Abstract
Sphingosine-1-phosphate (S1P) is a potent bioactive sphingolipid involved in cell proliferation, angiogenesis, inflammation and malignant transformation among other functions. S1P acts either directly on intracellular targets or activates G protein-coupled receptors, specifically five S1P receptors (S1PRs). The identified S1PRs differ in cellular and tissue distribution, and each is coupled to specific G proteins, which mediate unique functions. Here, we describe functional characteristics of all five receptors, emphasizing S1PR2, which is critical in the immune, nervous, metabolic, cardiovascular, musculoskeletal, and renal systems. This review also describes the role of this receptor in tumor growth and metastasis and suggests potential therapeutic avenues that exploit S1PR2.
Keywords: cancer, ceramide, ezrin, JTE013, S1P knockout mouse, S1PR2, SID46371153, sphingolipid diseases, sphingolipids, sphingosine 1-phosphate
Introduction
Lipids not only provide structure to the cell membrane, but also exhibit bioactive roles, such as interaction with and activation of different pathways (e.g. phosphatidylinositol 4,5-bisphosphate, diacylglycerol, lysophosphatidic acid and several sphingolipids). The sphingolipid family is comprised of multiple members: sphingomyelins and glycosphingolipids, which are major components of biological membranes. Other sphingolipids, such as ceramide, ceramide 1-phosphate, sphingosine and sphingosine 1-phosphate (S1P) are often generated from the large pool of membrane sphingolipids in response to cellular signaling, and they contribute to the determination of cell fate.
Sphingolipid biosynthesis [1,2] normally occurs by condensation of coenzyme A-activated palmitoyl group transfer and l-serine to form 3-ketosphinganine. More recently, other amino acid-based sphingolipids have been described such as l-alanine or l-glycine, to form 1-deoxy- or 1-deoxy-methyl-derivatives, respectively [3,4]. 3-Ketosphinganine is reduced to sphinga-nine, and N-acylated with different fatty acids to generate dihydroceramides. Desaturation of the alkyl chain results in de novo synthesis of ceramide, which is considered to be a central component of sphingolipid metabolism. Complex sphingolipids, such as sphingomyelin and glycosphingolipids are generated from ceramide [5], which can be cleaved to form sphingo-sine, or phosphorylated, yielding ceramide 1-phosphate. Sphingosine can also be phosphorylated to form S1P, one of the most studied sphingolipids due to its bioactive roles in cellular biology and physiology (cellular proliferation, inflammation, migration and angiogenesis).
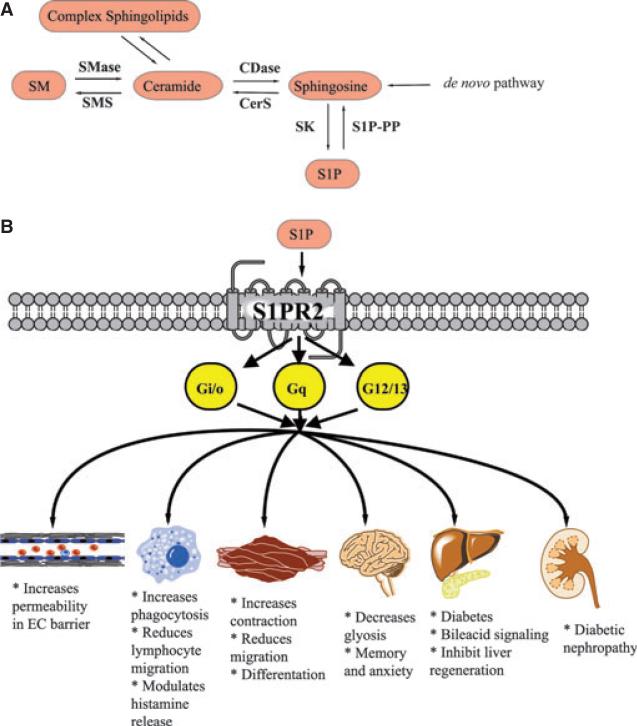
Intracellular and extracellular S1P are under tight control by several enzymes. Specifically, hydrolysis of complex sphingolipids is controlled by sphingomyelinases and glycosidases. Subsequently, ceramidases can hydrolyze ceramide to produce sphingosine, a direct precursor of S1P by the action of sphingosine kinases [6]. S1P is also regulated by enzymes responsible for its degradation (S1P phosphatases and S1P lyase). The biological roles of S1P are mediated either directly by intracellular targets [7], or by the action of five different transmembrane G protein coupled receptors (S1PR1–5) [8], which belong to the endothelial differentiation gene (EDG) family of receptors. S1P receptors participate in cellular responses based on the cell type and downstream available effectors. Figure 1 offers a depiction of the sphingolipid metabolic pathway.
Fig. 1.
(A) Schematic representation of the sphingolipids metabolic pathway. (B) The different biological functions downstream of S1PR2.
In this review, the functional roles of S1P receptors are described, prefaced with a brief history of their discovery. S1PR1 and S1PR3 have been extensively stud ied and is only discussed briefly here. S1PR4 and S1PR5, which are less well characterized, are discussed more comprehensively. The main focus of this review is on the S1PR2 receptor: specifically its normal physiological functions, and its role in pathophysiology and disease. Issues and apparent controversies surrounding the S1PR2 receptor are also discussed.
S1P transporters
Before delving into S1PR activation, an understanding is needed of how S1P relocates to the cell exterior to activate its receptors in an autocrine or paracrine manner. Unlike sphingosine, S1P cannot freely traverse the lipid bilayer to leave the cell [1]. Its polar nature prevents this; thus, it requires a specific transport mechanism. To date, two mechanisms have been proposed for S1P transport out of the cell. First, several members of the ATP-binding cassette family of transporters have been thought to participate in this translocation [9,10]. Cystic fibrosis transmembrane receptor has been implicated in S1P transport as well as lysophosphatidic acid and dihydro-S1P in C127/cystic fibrosis trans-membrane receptor cells [10]. ABCC1, however, has been described in mast cells, and its inhibition affected the migratory capabilities of mast cells during inflammation [9]. The second mechanism proposed is through the newly identified spinster-2 transporter in vascular endothelial cells. Mice lacking this protein have 60% less circulating S1P, and they have defective lymphocyte egress [11].
