Abstract
BACKGROUND
Lenalidomide, an immunomodulatory agent, has activity in lymphoproliferative disorders. The authors, therefore, evaluated its effects on T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia (CLL).
METHODS
To study the immunomodulatory effects of lenalidomide in CLL, the authors recruited 24 patients with untreated CLL enrolled in a phase 2 clinical trial of lenalidomide and obtained peripheral blood specimens for immunologic studies consisting of enumeration of T cells and assessing their ability to synthesize cytokines after activation through T-cell receptor (TCR).
RESULTS
After 3 cycles of therapy, patients had a significant reduction in percentage (%) and absolute lymphocyte count (ALC) and an increase in percentage of T cells, percentage of activated CD8+ T cells producing IFN-γ, and percentage of regulatory T (TR) cells when compared with their respective levels before treatment. After 15 cycles of treatment, responder patients had significant reduction in percentage of lymphocytes and ALC, percentage of activated CD4+ T cells producing IL-2, IFN-γ, or TNF-α, and percentage of TR cells when compared with their perspective levels after 3 cycles of treatment. Furthermore, the numbers of activated CD4+ T cells producing IL-2, IFN-γ, or TNF-α, activated CD8+ T cells producing IFN-γ, and TR cells normalized to the range of healthy subjects.
CONCLUSIONS
Treatment with lenalidomide resulted in the normalization of functional T-cell subsets in responders, suggesting that lenalidomide may modulate cell-mediated immunity in patients with CLL.
Keywords: chronic lymphocytic leukemia, immunomodulatory agents, T cells, cytokines
Lenalidomide
((RS)-3-(4-amino-1-oxo-3H-isoindol-2-yl)piperidine-2,6-dione) has shown clinical activity in several hematological disorders, including multiple myeloma (MM), myelodysplastic syndrome (MDS), and chronic lymphocytic leukemia (CLL).1,2 The relevant mechanisms of action of lenalidomide vary by disease setting. In multiple myeloma, lenalidomide increases the apoptotic rate of neoplastic plasma cells, inhibits cell adhesion, and induces changes in the bone marrow microenvironment by inhibiting adhesion of myeloma cells to the bone marrow stromal cell3; whereas in del(5q) MDS, lenalidomide directly affects erythroid progenitors by upregulating activin A and the tumor suppressor gene SPARC (secreted protein acidic and rich in cysteine).4 In CLL, lenalidomide’s mechanisms of action are not fully understood, and several possibilities are currently being considered, including suppression of cytokines such as tumor necrosis factor-alpha (TNF-α), inhibition of angiogenesis, and activation of natural killer cells.5
With new agents available to patients with CLL, the management of CLL becomes increasingly personalized.6 Lenalidomide offers a beneficial effect in patients with refractory/relapsed CLL, yet fatigue, thrombocytopenia, and neutropenia were frequently observed, and tumor flare reactions, characterized by painful lymphadenopathy with or without fever and bone pain, were reported in a subset of patients.7,8 Nevertheless, a recent trial with the combination of lenalidomide and rituximab demonstrated superior treatment effects to lenalidomide alone and no increase in toxicity.9 Unfortunately, lenalidomide in combination with fludarabine and rituximab in previously untreated CLL patients was very poorly tolerated when administered concurrently.10
The stimulatory effects of lenalidomide on both humoral11 and cellular immunity12,13 have been attributed to its ability to restore T-cell immune synapse formation12,13 and the CD154 expression on B-CLL cells with subsequent activation phenotype.11 Furthermore, it has been suggested that lenalidomide-associated immune activation is responsible for tumor flare reactions, a unique phenomenon seen only in CLL patients. To determine the effect of lenalidomide treatment on cell-mediated immunity of treatment-naive CLL patients, we measured changes in the immunophenotypes of T cells, including regulatory T (TR) cells, and the ability of CD4+ and CD8+ T cells to synthesize cytokines after activation through the T-cell receptor (TCR) with immobilized anti-CD3 antibodies. Cytoplasmic cytokine staining of cells identified by their surface antigens provided unique opportunities to detect cytokine expression within individual cells and to gather important information regarding the functionality of T cells.
