuropathies suggested a more focal nature of the tumor. Patients with diffuse intrinsic pontine gliomas, on the other hand, more often have bilateral cranial nerve deficits and weakness at presentation. Ataxia, however, is common to both types. It is true that diffuse intrinsic pontine gliomas have a very short clinical history at presentation, but patients with low-grade focal brainstem gliomas may have a brief clinical history if the onset of obstructive hydrocephalus brings them to clinical attention.
Prognostic Factors
Several purported patient- and treatment-related prognostic factors for patients with focal brainstem tumors have been reported in the literature. High-grade pathology (WHO Grade III/IV) has been consistently found to be a predictor of disease progression and death, whereas patients with pilocytic astrocytomas have the most favorable prognosis.6,12,16,18,20,27 Fisher et al.12 found that pontine location, engulfment of the basilar artery, symptom duration less than 6 months, fibrillary pathology, and abducens nerve palsy on presentation were all associated with worse survival. At 5 years, the survival of patients with pilocytic astrocytoma was 95%, compared with a dismal 15% for patients with fibrillary astrocytomas; however, the researchers did not differentiate focal and diffuse tumors within their patient population and also combined WHO Grade II–IV tumors as fibrillary astrocytomas. Patients with rapid onset of symptoms (generally considered < 6 months) have a poorer outcome, whereas enhancing lesions, which may be a surrogate for pilocytic astrocytoma, correlate with longer survival than nonenhancing lesions.18,20,28 Lesniak et al.18 also suggested that patients with enhancing medullary tumors experienced longer survival than those with tumors in the pons or midbrain.
In our study, age, sex, WHO grade, the tumor’s relation to the brainstem border, location of tumor, type of treatment, and extent of resection were not significant predictors of EFS. The population studied by Fried et al.13 most closely resembled our patient population, and they also found that tumor location, size, and extent of surgery were not predictive of tumor progression. They did find that the progression rate tended to be higher for patients younger than 3 years of age (74%) than for children older than 3 years (54%).
Two patients in our study developed radiographically apparent malignant transformation 5.3 and 10.3 months after radiotherapy, which was confirmed with repeat tissue acquisition. These short time frames would be unusual for true spontaneous malignant degeneration or radiation-induced malignant transformation. Thus, it is more likely that these patients already harbored high-grade malignancies that the biopsies failed to show because of sampling error. Only 2 patients died of disease progression. One death occurred in a child who received only chemotherapy after biopsy and who died of disease 5.9 years later. The second death occurred in a child who underwent GTR on the protocol and suffered distant progression 7.1 months after diagnosis; she then died of a progressive tumor obstructing the fourth ventricle 2 years after diagnosis.
Treatment Options
Surgery has an important role in the management of focal brainstem gliomas. Magnetic resonance imaging and other imaging techniques such as spectroscopy, perfusion, and PET are performed in an attempt to predict the histology of focal brainstem lesions, but a stereotactic biopsy is necessary to distinguish a focal low-grade tumor from a focal malignant tumor and can be conducted with low morbidity.6,14,17,22 Many investigators have advocated resection as the primary treatment for focal brainstem tumors.3,9,11,15,16,18,20,21,23,24,27 Some researchers have found that the extent of resection is an important prognostic factor for survival, contrary to our findings.16,24,25
Radiation therapy has not been widely accepted as a primary treatment modality in focal tumors, but instead is typically used as adjuvant therapy for residual tumor or progressive/recurrent disease.11,24 There have been a few reports on the use of stereotactic radiosurgery with these tumors.19,30 Yen et al.30 treated 20 patients, both adults and children, with Gamma Knife surgery. The mean tumor volume in this study was 2.5 cm3, and the mean dose was 10–18 Gy using an average of 4 isocenters. The tumor disappeared in 4 patients, shrank in 12, and progressed in 4, with 1 of those patients eventually succumbing to the disease. The researchers concluded that Gamma Knife surgery may be an effective primary treatment or adjunct to open surgery for focal brainstem gliomas. Although we have no experience with the use of radiosurgery in patients with focal brainstem gliomas, we would agree that it may have a role, but further research is needed. Conversely, Jallo et al.15 argued that adjuvant therapy has produced minimal improvement in the long-term survival of patients with low-grade brainstem gliomas. Thus, they advocated second-look surgery for patients with delayed recurrent growth resulting in new symptoms or when the initial resection was halted prematurely because of transient intraoperative injury confirmed by monitoring.