S1P receptors
Before 1995, S1P-mediated actions on cellular processes such as proliferation, cell movement and intra-cellular calcium levels were thought to be primarily related to its intracellular second messenger effects. Also during that year – and thereafter –evidence accumulated that this sphingolipid acts on G protein-coupled receptors. Goodemote et al. [12] noted that some S1P-specific effects in Swiss 3T3 fibroblasts, such as DNA synthesis and calcium release, were inhibited by pertussis toxin. One year later, Van Koppen and coworkers [13], reported that this toxin inhibited S1P-mediated increases in intracellular calcium and adenylyl cyclase inhibition. These effects were observed in a myriad of cell types [13] and it was suggested that S1P can act extracellularly at G protein-coupled receptors. S1PR1, previously known as the endothelial differentiation gene (EDG-1), was cloned in the 1990s as an early inducible gene in endothelial cells in response to phorbol myristate acetate. S1PR1 induction gives rise to endothelial differentiation of capillary-like tubular structures [14]. The ligand for S1PR1 remained unidentified for 8 years after the receptor had been cloned, until Lee et al. [15] identified S1P to be a potent (Kd = 8.1 nm) and specific ligand for this receptor. The second S1PR was cloned in rat aortic smooth muscle cells in 1993, referred to as S1PR2 (also known as EDG-5) [16]. S1PR3 (EDG-3) was cloned 3 years later by Yamaguchi and colleagues [17]. S1PR4 (EDG-6) was cloned in 1998 from human and murine dendritic cells [18]. Interestingly, S1P was reported to bind each of these receptors with high specificity, and with similar high affinities [Kd = 27 nm (S1PR2), 23 nm (S1PR3) and 63 nm (S1PR4)] [19].
The tissue and cellular distributions differ for each of these receptors, and these differences are summarized in Table 1. These factors, along with differences in the G proteins to which each of receptor is coupled, account for differences in biological functions associated with the receptors, a topic discussed below.
Table 1.
Distribution of the different S1P receptors.
| S1PR | Human chromosomal location | Distribution |
References | |
|---|---|---|---|---|
| Tissue | Cellular | |||
| S1PR1 (EDG1) | 1p21 | Most tissues, highest in CD19+ B cells and cerebellum | Plasma membrane, caveolae, cytoplasmic vesicles, nucleus, perinuclear region | [20,84] |
| S1PR2 (EDG5) | 19p13.2 | Most tissues | Plasma membrane, cytoplasm | [20] |
| S1PR3 (EDG3) | 9q22.1-q22.2 | Highest in heart, lung, spleen, kidney, intestine, diaphragm | Plasma membrane | [20,80] |
| S1PR4 (EDG6) | 19p13.3 | Highly expressed in lymphoid tissues and blood cells, especially CD19+ B cells and lung | N/A | [20,85] |
| S1PR5 (EDG8) | 19p13.2 | Mostly in brain, skin and natural killer cells | N/A | [85,86] |
Signaling pathways associated with S1PRs
S1PRs can be coupled to different G proteins depending on the cell type where the receptor is located and the specific receptor involved. Thus, activation of different pathways and subsequent engagement of different biological responses occurs in response to receptor activation. Whereas S1PR1 has been reported only to be linked to GαI, S1PR2 and S1PR3 have been reported to be coupled to different G proteins, depending on the cell type and the stimulus that activates the receptor. Thus, G protein associations for S1PR2 and S1PR3 include GαI, Ga12/13 and GαQ. By contrast, S1PR4 and S1PR5 can only activate GαI and Gα12/13 [20]. Each of these G proteins activates diverse pathways involved in cell proliferation, differentiation, and cell motility and morphology. GaI recruitment can, in turn, activate several pathways, among which is the small GTPase Rac, a well-known regulator of the cellular cytoskeleton and a promoter of cell migration. GaI can also enhance cell survival and inhibition of apoptosis by activating the AKT pathway. Cell survival is supported by way of coupling to the GTPase Ras, which subsequently activates the extracellular-signal-regulated kinase (ERK) pathway [7]. GαQ recruitment typically activates phospholipase C, which, in turn, promotes cell survival via several mechanisms through calcium release and activation of PKC and downstream targets, among which is the NF-κB pathway [20]. Gα12/13 recruitment, however, activates a critical GTPase involved in cellular dynamic modulation: Rho GTPase. The main functional consequences of Rho activation are inhibition of migration, compromised endothelial barrier integrity and smooth muscle contraction [21]. Examination of each G protein coupled to S1P receptors, and the different pathways that can be activated by each individual G protein, suggests diverse potential combinations and responses arising from receptor activation. This does not even account for the activation of several receptors within a single cell.
S1P receptors: roles in health and disease
The body of work relating to the roles of the different S1PRs in normal physiology and in disease continues to grow. Important insights were elucidated about the physiological roles of individual S1PRs from S1PR knockout mice that revealed functional redundancy of several receptors with the exception of S1PR1. Knockout of S1PR1 in mice was embryonically lethal, giving rise to major vascular abnormalities. Table 2 summarizes known phenotypic abnormalities, if any, for each of S1PR knockout mouse strains. When these knockout mice, or cells arising from these mice, were subjected to stress, the necessity of these receptors was clear. Various pathophysiological conditions in which these receptors are important are briefly presented below.
Table 2.
Lethality and phenotype of the S1P receptors knockout mice.
| S1PR KO embryo | Lethality | Phenotype | References |
|---|---|---|---|
| S1PR1–/– | Normal up to E11.5 | Abnormal yolk sacs, edematous, lack blood | [87] |
| All die before E14.5 | Normal vasculature | ||
| Intraembryonic bleeding | |||
| Underdeveloped, rounded limbs | |||
| Defective blood vessel maturation due to defective vascular smooth muscle migration and enforcement of vessels walls | |||
| S1PR2–/– | Normal at birth | Sporadic and spontaneous seizures between weeks 3 and 7 | [88-90] |
| 14% die between week 3 and 7 due to lethal seizures | Increased excitability of the pyramidal cortical neurons | ||
| Decreased renal and mesenteric vascular resistance | |||
| Deaf by one month of age due to vasculature disturbances within the stria vascularis | |||
| S1PR3–/– | Normal | Normal | [91] |
| Smaller litter size | |||
| S1PR4–/– | Normal | 9% of megakaryocytes have aberrant nongrained cytoplasm | [92] |
| Aberrant terminal megakaryocyte maturation but not affecting bleeding time | |||
| S1PR5–/– | Normal | Aberrant natural killer cell homing and migration to site of inflammation | [86,93] |
| Lack of Ly6C-patrolling monocytes in the periphery |
S1PR1
Other than being essential for life (noted in S1PR1 knockout mouse embryos), S1PR1 gained scientific interest due to its involvement in multiple physiological functions. This receptor is essential for T- and B-lymphocyte egress from lymphoid tissue [22], and is differentially regulated during immune response development. S1PR1 upregulation allows T cells to migrate from the thymus to the lymph nodes, where the receptor is downregulated to mediate T-cell retention in secondary lymphoid organs [22]. The inhibition of this receptor using the immunosuppressant drug FTY720 has proven to be beneficial for patients with relapse–remitting multiple sclerosis [23]. This compound is a sphingosine analog that binds all S1PRs with the exception of S1PR2 after being phosphorylated by sphingosine kinase 2. FTY720-mediated activation of S1PR1 leads to its ubiquitin-dependent degradation, which causes lymphocyte sequestration and thus immunosuppression [24,25]. It is currently used in the clinic, and is known as fingolimod [23].