MATERIALS AND METHODS
Patients and Treatment
Sixty treatment-naive CLL patients were enrolled in a phase 2 clinical trial of lenalidomide; 24 patients agreed to provide 15 mL of peripheral blood for optional immunologic studies before treatment after receiving 3 cycles and 15 cycles of treatment. To be eligible for this study, patients had to be aged 65 years or older and have standard indications for treatment according to National Cancer Institute (NCI) and International Workshop on CLL (IWCLL) guidelines.14 Treatment consisted of lenalidomide 5 mg daily continuous for each 28-day cycle. Completion of 2 28-day cycles (56 days) was required before escalating the daily lenalidomide dose to 10 mg, and then further escalation up to 25 mg daily by increments of 5 mg per cycle was allowed. The treatment was continued until disease progression. This study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board and registered at clinical trial.gov as NCT00535873– Lenalidomide as Initial Treatment of Patients with Chronic Lymphocytic Leukemia (CLL) Aged 65 and Older. All patients provided written informed consent in accordance with the Declaration of Helsinki before participation in this study. Patients were evaluated for clinical response according to the National Cancer Institute Working Group (NCI-WG) criteria15 after 3 cycles of treatment and every 6 cycles thereafter when they remained on treatment. In addition, we recruited 22 agematched healthy subjects to participate in the study as a control group for data analyses.
Enumeration of T-Cell Subsets and TR Cells
We enumerated lymphocyte subsets as previously described.16 Briefly, 100 µL aliquots of peripheral blood were incubated with mouse monoclonal antibody reagents to detect T-cell subsets (CD3, CD4, and CD8). Surface expressions of CD3, CD4, and CD25 were determined by using mouse antibodies conjugated with peridinin chlorophyll protein, fluorescein isothiocyanate, and phycoerythrin, respectively.
Expression of the Forkhead box protein P3 (FoxP3) is important in the differentiation of natural TR cells.17 TR cells were enumerated in peripheral blood mononuclear cells (PBMC) by using the FoxP3 staining kit (BD Pharmingen, San Diego, California) according to the manufacturer’s recommended protocol. All reagents were purchased from BD Biosciences (San Jose, California). Mouse immunoglobulin isotype controls were used to control autofluorescence.
Measurement of Cytokines Synthesis by Activated T Cells
PBMC were isolated from whole blood by Ficoll-Hypaque density-gradient centrifugation and stored at −80°C in fetal bovine serum (FBS) with 10% dimethyl sulfoxide. Upon thawing, PBMC were suspended in complete medium consisting of RPMI-1640 (Whittaker Bioproduct, Walkersville, Maryland) supplemented with 10% heatinactivated FBS, 100 U/mL of penicillin (Whittaker Bioproduct), 100 µg/mL of streptomycin (Whittaker Bioproduct), and 2 mM glutamine (GIBCO, Grand Island, New York). PBMC (6 × 106) were activated through the T-cell receptor (TCR) by anti-CD3 antibodies that were immobilized to the surface of a 24-well plate (Costar, Cambridge, Massachusetts). Briefly, the bottom of each well of a 24-well plate was coated with 0.75 µg of anti-CD3 (Coulter Immunology, Hialeah, Florida), and the plate was incubated at 37°C for 6 hours. Thereafter, the plates were air-dried with the plate lid off in a laminar flow hood. One mL of complete medium, 1.5 µg of anti- CD28 (BD Biosciences, San Jose, California), and 106 PBMC were added to each well coated with anti-CD3, and the plate was incubated at 37°C for 16 to 18 hours. Ten µg of brefeldin A were added to each milliliter of PBMC culture for the last 3 hours of the incubation period. Next, a total of 6×106 activated PBMC were harvested and treated with monoclonal antibody reagents for the detection of the intracellular cytokines produced by activated T cells as previously described.16,18
Statistical analysis
The nonparametric Wilcoxon signed-rank test was used to compare the differences in levels of white blood cell count (WBC), percentage and absolute lymphocyte counts, T-cell subsets (including TR cells), and activated T cells synthesizing cytokines at pretreatment versus their respective levels after 3 cycles of treatment as well as for comparing their levels after 3 cycles versus after 15 cycles of treatment. The Mann-Whitney test was used to compare the differences in median of WBC, percentage and absolute count of lymphocytes, T-cell subsets (including TR cells), and TCR-activated T cells producing cytokines IL-2, IL-10, IFN-γ, or TNF-α between healthy subjects and patients at pretreatment, after 3 cycles or after 15 cycles of treatment.
RESULTS
Patient Population
The clinical characteristics of the 24 patients studied are listed in Table 1. Six patients did not respond to treatment and were taken off study after 3 cycles. Eighteen patients had partial responses (PR) after 3 or 9 cycles of treatment including 3 nodal partial responses (nPR) after 9 cycles of therapy. Four patients had complete responses (CR), and 2 patients had incomplete CR (CRi) after 15 or 21 cycles of treatment.
Table 1.