We have shown that surgery and radiotherapy are effective in providing local control and thus survival in the majority of patients, regardless of the extent of resection. The role of radiotherapy is to provide enhanced local control when either clinical symptoms or imaging-based evidence of progression demands more aggressive intervention, and in this cohort, it proved necessary in most cases. Only 4 of 24 treatment failures included radiation therapy shortly after resection; however, 19 of the remaining 20 treatment failures occurred within the first 2 years of diagnosis, with late progression (> 11.2 months after therapy) occurring in only 3 children. Treatment failure after radiation therapy appeared to portend a poor prognosis, as these patients tended to have rapid treatment failure (5–10 months after completion of radiation therapy) and most eventually died of their disease.
Treatment Recommendations
Based on our experience, we believe that patients with symptomatic or radiographically progressive focal brainstem gliomas should be treated using either biopsy as well as conformal irradiation, or surgery (Figs. 2 and 3). Surgery should be pursued if the tumor is considered accessible, and resectable with the family understanding and accepting the risks of new and potentially permanent neurological deficits. For patients who are not operative candidates or whose parents are not willing to accept the surgical morbidity risk, a multipass stereotactic biopsy should be performed, followed by conformal radiation therapy when indicated by either clinical progression or imaging-based progression with potential for worsening morbidity. It is unclear whether radiation administered after resection confers additional survival benefit. Our findings support clinical and radiographic surveillance, recognizing that postoperative progression occurs early and can be managed secondarily with radiation treatment.
Figure 2.
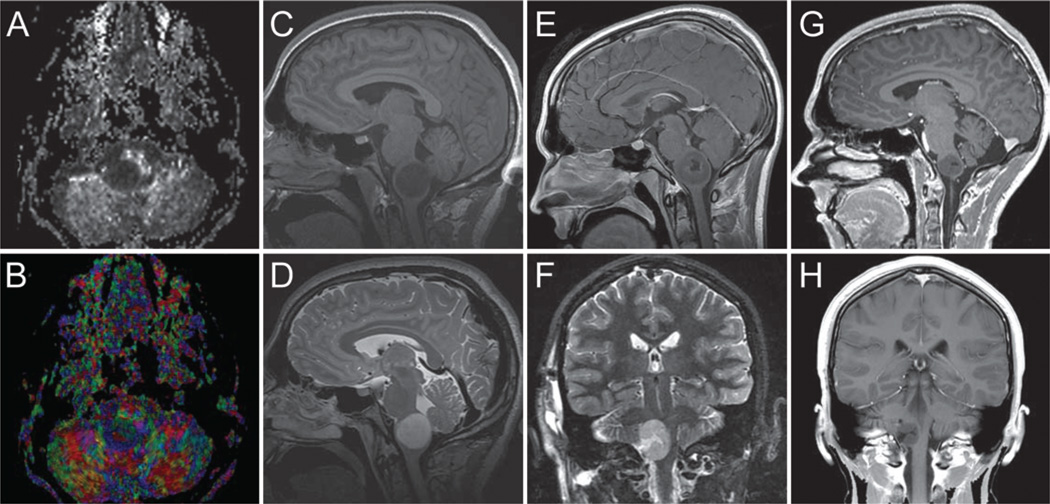
Images obtained in a 17-year-old boy who presented with medullary WHO Grade II astrocytoma. Three separate time points are selected for demonstration. A and B: Preoperative fractional anisotropy grayscale and color maps showing baseline imaging. C and D: Preoperative sagittal T1-weighted noncontrast (C) and T2-weighted MR images (D). E and F: Postoperative, preirradiation sagittal T1-weighted MR image obtained after contrast administration (E) and coronal T2-weighted MR image (F) obtained 1 week later. G and H: Postirradiation sagittal (G) and coronal (H) T1-weighted MR images obtained after contrast administration 21 months later.
Figure 3.