In addition, S1PR1 (along with S1PR3) is not only responsible for proper angiogenesis, but is also needed for lymphangiogenesis through the phospholipase C/calcium pathway [26]. Moreover, S1PR1 is critical for mediating S1P signaling in proinflammatory pathways such as cyclo-oxygenase-2 activation in rheumatoid arthritis synoviocytes [27], and for intracellular adhesion molecule (ICAM)-1 expression in human endothelial cells [28]. Finally, S1PR1 is reported to be a pro-tumorigenic receptor, promoting cancer cell migration and invasion in addition to tumor neovascularization. For example, in Wilms’ tumor, S1P-mediated exacerbations in malignancy occur through S1PR1 activation of phosphatidylinositol 3-kinase and the promigratory Rac pathway [29]. Also, S1PR1 knockdown in vivo dramatically inhibited tumor growth of implanted Lewis lung carcinoma cells by inhibiting new blood vessel formation within the growing tumor mass [30].
S1PR3
Studies that address the functional capabilities of S1PR3 alone have been historically scarce; only now is research being reported about this receptor. Several published observations suggest that S1PR3-mediated functions occur in coordination with S1PR1 or S1PR2. By itself, S1PR3 was revealed to have several important functions. Its expression in dendritic cells is essential for switching immune reactions to T-helper cell (TH1) responses. This immune conversion was evident when dendritic cells deficient in this receptor were implanted into wild-type mice. The receptor activated the Th2/interleukin-4 response and provided renal protection against ischemic reperfusion injury in the mice [31]. Moreover, S1PR3 is upregulated in astrocytes in multiple sclerosis [32,33], which is necessary for maintaining astrocyte activation, although the biological significance of this has yet to be determined: it might be detrimental or beneficial for disease progression [33]. Because of the tight association of the S1PR3 receptor with some aspects of inflammation, S1PR3 has been proposed to be a potential biomarker to measure acute lung injury. S1PR3 is shed into the blood during sepsis, lowering blood vessel resistance and increasing vessel permeability [34]. In cardiovascular disease, S1PR3 seems to be essential for macrophage recruitment to atherosclerotic lesion sites, as well as promoting secretion of tumor necrosis factor and monocyte chemoattractant protein-1. By contrast, S1PR3 exerts an antiproliferative and antimigratory effect on smooth muscle cells after an arterial insult [35]. In addition, S1P-mediated decreases in coronary blood flow are strictly caused by S1PR3 agonism. In fact, S1PR3 engagement leads to coronary smooth muscle contraction via increases in intracellular calcium and Rho activation [36]. The role of S1PR3 in tumorogenesis has also been addressed: S1PR3 signaling was found to be closely associated with epithelial growth factor (EGF) signaling. In lung cancer, S1PR3 increased expression of the EGF receptor in a Rho-dependent manner leading to increased proliferation and anchorage-independent growth [37]. In breast cancer, however, S1PR3 transactivates the EGF receptor in response to estrogen stimulation, and thus amplifies its signaling. In both cases, S1PR3 could be a potential target for cancer therapy [38].
S1PR4
S1PR4 is mainly expressed in the hematopoietic system, thus most functions attributed to it are described for that particular system. Physiological roles for the receptor are unclear at this time. However, the few studies in the literature offer the following observations about the receptor. First overexpression of this receptor in Chinese hamster ovary cells induced cell rounding and stress-fiber formation via coupling with Gi and Gα12/13. Overexpression of S1PR4 in Jurkat T cells, by contrast, induced cell motility even in the absence of exogenous S1P [39]. Later studies disproved the role of S1PR4 in affecting T-cell motility in mice. T cells expressing S1PR4, but not S1PR1, failed to migrate not only towards S1P, but also toward several chemotactic stimuli [40]. S1PR4, however, was essential in promoting inhibitory effects of S1P in T cells. S1PR4 inhibited T-cell proliferation, switching the cells to an inhibitory phenotype that secretes interleukin-10, but not interleukin-2, interleukin-4 or interferon-gamma [40]. A recent study using S1PR4 knockout mice by Schulze et al. suggested that S1PR4 had only a modest effect on T-cell function [41]. Rather, the main defect in the knockout mouse was found within dendritic cell differentiation and cytokine secretion. Specifically, dendritic cells could no longer switch T cells to the Th17 phenotype, thereby switching the immune response to a Th2 response [41]. Finally, S1PR4 inhibition may have a therapeutic value: S1PR4 knockout mice were protected from dextran sulfate-induced colitis [41].