Patient Characteristics
| Case No. |
Age, y |
Sex | ALC at Start |
Rai Stage |
Lenalidomide- Tolerated Dose |
B2M, mg/L |
NCI Response Criteria |
FISH Hierarchy |
% CD19+ CD38+ in Bone Marrow |
IGVH Status |
ZAP70 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | 115,200 | 4 | 10.0 | 4.8 | NR | ATM | 96.8 | Unmutated | Pos |
| 2 | 79 | M | 40,480 | 1 | 10.0 | 5.4 | nPR | ATM | 81.2 | Unmutated | ND |
| 3 | 82 | W | 14,469 | 4 | 5.0 | 2.1 | NR | D13 | 3.7 | Mutated | Neg |
| 4 | 67 | W | 95,060 | 3 | 10.0 | 2.9 | CRi | D13 | 3.2 | Unmutated | Pos |
| 5 | 82 | M | 87,040 | 2 | 2.5 | 4.3 | PR | T12 | 4.1 | Unmutated | ND |
| 6 | 72 | W | 102,238 | 2 | 5.0 | 3.8 | PR | D13 | 0.5 | Mutated | Neg |
| 7 | 69 | M | 152,902 | 2 | 5.0 | 4.7 | PR | ATM | 61 | Unmutated | Pos |
| 8 | 81 | W | 76,648 | 0 | 10.0 | 2.9 | NR | p53 | 10.8 | Mutated | Neg |
| 9 | 70 | M | 40,032 | 1 | 5.0 | 2.5 | CR | ATM | 33.9 | Unmutated | ND |
| 10 | 76 | W | 50,850 | 4 | 7.5 | 4.2 | PR | NEG | 11.4 | Mutated | Pos |
| 11 | 77 | W | 31,096 | 2 | 5.0 | 6.9 | NR | p53 | 55.9 | ND | Pos |
| 12 | 69 | W | 126,852 | 2 | 10.0 | 3.9 | PR | T12 | 2 | Mutated | Neg |
| 13 | 66 | M | 2530 | 2 | 5.0 | 4.9 | PR | T12 | 92.5 | Unmutated | Neg |
| 14 | 68 | W | 148,010 | 1 | 10.0 | 3.1 | CR | ATM | 40.5 | Unmutated | Pos |
| 15 | 83 | W | 104,256 | 3 | 10.0 | 9.8 | NR | p53 | 55.6 | Unmutated | ND |
| 16 | 74 | M | 146,168 | 1 | 5.0 | 6 | CR | D13 | 4.4 | Unmutated | ND |
| 17 | 67 | M | 179,046 | 1 | 10.0 | 3.6 | CR | ATM | 93.1 | Unmutated | Pos |
| 18 | 72 | M | 113,181 | 1 | 5.0 | 10.2 | PR | ATM | 59.1 | ND | Neg |
| 19 | 66 | W | 32,263 | 1 | 10.0 | 4.5 | PR | T12 | 21.1 | Unmutated | Pos |
| 20 | 66 | M | 98,784 | 3 | 10.0 | 2.7 | PR | D13 | 49.1 | Mutated | Pos |
| 21 | 69 | W | 84,265 | 1 | 2.5 | 2.4 | PR | NEG | 7.3 | Unmutated | Pos |
| 22 | 71 | M | 222,586 | 4 | 5.0 | 8 | PR | ATM | ND | Unmutated | Pos |
| 23 | 71 | M | 3185 | 4 | 2.5 | 3.1 | CRi | D13 | 11.5 | Mutated | ND |
| 24 | 76 | M | 91,296 | 4 | 5.0 | 4.8 | NR | D13 | 4.9 | Mutated | Neg |
ALC, absolute lymphocyte count (per µL); Rai Stage, use of the cytologic Rai Classification system for chronic lymphocytic leukemia; NCI, National Cancer Institute; FISH, fluorescence in situ hybridization; IGVH, IgVH gene mutation analysis; M, men; NR, ; ATM, microarray ATM gene analysis; nPR, nodal partial response; ND, not done; W, women; D13, ; CRi, incomplete complete response; PR, partial response; CR, complete response.
Nine of 24 patients had Rai stages III or IV (Rai Classification system for CLL), and the median serum beta-2 microglobulin (β2M) was 4.2 mg/dL (range, 2.1 to 12.0 mg/dL). Immunoglobulin heavy-chain (IGVH) variable gene sequencing was determined on leukemia cells of 22 patients and 14 (64%) had unmutated (≤2% deviation from germline) IGVH. Expression of ZAP-70 was measured by immunocytochemistry in 18 patients, of whom 10 (56%) were positive. Twenty-two patients had cytogenetic abnormalities by fluorescence in situ hybridization (FISH) testing, 7 had chromosome 13q deletion, and 8 had chromosome 11q deletion. Peripheral blood was collected from patients before initiation of therapy (n = 24), after 3 cycles (n = 24), and again after 15 cycles (n = 17) of treatment.