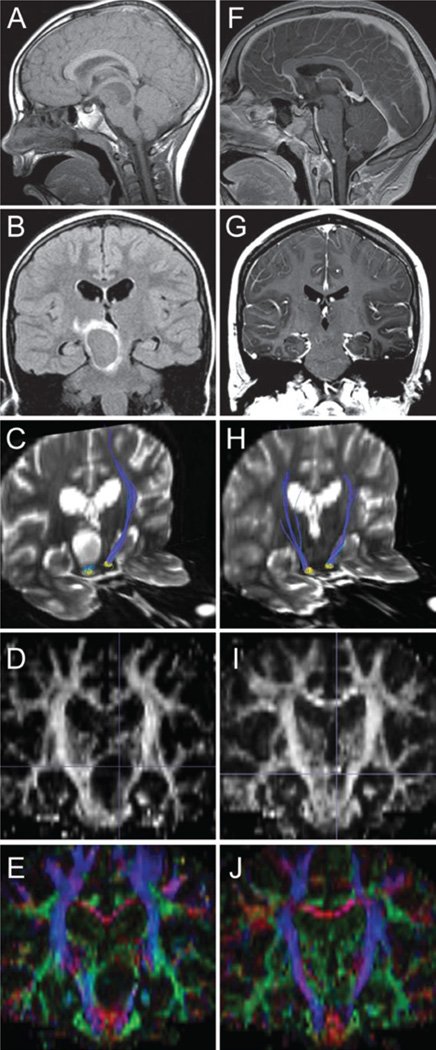
Images obtained in a 10-year-old boy who presented with a mesencephalic WHO Grade I pilocytic astrocytoma. A–E: Preoperative images included sagittal T1-weighted noncontrast MR image (A), coronal T2-weighted FLAIR (B), diffusion tensor image of bilateral corticospinal tract (blue) with ipsilateral tract thinning (C), coronal fractional anisotropy (D), and coronal color map (E). F–J: Postoperative images, obtained 4 years after the preoperative images, included sagittal T1-weighted postcontrast (F), coronal T1-weighted postcontrast (G), diffusion tensor image of bilateral corticospinal tracts (blue) demonstrating recovery of ipsilateral tract thickness (H), coronal fractional anisotropy (I), and coronal color map (J).
Our treatment recommendations are somewhat contrary to those put forth by Fried et al.13 Although we agree that some patients may be observed initially, they cautioned that aggressive resection or upfront radiotherapy may not be justified based on data suggesting no clear outcome decrement in children treated with upfront chemotherapy. However, they acknowledge a potential bias because upfront chemotherapy is more commonly associated with worse disease control and its role in younger children has been established more for toxicity reduction than disease control benefit. In our experience, the role of chemotherapy in low-grade gliomas is poorly defined, and its incorporation has occurred in a small subset of our patients. Nonetheless, it is viewed as a treatment option for younger children, patients with the potential for high surgical morbidity, and when the family does not want to pursue radiation treatment.
Strengths and Limitations
The strengths of our study are the large number of patients treated at a single institution and the long clinical follow-up duration. Our study suffers from the limitations inherent in a long-term retrospective review. In addition, the treatment was not standardized and was administered over several treatment eras, which we defined as the decade in which each patient was treated. Finally, sampling error (misidentifying a more aggressive tumor) may be an additional confounding factor, which was probably related to the deaths of 2 of our patients.
Conclusions
Focal brainstem tumors are a rare, heterogeneous group both pathologically and radiographically, but are associated with excellent long-term survival. It is critical that these focal low-grade tumors be recognized and distinguished from diffuse tumors, especially diffuse intrinsic pontine gliomas. Resection and conformal radiation therapy are viable frontline treatment options, but short-interval follow-up is necessary during at least the first 2 years after diagnosis. Radiation serves as an excellent treatment for patients whose disease progresses after surgery. Future research will hopefully unlock the molecular mysteries of these tumors so that treatment can be tailored to the tumor biology in conjunction with advances in current clinical management.
Acknowledgment
The authors thank Kristin Kraus, M.Sc., for editorial assistance.
Disclosure
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and by Cancer Center Support Grants CA23099 and CA21765 from the NIH. Dr. Boop is Secretary of the AANS and Treasurer of the International Society for Pediatric Neurosurgery.
Abbreviations used in this paper
- EFS
event-free survival
- GTR
gross-total resection
- NTR
near-total resection
- STR
subtotal resection
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Pai Panandiker, Klimo, Thompson, Boop, Kun, Sanford. Acquisition of data: Pai Panandiker, Klimo, Ellison, Sanford. Analysis and interpretation of data: Pai Panandiker, Klimo, Thompson, Boop, Ellison, Kun, Sanford. Drafting the article: Pai Panandiker, Klimo, Boop, Qaddoumi, Gajjar, Armstrong, Kun. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Pai Panandiker. Administrative/technical/material support: Ogg.