S1PR5
S1PR5 has been studied in a limited fashion, chiefly investigated in the brain and within natural killer cells, where it is highly expressed. Some studies also implicate S1PR5 in tumorigenesis. In the brain, S1PR5 expression is limited to oligodendrocytes and their precursors. The functional consequences of S1PR5 activation in these cells depend on their developmental stage. The S1PR5 receptor mediates process retraction [42] and impedes migration of immature oligodendrocytes [43] through a Rho kinase-dependent pathway, whereas S1PR5 mediates the survival of mature oligodendrocytes through an AKT-dependent pathway [42]. S1PR5 is gaining attention due to the high expression of S1PR5 in natural killer cells and its essential role in natural killer egress into the lymphatic system [44] (see Table 2). The Duane mouse, an animal strain with a mutation in the Tbx21 gene encoding the T-bet transcription factor, is similar in phenotype to the S1PR5 knockout mice. The Duane mouse was reported to have dramatically fewer S1PR5 transcripts and proteins, and T-bet was shown to induce S1PR5 expression by binding to its promoter region [45]. Furthermore, S1PR5 is reported to be key for maintaining blood–brain barrier integrity as well as its immunological quiescence [46]. In fact, S1PR5 increases blood–brain barrier tight junctions, decreasing its permeability. S1PR5 also lowers monocyte transendothelial migration into the brain parenchyma, which decreases inflammation [46]. Also, S1PR5 can decrease inflammation by inhibiting NF-jB activation, and subsequent cytokine secretion [46]. Finally, studies to investigate the effect of S1PR5 signaling in tumor development are scarce and conflicting. Autophagy, a mechanism by which some cancers thrive during conditions of scarce nutrient supply, is induced by S1P in prostate cancer cells in an S1PR5-dependent manner [47]. By contrast, S1PR5 inhibits migration and proliferation of esophageal cancer cells, and, in fact, these cancerous cells may downregulate the S1PR5 receptor to escape cellular control (suggested by Hu et al. [48]). Further studies are needed to elucidate the function of this receptor in malignancy because it may represent a potential target for chemotherapy.
Roles of S1PR2
S1PR2 in mediating different cellular functions and pathologies
Endothelial cell functions. S1PR2 has been implicated in increasing the capillary paracellular permeability. In fact, S1PR2 stimulation causes stress-fiber formation and disrupts adherens junctions between endothelial cells [49]. This effect has been attributed to the ability of S1PR2 to couple to the Rho–Rho-associated protein kinase (ROCK) pathway which will, in turn, activate phosphatase phosphatase and tensin homolog, thereby inhibiting the phosphatidylinositol 3-kinase pathway [49]. tumor necrosis factor- and lipopolysaccharide-induced swelling and edema have been reported to arise from increased dermal microvasculature permeability attributed to increased S1PR2 expression [50]. S1PR2 is also known to modify endothelial function with respect to wound healing: S1PR2 expression increases in senescent endothelial cells, as reported by Lu and colleagues [51]. This increased expression is responsible for malfunctions of endothelial cells associated with aging, such as impaired wound healing due to decreased tube formation and impaired endothelial cell migratory capabilities [51]. These impairments were reversed by the expression of a dominant-negative phosphatase and tensin homolog [52], which confirms this phosphatase as a downstream target of S1PR2 in endothelial cells. S1PR2 is also upregulated by hypoxic stress in the retina, ultimately leading to defective neovascularization [53]. In fact, a component of this retinopathy is due to inhibition of endothelial nitrous oxide synthase expression and induction of cyclo-oxygenase-2 by S1PR2 [53]. This validates S1PR2 as a regulator of the inflammatory response, a topic that is explored in greater detail later in this review.
Metabolic functions
Evidence is emerging that S1PR2 functions in the liver and pancreas which suggests important metabolic dimensions for this receptor. S1PR2 blockade has been involved in protection against streptozocin-induced diabetes in mice. In fact, S1PR2−/− mice were protected from pancreatic beta cells apoptosis, had higher insulin and lower glucose [54]. It has also been described as the receptor for conjugated bile acids in the liver [55]. Their ligation to this receptor was shown to regulate multiple hepatic metabolic pathways including glucose control, bile acid synthesis and lipid metabolism through the activation of AKT and ERK1/2 pathways. Interestingly, these actions are mediated through S1PR2 activation [55]. Actions of S1PR2 on hepatocytes are not only limited to regulating their metabolic functions, but also extend to the regulation of hepatocyte regeneration after injury [56]. In fact, the liver of S1PR2−/− mice was less fibrotic and had increased regeneration after hepatic injection with the model hepatotoxicant, carbon tetrachloride. Thus, targeting this receptor in patients suffering from the early stages of liver cirrhosis may be a future experimental goal.
Allergy and immunity
The role of S1PR2 in anaphylaxis is unclear. One laboratory has reported that it is essential for mast cell activation and degranulation in response to FceRI cross-linking, and that its activation leads to release of histamine, the chemokine CCL2 and tumor necrosis factor, which aggravates anaphylactic shock [57]. Another group reported that S1PR2 signaling contributed to histamine clearance, and actually shortened the duration of shock [58]. Such discrepancies have uncertain origins, but an agonist or an antagonist for treatment of type 1 allergic reactions may warrant further study. S1PR2 functions are not limited to mast cells, but they extend to macrophages. Whereas S1P has been shown to mediate lymphocyte egress and migration through S1PR1, S1P inhibits macrophage migration to the inflammation site via S1PR2 activation [59]. This response is partly achieved by cAMP production, which activates protein kinase A [59]. In contrast, S1P enhances phagocytosis of Cryptococcus neoformans by macrophages via S1PR2-mediated expression of FC gamma receptors 2 and 3 [60].
Muscle functions
S1PR2 studies in muscle cells are extensive. This receptor has been implicated in muscle cell chemotaxis [61], proliferation [28,62,63], differentiation [63,64] and contraction [65,66]. Early studies of S1PR2 in muscle cells were reported in 2002 [67]. These findings revealed that, along with S1PR3, S1PR2 is responsible for the calcium peak observed in myoblasts after S1P treatment [67], but no definitive mechanism was proposed to explain this. By contrast, investigators reported that S1P inhibited migration of vascular smooth muscle cells via action on S1PR2 [61]. The mechanism behind this was reported to be coupling of the receptor to Gq and G12/13 for the purpose of activating small GTPase Rho and inhibiting Rac, leading to cell migration arrest [61]. This activity is not limited to smooth muscle cells, but also occurs in other cell types, which are discussed later. Increased smooth muscle proliferation and migration are usually observed in neo-intimal lesions, giving rise to blood vessel blockade and thrombosis. Therefore, S1PR2 activity was thought to be beneficial for preventing the formation and growth of these lesions due to Rho-dependent reduced arterial smooth muscle cell proliferation and migration [28,62]. S1PR2 can also induce the transcription of smooth muscle differentiating genes, such as alpha-actin, by promoting binding of serum response factor to the promoter of these genes [62]. The study of mechanism of RhoA activation by S1PR2 revealed that a RhoA-specific guanine exchange factor, referred to as leukemia-associated Rho guanine exchange factor, activated RhoA, to promote expression of smooth muscle differentiating genes [64]. The role of S1PR2 in skeletal and muscle stem cells (satellite cells) was different from its role in vascular smooth muscles. In fact, S1P-mediated muscle regeneration and proliferation after injury was shown to depend on S1PR2 signaling [63]. Moreover, S1PR2 activated signal transducer and activator of transcription 3, which subsequently repressed cell cycle inhibitors p21 and p27 [63]. Finally, although S1PR2 stimulation caused smooth muscle contraction [65,66], the mechanism by which this happens depends on the tissue of origin. For example, in bronchial smooth muscle cells, S1P/S1PR2-mediated bronchoconstriction occurs through the Rho/ROCK pathway [65], whereas in mesenteric vascular smooth muscles, S1P/S1PR2-mediated vasoconstriction occurs via Rho-independent activation of the p38-MAPKinase pathway [66].