On the basis of the 60 patients enrolled in the study, the most common toxicity was grade 3–4 neutropenia, which occurred in 34% of evaluable treatment cycles. Other hematological toxicities were less common with grade 3 or 4 thrombocytopenia and anemia occurring in only 12% and <1% of cycles, respectively. Grade 1 and 2 toxicities were more common and included fatigue (75%), diarrhea (52%), constipation (48%), rash (47%), and/or pruritus (42%). Grade 1 or 2 tumor flare reactions occurred in 48% of patients, but these were generally mild and treated with short administration of nonsteroidal anti-inflammatory drugs, steroids, or dose adjustment of lenalidomide. There were no grade 3 or 4 episodes of tumor flare or tumor lysis in this study. (Xavier Badoux et al, personal communication).
Lenalidomide Significantly Lowered the Absolute Count of Lymphocytes and T Cells
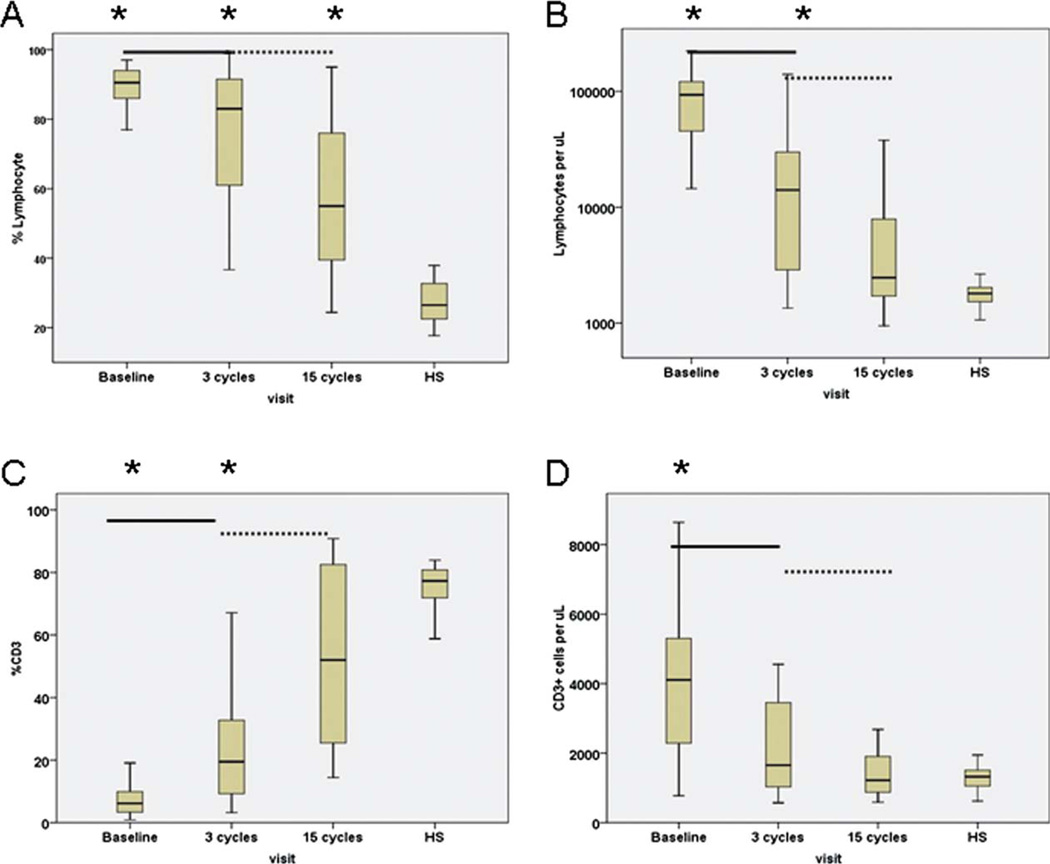
The median WBC at start of treatment was 100.2 × 103/µL. Along with significant reductions in WBC (11.9 × 103/µL) after 3 cycles of treatment (P = .000), there were significant decreases in the ALC as well as absolute count of T cells of CLL patients (P = .000 and P = .009, respectively; Fig. 1). After 15 cycles of treatment, patients had further reductions in WBC (4.8 × 103/µL) and ALC (2.5 × 103/µL) with median values within the normal range for healthy subjects established at the University of Texas M D Anderson Cancer Center (Fig. 1). In addition, the median number of T cells of CLL patients was also within the normal range of healthy subjects.
Figure 1.