References
- 1.Barkovich AJ, Krischer J, Kun LE, Packer R, Zimmerman RA, Freeman CR, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990–1991;16:73–83. doi: 10.1159/000120511. [DOI] [PubMed] [Google Scholar]
- 2.Boydston WR, Sanford RA, Muhlbauer MS, Kun LE, Kirk E, Dohan FC, Jr, et al. Gliomas of the tectum and periaqueductal region of the mesencephalon. Pediatr Neurosurg. 1991–1992;17:234–238. doi: 10.1159/000120603. [DOI] [PubMed] [Google Scholar]
- 3.Bricolo A, Turazzi S, Cristofori L, Talacchi A. Direct surgery for brainstem tumours. Acta Neurochir Suppl (Wien) 1991;53:148–158. doi: 10.1007/978-3-7091-9183-5_25. [DOI] [PubMed] [Google Scholar]
- 4.Broadway SJ, Ogg RJ, Scoggins MA, Sanford R, Patay Z, Boop FA. Surgical management of tumors producing the thalamopeduncular syndrome of childhood. Clinical article. J Neurosurg Pediatr. 2011;7:589–595. doi: 10.3171/2011.4.PEDS119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choux M, Lena G, Do L. Brainstem tumors. In: Choux M, Di Rocco C, Hockley A, et al., editors. Pediatric Neurosurgery. New York: Churchill Livingstone; 2000. pp. 471–491. [Google Scholar]
- 6.Dellaretti M, Touzet G, Reyns N, Dubois F, Gusmão S, Pereira JL, et al. Correlation among magnetic resonance imaging findings, prognostic factors for survival, and histological diagnosis of intrinsic brainstem lesions in children. Clinical article. J Neurosurg Pediatr. 2011;8:539–543. doi: 10.3171/2011.9.PEDS1167. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–1272. doi: 10.1200/JCO.2005.04.6599. [DOI] [PubMed] [Google Scholar]
- 8.Epstein F, McCleary EL. Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg. 1986;64:11–15. doi: 10.3171/jns.1986.64.1.0011. [DOI] [PubMed] [Google Scholar]
- 9.Epstein F, Wisoff JH. Intrinsic brainstem tumors in childhood: surgical indications. J Neurooncol. 1988;6:309–317. doi: 10.1007/BF00177425. [DOI] [PubMed] [Google Scholar]
- 10.Fangusaro J. Pediatric high-grade gliomas and diffuse intrinsic pontine gliomas. J Child Neurol. 2009;24:1409–1417. doi: 10.1177/0883073809338960. [DOI] [PubMed] [Google Scholar]
- 11.Farmer JP, Montes JL, Freeman CR, Meagher-Villemure K, Bond MC, O’Gorman AM. Brainstem gliomas. A 10-year institutional review. Pediatr Neurosurg. 2001;34:206–214. doi: 10.1159/000056021. [DOI] [PubMed] [Google Scholar]
- 12.Fisher PG, Breiter SN, Carson BS, Wharam MD, Williams JA, Weingart JD, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89:1569–1576. doi: 10.1002/1097-0142(20001001)89:7<1569::aid-cncr22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Fried I, Hawkins C, Scheinemann K, Tsangaris E, Hesselson L, Bartels U, et al. Favorable outcome with conservative treatment for children with low grade brainstem tumors. Pediatr Blood Cancer. 2012;58:556–560. doi: 10.1002/pbc.23200. [DOI] [PubMed] [Google Scholar]
- 14.Gonçalves-Ferreira AJ, Herculano-Carvalho M, Pimentel J. Stereotactic biopsies of focal brainstem lesions. Surg Neurol. 2003;60:311–320. doi: 10.1016/s0090-3019(03)00379-3. [DOI] [PubMed] [Google Scholar]
- 15.Jallo GI, Biser-Rohrbaugh A, Freed D. Brainstem gliomas. Childs Nerv Syst. 2004;20:143–153. doi: 10.1007/s00381-003-0870-6. [DOI] [PubMed] [Google Scholar]