Neuronal functions
Blockade of S1PR2 by a specific inhibitor, JTE013, was revealed to significantly augment the migration of neural progenitor cells toward areas of brain infarction [68]. This was proven in in vitro models of cell migration as well as in in vivo mouse models, in which JTE013 was administered to brain ventricles [68]. Thus, JTE013 may be a therapeutic target for stroke patients. Subsequent studies using S1PR2−/− mice are intriguing; S1PR2 in the brain is chiefly expressed in the hippocampus, and its absence caused extensive gliosis in this area. Thus, the mice were not only susceptible to lethal seizures, but also they suffered from CNS functional impairments such as deficits in spatial working memory and increased anxiety [69].
Kidney functions
The role of S1PR2 in the kidney is uncertain, as well. In the context of diabetic nephropathy, S1PR2 is up-regulated in mesengial cells compared with normal rats, leading to stimulation of fibronectin synthesis and accumulation in the mesengium, which is a hallmark of this disease [70]. S1PR2 in mesengial cells is tightly coupled to the ERK–MAP kinase pathway which is essential for fibronectin upregulation [70]. By contrast, S1PR2 has been shown to exacerbate ischemia–reper-fusion injury in renal proximal tubules [71]. For the first time, studies revealed that S1PR2 inhibition induces sphingosine kinase 1 expression and S1P secretion [71]. Furthermore, the protective effects of this antagonism were thought to be due to increased S1P production and its interaction with S1PR1, rather than arising from S1PR2 alone [71]. These data were the first experiments to use the novel S1PR2 agonist, SID46371153 [71].
S1PR2 in cancer
S1PR2 as an anticancer receptor
Most scientific evidence supports an antimigratory effect of S1PR2, and it is also thought to be antitumorigenic, inhibiting cells from undergoing distant metastasis. Early reports regarding S1PR2 and tumor formation emerged in 2003, specifically in a mouse melanoma model [72,73]. Melanoma B16F10 cells chiefly express S1PR2, and their migration was enhanced by receptor blockade with JTE013. Interestingly, S1PR2 not only inhibited migration via activation of RhoA, but also by simultaneously inhibiting the promigratory G protein Rac in response to S1P [73]. To further explore the role of this G-protein-coupled receptor in metastasis, S1P pretreatment of these melanoma cells dramatically decreased lung metastasis in an S1PR2-dependent manner in tail-vein-injection mouse models [72]. The physiological role of such a system warrants investigation; S1P is abundant in plasma, and its presence does not inhibit melanoma metastasis, as pretreatment with S1P did. The antitumorigenic actions of S1PR2 were further explored in glioblastomas [21]. The antimigratory phenotype of S1PR2 was discerned in glioblastoma cell lines that express this receptor by way of activation of RhoA and its downstream effector, ROCK, without the need for inhibiting Rac signaling [21]. Interestingly, the antitumorigenic capabilities of S1PR2 are not limited to its antimigratory effect, but are also related to its ability to inhibit proliferation and growth as observed in Wilms’ tumor [74]. In fact, S1P treatment of WiT49 cells prompted expression of the antiproliferative connective tissue growth factor in an S1PR2-dependent manner. These data represent some of the few studies to prove that S1PR2 can induce gene expression though the ROCK/c-jun axis [74]. Later reports showed that S1PR2 knockout mice develop diffuse large B-cell lymphomas at advanced ages [75], thereby identifying a novel role for S1PR2 in B-cell germinal-centered hematopoiesis. Interestingly, a quarter of patients with such a malignancy were found to harbor a mutation in S1PR2 [75]. Taken together, this set of data suggests important anti-tumor functions of S1PR2.
S1PR2 as a pro-cancerous receptor
The first report implicating S1PR2 as a pro-cancerous receptor dates back to 2000 when studies were conducted in hepatoma and Jurkat cells [76]. Cotransfection of S1PR2 and S1PR3 in these cells led to S1P-mediated cellular proliferation and inhibition of apoptosis. These effects were attributed to activation of the ERK/MAPKinase pathway, along with the immediate initiation of c-jun and c-fos transcription in a Rho-dependent manner [76]. Pro-tumorigenic effects of this receptor are not only linked to its ability to affect cell movement, but also its effects on protein stability as observed in chronic myeloid leukemia [77]. In fact, S1PR2 inactivates the phosphatase PP2A, which normally dephosphorylates the constitutively active bcr-abl, and tags it for proteosomal degradation. Reports support the idea that S1PR2 may constitute a novel target in chronic myelogenous leukemia patients who were resistant to imatinib treatment [77]. Finally, S1PR2 was shown to enhance tumorigenesis by downregulating tumor suppressor proteins as reported in bladder cancer [78]. Although detailed mechanistic studies of such down-regulation were not addressed, inhibition of S1PR2 dramatically increased tumor suppressor breast carcinoma metastasis suppressor 1 (brms1), leading to a decrease in prostate cancer growth and lung metastasis [78].
Lastly, our group has very recently identified a novel signaling function that is specific for S1PR2. We showed that S1PR2 is essential for the activation of the ezrin–radixin–moesin family of proteins in response to S1P and EGF. Ezrin–radixin–moesin proteins are adaptor molecules linking the actin cytoskeleton with the plasma membrane, and have been implicated in filopodia and lamellipodia formation, thus cancer invasion. Inhibition of this receptor in HeLa cells caused a dramatic decrease in the EGF-induced invasion in an ezrin–radixin–moesin-dependent manner. The identification of S1PR2 as one of the effector arms in the EGF-induced malignancies could constitute an important target for therapy [79].