Decreases in (a) percentages and (b) numbers of lymphocytes and increases (c) in percentage and (d) number of T cells with lenalidomide are shown. Data obtained from healthy subjects (HS) are shown on the right side of each figure. Asterisks represent significant differences between healthy subjects and those of CLL patients at the designated time points during treatment. Solid line represents significant differences between pretreatment versus after 3 cycles of therapy; dashed line represents significant differences between after 3 cycles versus after 15 cycles of therapy.
Lenalidomide Significantly Lowered the Percentage of Lymphocytes and Increased the Percentages of T Cells
The median percentage of lymphocytes at the start of treatment was 90.5%. After 3 cycles of therapy, there was a significant decreases in the percentage of lymphocytes (P = .004), and further reduction was observed after 15 cycles (P = .002; Fig. 1). However, despite significant reductions in percentage of lymphocytes, patients still had significantly higher median percentage of lymphocytes than healthy subjects (55.0% vs 26.5%, P = .000).
The median percentage of T cells at the start of treatment was significantly lower than that of healthy subjects but had significantly increased after 3 cycles of therapy (P = .000) with an additional increase after 15 cycles of treatment that reached the median value (52%) of T cells that was comparable to that of healthy subjects (Fig. 1).
Lenalidomide Affects Cytokine Production by Anti-CD3 Activated CD4+ T Cells
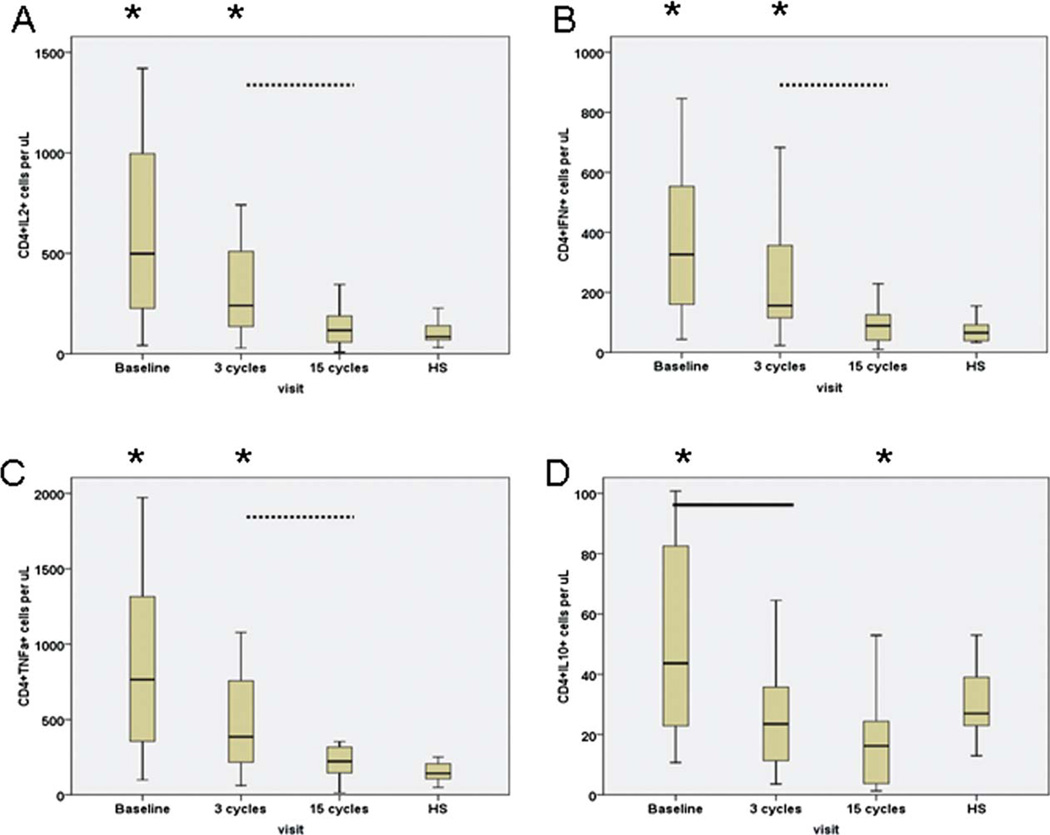
The median percentages and numbers of activated CD4+ T cells that synthesized IL-2, IFN-γ or TNF-α before treatment were higher than those of healthy subjects (Table 2 and Fig. 2). After 3 cycles of treatment, the percentages and numbers of CD4+ T cells producing these cytokines were similar to their baseline levels. However, percentages and numbers of CD4+ T cells producing IL-2, IFN-γ, or TNF-α decreased significantly after 15 cycles of treatment (P < .02), and their median values were equivalent to those of healthy subjects.
Table 2.