- 16.Kestle J, Townsend JJ, Brockmeyer DL, Walker ML. Juvenile pilocytic astrocytoma of the brainstem in children. J Neurosurg. 2004;101(1 Suppl):1–6. doi: 10.3171/ped.2004.101.2.0001. [DOI] [PubMed] [Google Scholar]
- 17.Kwon JW, Kim IO, Cheon JE, Kim WS, Moon SG, Kim TJ, et al. Paediatric brain-stem gliomas: MRI, FDG-PET and histological grading correlation. Pediatr Radiol. 2006;36:959–964. doi: 10.1007/s00247-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 18.Lesniak MS, Klem JM, Weingart J, Carson BS., Sr Surgical outcome following resection of contrast-enhanced pediatric brainstem gliomas. Pediatr Neurosurg. 2003;39:314–322. doi: 10.1159/000075260. [DOI] [PubMed] [Google Scholar]
- 19.Liao CH, Pan DH, Yang HC, Wu HM, Ho DM, Wong TT, et al. Gamma Knife radiosurgery as a treatment modality for low-grade pediatric brainstem gliomas: report of two cases. Childs Nerv Syst. 2012;28:175–178. doi: 10.1007/s00381-011-1620-9. [DOI] [PubMed] [Google Scholar]
- 20.Mauffrey C. Paediatric brainstem gliomas: prognostic factors and management. J Clin Neurosci. 2006;13:431–437. doi: 10.1016/j.jocn.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Mehta VS, Chandra PS, Singh PK, Garg A, Rath GK. Surgical considerations for ‘intrinsic’ brainstem gliomas: proposal of a modification in classification. Neurol India. 2009;57:274–281. doi: 10.4103/0028-3886.53272. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Gómez JL, Rodríguez-Alvarez CA, Marhx-Bracho A, Rueda-Franco F. Stereotactic biopsy for brainstem tumors in pediatric patients. Childs Nerv Syst. 2010;26:29–34. doi: 10.1007/s00381-009-1000-x. [DOI] [PubMed] [Google Scholar]
- 23.Recinos PF, Sciubba DM, Jallo GI. Brainstem tumors: where are we today? Pediatr Neurosurg. 2007;43:192–201. doi: 10.1159/000098831. [DOI] [PubMed] [Google Scholar]
- 24.Sandri A, Sardi N, Genitori L, Giordano F, Peretta P, Basso ME, et al. Diffuse and focal brain stem tumors in childhood: prognostic factors and surgical outcome. Experience in a single institution. Childs Nerv Syst. 2006;22:1127–1135. doi: 10.1007/s00381-006-0083-x. [DOI] [PubMed] [Google Scholar]
- 25.Schild SE, Stafford SL, Brown PD, Wood CP, Scheithauer BW, Schomberg PJ, et al. The results of radiotherapy for brainstem tumors. J Neurooncol. 1998;40:171–177. doi: 10.1023/a:1006193306286. [DOI] [PubMed] [Google Scholar]
- 26.Stroink AR, Hoffman HJ, Hendrick EB, Humphreys RP. Diagnosis and management of pediatric brain-stem gliomas. J Neurosurg. 1986;65:745–750. doi: 10.3171/jns.1986.65.6.0745. [DOI] [PubMed] [Google Scholar]
- 27.Teo C, Siu TL. Radical resection of focal brainstem gliomas: is it worth doing? Childs Nerv Syst. 2008;24:1307–1314. doi: 10.1007/s00381-008-0647-z. [DOI] [PubMed] [Google Scholar]
- 28.Ueoka DI, Nogueira J, Campos JC, Maranhão Filho P, Ferman S, Lima MA. Brainstem gliomas—retrospective analysis of 86 patients. J Neurol Sci. 2009;281:20–23. doi: 10.1016/j.jns.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Weiner HL, Freed D, Woo HH, Rezai AR, Kim R, Epstein FJ. Intra-axial tumors of the cervicomedullary junction: surgical results and long-term outcome. Pediatr Neurosurg. 1997;27:12–18. doi: 10.1159/000121219. [DOI] [PubMed] [Google Scholar]
- 30.Yen CP, Sheehan J, Steiner M, Patterson G, Steiner L. Gamma knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:8–17. doi: 10.3171/jns.2007.106.1.8. [DOI] [PubMed] [Google Scholar]