In conclusion, the effects of S1PR2 on tumor growth and progression are cell-type specific. Possibly, the specific G protein coupled to that receptor may dictate its biological functions. Therefore, inhibition or stimulation of S1PR2 can prove to be beneficial or harmful, depending on the tumor being studied. Contrasting data presented previously suggest that several questions remain unanswered. One such question is whether JTE013 in patients with leukemia will predis-pose them to develop secondary types of cancer such as Wilms tumor or lymphomas.
S1PR2 agonist and antagonist
JTE013 is the most used S1PR2 antagonist. It was synthesized at the Central Pharmaceutical Institute in Japan during 2001 [80]. It is a pyrazolopyridine derivative, that was shown to antagonize the binding of radiolabeled S1P in Chinese hamster ovary cells over-expressing S1PR2 [81]. The specificity of JTE013 to S1PR2 is debatable. This compound did not inhibit S1P-induced calcium mobilization in HCT4 cells over-expressing S1PR2, but also in those overexpressing S1PR4 [82]. Its action on S1PR4 have also been eluded in MDA-MB-453 cells. These cells express endogenous S1PR2 and S1PR4. JTE-013 inhibited S1P-induced ERK phosphorylation in these cells, whereas the use of S1PR2 siRNA did not [82]. Furthermore, JTE013 was still effective in abolishing S1P-induced vasoconstriction in S1PR2 knockout mice [81]. Therefore, the use of JTE013 as the only method to prove S1PR2-mediated events is not sufficient, and could explain the discrepancies in the literature regarding this receptor.
However, SID46371153 is the first novel reported S1PR2 agonist [83]. It was discovered after a high throughput screening of thousands of compounds in 2007. Its specificity for S1PR2 was demonstrated using Chinese hamster ovary cells overexpressing S1PR2 and the cAMP response element-beta lactamase (CREBLA) reporter construct [83]. It has an EC50 of 0.72 μm [83]. This compound has only been used once so far in-vivo studies assessing ischemic–reperfusion injury in kidneys as mentioned above [71].
Conclusions
S1PR2 has diverse functions and has been implicated in many organ-system pathologies. However, a simple single conclusion cannot be drawn about the receptor with respect to any organ or system. The literature contains conflicting data that diverge to the degree that we cannot generalize much about the receptor and its effects. Unique data reported in the literature can arise from the use of different cell lines, even if the cells are from the same organ system. Also, S1PR2 in each cell line may be coupled to a different G protein, and therefore a different signaling pathway. Also, the use of S1P receptor inhibitors for studies may have various specificities, with multiple non-specific functions. Thus, investigations should be consistent with respect to cells used for studies. For example, they should probably be derived from S1PR2−/− mice. In addition, S1P recep-have redundant functions as evidenced by studies in knockout mice. Thus data should be assessed carefully when results suggest that receptor inhibition studies reveal no discernible effects. Future studies are needed to clarify downstream effects of S1PR2.
Acknowledgements
This manuscript is based upon work supported in part by a MERIT Award, [BX000156-01A1] (LMO) by the Office of Research and Development, Department of Veterans Affairs, Northport VA Medical Center, Northport, NY. The content of this material does not represent the views of the Department of Veterans Affairs or the United States Government; and National Cancer Institute [PO1-CA97132 (LMO)] and National Institutes of Health National Institute of General Medical Sciences [R01-GM062887 (LMO)].
Abbreviations
- EDG
endothelial differentiation gene
- EGF
epithelial growth factor
- ERK
extracellular-signal regulated kinases
- S1PR
sphingosine-1-phosphate receptor
- S1P
sphingosine-1-phosphate
- TH
T-helper cell
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Garnier-Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J Biol Chem. 2009;284:4786–4795. doi: 10.1074/jbc.M808798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penno A, Reilly MM, Houlden H, Laura M, Rentsch K, Niederkofler V, Stoeckli ET, Nicholson G, Eichler F, Brown RH, Jr, et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem. 2010;285:11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingwood CA. Glycosphingolipid functions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004788. doi:10.1101/cshperspect.a004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gault CR, Obeid LM. Still benched on its way to the bedside: sphingosine kinase 1 as an emerging target in cancer chemotherapy. Crit Rev Biochem Mol Biol. 2011;46:342–351. doi: 10.3109/10409238.2011.597737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 8.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA, Obeid LM. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 11.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodemote KA, Mattie ME, Berger A, Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995;270:10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- 13.van Koppen C, Meyer zu Heringdorf M, Laser KT, Zhang C, Jakobs KH, Bunemann M, Pott L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- 14.Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- 15.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki H, Ishizaka N, Sakurai T, Kurokawa K, Goto K, Kumada M, Takuwa Y. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system. Biochem Biophys Res Commun. 1993;190:1104–1109. doi: 10.1006/bbrc.1993.1163. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi F, Tokuda M, Hatase O, Brenner S. Molecular cloning of the novel human G protein-coupled receptor (GPCR) gene mapped on chromosome 9. Biochem Biophys Res Commun. 1996;227:608–614. doi: 10.1006/bbrc.1996.1553. [DOI] [PubMed] [Google Scholar]
- 18.Graler MH, Bernhardt G, Lipp M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–169. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel S. Sphingosine 1-phosphate: a ligand for the EDG-1 family of G-protein-coupled receptors. Ann NY Acad Sci. 2000;905:54–60. doi: 10.1111/j.1749-6632.2000.tb06537.x. [DOI] [PubMed] [Google Scholar]
- 20.Aarthi JJ, Darendeliler MA, Pushparaj PN. Dissecting the role of the S1P/S1PR axis in health and disease. J Dental Res. 2011;90:841–854. doi: 10.1177/0022034510389178. [DOI] [PubMed] [Google Scholar]