Percentages of TCR-Activated CD4+ and CD8+ T Cells That Synthesized IL-2, IFN-γ, TNF-α, or IL-10 at Pretreatment, After 3 cycles, and After 15 cycles of Lenalidomide Therapy
| Treatment With Lenalidomide | Healthy Subjects n=22 | |||
|---|---|---|---|---|
| Pretreatment n=24 Median (Mean±SEM) |
After 3 Cycles n=24 Median (Mean±SEM) |
After 15 Cycles n=17 Median (Mean±SEM) |
Median (Mean±SEM) | |
| % CD4+IL-2+ | 29.1a (26.6±2.2) | 30.3a (26.9±2.2) | 14.7b (14.2±1.8) | 19.3 (17±1.5) |
| % CD4+IFN-γ+ | 20.2a (18.3±1.7) | 16.4 (17.6±1.3) | 11.4b (12.0±2.7) | 13.4 (13.9±1.8) |
| % CD4+TNF-α+ | 43.9a (39.3±2.9) | 39.7a (29.1±2.3) | 28.5b (26.8±2.9) | 27.8 (26.8±1.8) |
| % CD4+IL-10+ | 2.6a (2.8±0.3) | 1.9a,c (2.2±0.2) | 1.7a (2.0±0.4) | 4.7 (5.4±0.5) |
| % CD8+IL-2+ | 6.7 (7.9±1.4) | 8.7 (9.8±1.5) | 2.0a,b (2.8±0.6) | 8.0 (8.0±1.1) |
| % CD8+IFN-γ+ | 12.7 (14.4±1.8) | 20.1c (19.1±1.7) | 11.3 (13.2±2.3) | 13.6 (15.4±1.5) |
| % CD8+TNF-α+ | 13.9 (15.9±2.1) | 17.1 (18.7±1.9) | 10.6a,b (11.2±1.4) | 16.7 (7.4±1.6) |
| % CD8+IL-10+ | 1.0a (1.2±0.1) | 1.0a (1.2±0.2) | 1.4a (1.7±0.4) | 3.8 (4.9±0.7) |
SEM indicates standard error of the mean.
There were significant differences between CLL patients and healthy subjects with respect to cytokine synthesis by TCR-activated CD4+ or CD8+ T cells.
There were significant changes between the median percentages of TCR-activated CD4+ or CD8+ T cells producing cytokines by CLL patients after 15 cycles of treatment versus their respective levels after 3 cycles of treatment.
There were significant changes between the median percentages of TCR-activated CD4+ or CD8+ T cells producing cytokines by CLL patients after 3 cycles of treatment versus their respective levels before treatment.
Figure 2.
Lenalidomide modulates the absolute numbers of TCR-activated CD4+ T cells that synthesized (a) IL-2, (b) IFN-γ, (c) TNF-α, and (d) IL-10. Data obtained from healthy subjects are shown on the right side of each figure. Asterisks represent significant differences between responses of healthy subjects (HS) and those of CLL patients at the designated time points during treatment. Solid line represents significant differences in activated CD4+ T cells synthesizing cytokines between pretreatment versus after 3 cycles of therapy; dashed line represents significant differences between after 3 cycles versus after 15 cycles of therapy.
Compared with healthy subjects, the percentage of activated CD4+ T cells producing IL-10 was lower at pretreatment and after 3 cycles of therapy (P < .003). After 15 cycles of treatment, the percentage and number of CD4+ IL-10+ T cells were still significantly lower that those of healthy subjects (P < .003).
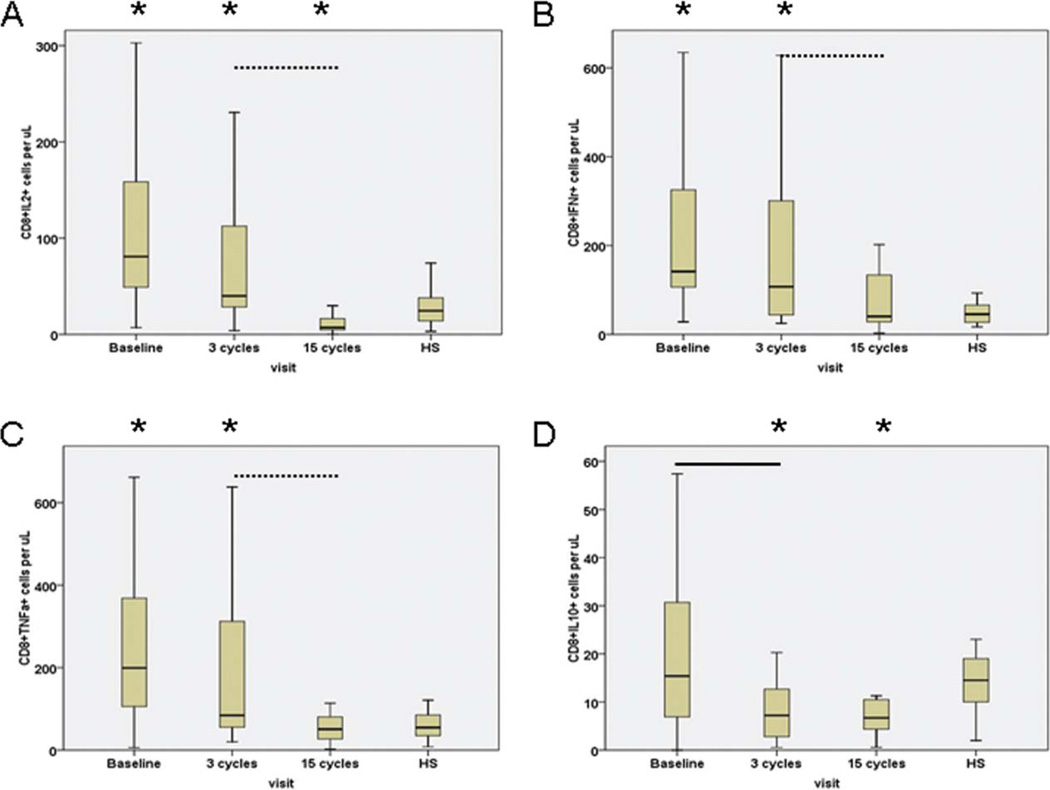
Cytokine Synthesis by Activated CD8+ T Cells Is Affected by Treatment With Lenalidomide
Whereas the percentages of activated CD8+ T cells synthesizing IL-2, IFN-γ, or TNF-α before initiation of treatment were similar to those of healthy subjects, interestingly the percentage of activated CD8+ T cells producing IFN-γ after 3 cycles of therapy was significantly increased (Table 2). Moreover, the median numbers of activated CD8+ T cells producing IL-2, IFN-γ, or TNF-α were significantly higher than those of healthy subjects at pretreatment and after 3 cycles of therapy, but they were significantly decreased after 15 cycles of therapy and became comparable to those of healthy subjects (Fig. 3).
Figure 3.
Lenalidomide modulates the absolute number of TCR-activated CD8+ T cells that synthesized (a) IL-2, (b) IFN-γ, (c) TNF-α, and (d) IL-10. Data obtained from healthy subjects are shown on the right side of each figure. Asterisks represent significant differences between responses of healthy subjects (HS) and those of CLL patients at the designated time points during treatment. Solid line represents significant differences between pretreatment versus after 3 cycles of therapy; dashed line represents significant differences between after 3 cycles versus after 15 cycles of therapy.
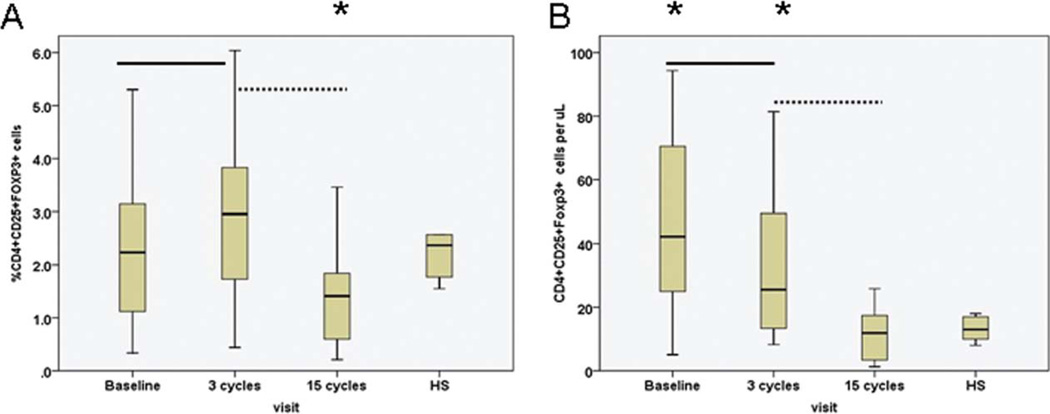
The Percentage and Absolute Number of TR Cells Normalized During Treatment With Lenalidomide
The median percentage of TR cells was 2.2% at pretreatment and significantly increased after 3 cycles of treatment (P = .015; Fig. 4). However, after 15 cycles of treatment, the median percentage and absolute number of TR cells decreased significantly and were equivalent to that of healthy subjects.
Figure 4.