- 21.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–3795. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 22.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 23.Yeh EA, Weinstock-Guttman B. Fingolimod: an oral disease-modifying therapy for relapsing multiple sclerosis. Adv Ther. 2011;28:270–278. doi: 10.1007/s12325-011-0004-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhi L, Kim P, Thompson BD, Pitsillides C, Bankovich AJ, Yun SH, Lin CP, Cyster JG, Wu MX. FTY720 blocks egress of T cells in part by abrogation of their adhesion on the lymph node sinus. J Immunol. 2011;187:2244–2251. doi: 10.4049/jimmunol.1100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoon CM, Hong BS, Moon HG, Lim S, Suh PG, Kim YK, Chae CB, Gho YS. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood. 2008;112:1129–1138. doi: 10.1182/blood-2007-11-125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthr Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy MA. Sphingosine 1-phosphate receptor 2 negatively regulates neointimal formation in mouse arteries. Circ Res. 2007;101:995–1000. doi: 10.1161/CIRCRESAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 29.Li MH, Sanchez T, Yamase H, Hla T, Oo ML, Pappalardo A, Lynch KR, Lin CY, Ferrer F. S1P/S1P1 signaling stimulates cell migration and invasion in Wilms tumor. Cancer Lett. 2009;276:171–179. doi: 10.1016/j.canlet.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajwa A, Huang L, Ye H, Dondeti K, Song S, Rosin DL, Lynch KR, Lobo PI, Li L, Okusa MD. Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1–Th2 polarity in kidney ischemia-reperfusion injury. J Immunol. 2012;189:2584–2596. doi: 10.4049/jimmunol.1200999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der Valk P, Reijerkerk A, et al. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58:1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- 33.Fischer I, Alliod C, Martinier N, Newcombe J, Brana C, Pouly S. Sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 are functionally upregulated on astrocytes under pro-inflammatory conditions. PLoS ONE. 2011;6:e23905. doi: 10.1371/journal.pone.0023905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, Sammani S, Chiang ET, Moreno-Vinasco L, Wade MS, Zhou T, et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Resp Cell Mol Biol. 2012;47:628–636. doi: 10.1165/rcmb.2012-0048OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 36.Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, Hasegawa T, Sasamori J, Konno T, Hayashi K, et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Mol Pharmacol. 2010;77:704–713. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- 37.Hsu A, Zhang W, Lee JF, An J, Ekambaram P, Liu J, Honn KV, Klinge CM, Lee MJ. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. Int J Oncol. 2012;40:1619–1626. doi: 10.3892/ijo.2012.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graler MH, Grosse R, Kusch A, Kremmer E, Gudermann T, Lipp M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J Cell Biochem. 2003;89:507–519. doi: 10.1002/jcb.10537. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005;19:1731–1733. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 41.Schulze T, Golfier S, Tabeling C, Rabel K, Graler MH, Witzenrath M, Lipp M. Sphingosine-1-phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25:4024–4036. doi: 10.1096/fj.10-179028. [DOI] [PubMed] [Google Scholar]
- 42.Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, Gudz TI. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 2007;21:1503–1514. doi: 10.1096/fj.06-7420com. [DOI] [PubMed] [Google Scholar]
- 44.Mayol K, Biajoux V, Marvel J, Balabanian K, Walzer T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood. 2011;118:4863–4871. doi: 10.1182/blood-2011-06-362574. [DOI] [PubMed] [Google Scholar]
- 45.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, Xu Y, Roots CM, Beilke JN, Banerjee A, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Doorn R, Lopes Pinheiro MA, Kooij G, Lakeman K, van het Hof B, van der Pol SM, Geerts D, van Horssen J, van der Valk P, van der Kam E, et al. Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. J Neuroinflammation. 2012;9:133. doi: 10.1186/1742-2094-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang CL, Ho MC, Lee PH, Hsu CY, Huang WP, Lee H. S1P(5) is required for sphingosine 1-phosphate-induced autophagy in human prostate cancer PC-3 cells. Am J Physiol. 2009;297:C451–C458. doi: 10.1152/ajpcell.00586.2008. [DOI] [PubMed] [Google Scholar]
- 48.Hu WM, Li L, Jing BQ, Zhao YS, Wang CL, Feng L, Xie YE. Effect of S1P5 on proliferation and migration of human esophageal cancer cells. World J Gastroenterol. 2010;16:1859–1866. doi: 10.3748/wjg.v16.i15.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscl Throm Vasc. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 50.Du J, Zeng C, Li Q, Chen B, Liu H, Huang X, Huang Q. LPS and TNF-alpha induce expression of sphingosine-1-phosphate receptor-2 in human microvascular endothelial cells. Pathology Res Pract. 2012;208:82–88. doi: 10.1016/j.prp.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Lu H, Yuan H, Chen S, Huang L, Xiang H, Yang G, Deng H, Zhou J. Senescent endothelial dysfunction is attributed to the up-regulation of sphingosine-1-phosphate receptor-2 in aged rats. Mol Cell Biochem. 2012;363:217–224. doi: 10.1007/s11010-011-1173-y. [DOI] [PubMed] [Google Scholar]
- 52.Estrada R, Zeng Q, Lu H, Sarojini H, Lee JF, Mathis SP, Sanchez T, Wang E, Kontos CD, Lin CY, et al. Up-regulating sphingosine 1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem. 2008;283:30363–30375. doi: 10.1074/jbc.M804392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imasawa T, Koike K, Ishii I, Chun J, Yatomi Y. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates streptozotocin-induced apoptosis of pancreatic beta-cells. Biochem Biophys Res Commun. 2010;392:207–211. doi: 10.1016/j.bbrc.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, Dent P, Spiegel S, Shi R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikeda H, Watanabe N, Ishii I, Shimosawa T, Kume Y, Tomiya T, Inoue Y, Nishikawa T, Ohtomo N, Tanoue Y, et al. Sphingosine 1-phosphate regulates regeneration and fibrosis after liver injury via sphingosine 1-phosphate receptor 2. J Lipid Res. 2009;50:556–564. doi: 10.1194/jlr.M800496-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, Ryan JJ, Milstien S, Spiegel S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–474. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, Watford W, Meylan F, Diesner SC, Li L, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1440. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McQuiston T, Luberto C, Del Poeta M. Role of sphingosine-1-phosphate (S1P) and S1P receptor 2 in the phagocytosis of Cryptococcus neoformans by alveolar macrophages. Microbiology. 2011;157:1416–1427. doi: 10.1099/mic.0.045989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takashima S, Sugimoto N, Takuwa N, Okamoto Y, Yoshioka K, Takamura M, Takata S, Kaneko S, Takuwa Y. G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on Rho but not Rho kinase. Cardiovasc Res. 2008;79:689–697. doi: 10.1093/cvr/cvn118. [DOI] [PubMed] [Google Scholar]
- 62.Grabski AD, Shimizu T, Deou J, Mahoney WM, Jr, Reidy MA, Daum G. Sphingosine-1-phosphate receptor-2 regulates expression of smooth muscle alpha-actin after arterial injury. Arterioscl Throm Vasc. 2009;29:1644–1650. doi: 10.1161/ATVBAHA.109.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loh KC, Leong WI, Carlson ME, Oskouian B, Kumar A, Fyrst H, Zhang M, Proia RL, Hoffman EP, Saba JD. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE. 2012;7:e37218. doi: 10.1371/journal.pone.0037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medlin MD, Staus DP, Dubash AD, Taylor JM, Mack CP. Sphingosine 1-phosphate receptor 2 signals through leukemia-associated RhoGEF (LARG), to promote smooth muscle cell differentiation. Arterioscl Throm Vasc. 2010;30:1779–1786. doi: 10.1161/ATVBAHA.110.209395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiba Y, Suzuki K, Uechi M, Kurihara E, Goto K, Sakai H, Misawa M. Downregulation of sphingosine-1-phosphate receptors in bronchial smooth muscle of mouse experimental asthma. Pharmacol Res. 2010;62:357–363. doi: 10.1016/j.phrs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Hoefer J, Azam MA, Kroetsch JT, Leong-Poi H, Momen MA, Voigtlaender-Bolz J, Scherer EQ, Meissner A, Bolz SS, Husain M. Sphingosine-1-phosphate-dependent activation of p38 MAPK maintains elevated peripheral resistance in heart failure through increased myogenic vasoconstriction. Circ Res. 2010;107:923–933. doi: 10.1161/CIRCRESAHA.110.226464. [DOI] [PubMed] [Google Scholar]
- 67.Meacci E, Cencetti F, Formigli L, Squecco R, Donati C, Tiribilli B, Quercioli F, Zecchi Orlandini S, Francini F, Bruni P. Sphingosine 1-phosphate evokes calcium signals in C2C12 myoblasts via Edg3 and Edg5 receptors. Biochem J. 2002;362:349–357. doi: 10.1042/0264-6021:3620349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411–3417. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- 69.Akahoshi N, Ishizaki Y, Yasuda H, Murashima YL, Shinba T, Goto K, Himi T, Chun J, Ishii I. Frequent spontaneous seizures followed by spatial working memory/anxiety deficits in mice lacking sphingosine 1-phosphate receptor 2. Epilepsy Behav. 2011;22:659–665. doi: 10.1016/j.yebeh.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Liu W, Lan T, Xie X, Huang K, Peng J, Huang J, Shen X, Liu P, Huang H. S1P2 receptor mediates sphingosine-1-phosphate-induced fibronectin expression via MAPK signaling pathway in mesangial cells under high glucose condition. Exp Cell Res. 2012;318:936–943. doi: 10.1016/j.yexcr.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Park SW, Kim M, Brown KM, D'Agati VD, Lee HT. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:266–280. doi: 10.1681/ASN.2011050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi H, Kitayama J, Takuwa N, Arikawa K, Inoki I, Takehara K, Nagawa H, Takuwa Y. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J. 2003;374:715–722. doi: 10.1042/BJ20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, Takehara K, Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J Biol Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- 74.Li MH, Sanchez T, Pappalardo A, Lynch KR, Hla T, Ferrer F. Induction of antiproliferative connective tissue growth factor expression in Wilms’ tumor cells by sphingosine-1-phosphate receptor 2. Mol Cancer Res. 2008;6:1649–1656. doi: 10.1158/1541-7786.MCR-07-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cattoretti G, Mandelbaum J, Lee N, Chaves AH, Mahler AM, Chadburn A, Dalla-Favera R, Pasqualucci L, MacLennan AJ. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res. 2009;69:8686–8692. doi: 10.1158/0008-5472.CAN-09-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem. 2000;275:288–296. doi: 10.1074/jbc.275.1.288. [DOI] [PubMed] [Google Scholar]
- 77.Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, Saddoughi SA, Apohan E, Sentelle RD, Smith C, Gault CR, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–5952. doi: 10.1182/blood-2010-08-300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ponnusamy S, Selvam SP, Mehrotra S, Kawamori T, Snider AJ, Obeid LM, Shao Y, Sabbadini R, Ogretmen B. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med. 2012;4:761–775. doi: 10.1002/emmm.201200244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Orr Gandy KA, Adada M, Canals D, Carroll B, Roddy P, Hannun YA, Obeid LM. Epidermal growth factor-induced cellular invasion requires sphingosine-1-phosphate/sphingosine-1-phosphate 2 receptor-mediated ezrin activation. FASEB J. 2013 doi: 10.1096/fj.13-228460. doi:10.1096/fj.13-228460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takabe K, Paugh SW, Milstien S, Spiegel S. ‘Inside-out’ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salomone S, Waeber C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol. 2011;2:9. doi: 10.3389/fphar.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long JS, Fujiwara Y, Edwards J, Tannahill CL, Tigyi G, Pyne S, Pyne NJ. Sphingosine 1-phosphate receptor 4 uses HER2 (ERBB2) to regulate extracellular signal regulated kinase-1/2 in MDA-MB-453 breast cancer cells. J Biol Chem. 2010;285:35957–35966. doi: 10.1074/jbc.M110.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen H. High throughput screening for S1P receptor agonists and antagonists, S1P2 agonist. The Scripps Molecular Screening Center Probe Report. The Scripps Research Institute Molecular Screening Center; La Jolla, CA.: 2011. [Google Scholar]
- 84.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 86.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, Anderson KJ, Roper SN, Lee N. An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci. 2001;14:203–209. doi: 10.1046/j.0953-816x.2001.01634.x. [DOI] [PubMed] [Google Scholar]
- 89.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol. 2007;292:R440–R446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 90.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 91.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, Kingsbury MA, Zhang G, Brown JH, Chun J. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/ EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 92.Golfier S, Kondo S, Schulze T, Takeuchi T, Vassileva G, Achtman AH, Graler MH, Abbondanzo SJ, Wiekowski M, Kremmer E, et al. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1-phosphate receptor S1P4. FASEB J. 2010;24:4701–4710. doi: 10.1096/fj.09-141473. [DOI] [PubMed] [Google Scholar]
- 93.Debien E, Mayol K, Biajoux V, Daussy C, De Aguero MG, Taillardet M, Dagany N, Brinza L, Henry T, Dubois B, et al. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur J Immunol. 2013 doi: 10.1002/eji.201343312. doi:10.1002/eji.201343312. [DOI] [PubMed] [Google Scholar]