(a) Percentage and (b) number of regulatory T cells (TR) of pretreatment CLL patients are shown after 3 cycles and after 15 cycles of lenalidomide therapy. Data obtained from healthy subjects (HS) are shown on the right side of each figure. Asterisks represent significant differences in TR cells between healthy subjects and CLL patients at the designated time points during treatment. Solid line represents significant differences in TR cells between pretreatment versus after 3 cycles of therapy; dashed line represents significant differences in TR cells after 3 cycles versus after 15 cycles of therapy.
DISCUSSION
Treatment of B-CLL patients with lenalidomide significantly decreased the total number of lymphocytes and T cells to levels observed in healthy subjects. Similarly and more importantly, after 15 cycles of treatment, the percentages of T-cell subsets that synthesized cytokines resembled those of healthy subjects. In particular, the numbers of CD4+ IL-2+ T-helper and CD8+ IFN−γ+ T cells were restored to the normal range for healthy subjects.
T-cell dysfunction in patients with CLL may contribute to the progression of disease,19 and others have reported that CLL patients have increased numbers of TR cells20 that are responsible for suppression of cytokine production. Multiple T-cell abnormalities including over-expression of CD3 zeta chain and ZAP-70 in CLL patients with indolent disease indicated a state of chronic and aberrant activation of T cells.21 Thus, it was not unexpected that T-cell responses before treatment, ie, percentage of activated CD4+ T cells producing IL-2, IFN-γ, or TNF-α, were significantly higher when compared with those of healthy subjects, and the heightened activation through the TCR could be related to the intrinsic activation of T cells by B-CLL cells. Despite reductions in absolute number of T cells after 3 cycles of therapy, the residual T cells retained their ability to synthesize cytokines. This was followed by significant reductions in the percentages of activated CD4+ and CD8+ T cells that synthesized IL-2, IFN-γ, or TNF-α after 15 cycles of therapy. In addition, the median numbers of activated CD4+ T cells that produced IL-2, IFN-γ, or TNF-α and activated CD8+ T cells that produced IFN-γ were equivalent to those of healthy subjects.
Gorgun and collaborators have suggested a role for leukemic B cells in T-cell dependent immune deficiency. They observed that coculturing B-CLL cells with normal T cells resulted in the inability of CD4+ T cells to differentiate into Th1 cells and CD8+ T cells with defective cytotoxic ability.22 Interestingly, in our study, there was a significant transient increase in percentages of activated CD8+ T cells producing IFN-γ after 3 cycles of treatment with lenalidomide (Fig. 3). These data suggest that cell-mediated cytotoxicity may be involved in the clinical activity of lenalidomide. This mechanism is supported by other studies reporting that the administration of lenalidomide to TCL1 mice reversed the dysfunctional immunological synapse formation between T cells and leukemic cells.12,22
The role of IL-10 in the pathogenesis of CLL is still controversial, as some investigators have reported IL-10 as a survival factor for B cells,23 and others have shown IL-10–induced apoptosis of CLL cells in vitro.24 Although the median percentage of IL-10–producing CD4+ T cells of CLL patients at pretreatment was significantly lower than that of the healthy subjects, the median level was further decreased after 3 cycles of treatment. These data tend to support the hypothesis that decreased IL-10 production may relate to increased apoptosis of CLL cells as suggested by another in vitro study.25
Natural TR cells are believed to arise as a suppressive population within the thymus,26 but there is evidence that they may also arise peripherally.27 In humans, TR cells compose about 1% to 2% of circulating CD3+ CD4+ T cells that coexpress the IL-2 receptor-α (IL-2Rα or CD25) at high density (CD25hi).28 CLL patients have been reported to have an increase in TR cells, which were reduced after thalidomide therapy.29 In our study, there was a transient increase (~18%) in the percentage of TR cells in the peripheral blood after 3 cycles of treatment (Fig. 4) that was followed by a significant decrease (~40%) in TR cells after 15 cycles of treatment (P = .001). The transient increase of TR cells after 3 cycles of therapy may be explained by the activation of CD4+ T cells through increased circulating tumor antigens30 associated with the destruction of B-CLL cells by lenalidomide.31 We suggest that whereas the transient increase in TR cells after 3 cycles of treatment may be beneficial by preventing the development of the tumor flare reaction in patients receiving lenalidomide, the subsequent reduction in percentages of TR cells after 15 cycles of therapy is consistent with the in vitro inhibitory effect of lenalidomide on TR cells.32 Interestingly, patients with complete responses had significantly higher percentages of CD4+ T cells and low percentages of TR cells when compared with patients who experienced partial response after 15 cycles of treatment.
Lastly, lenalidomide induced significant reductions in the level of serum cytokines, TNF-α, and its soluble receptor sTNF-R1, angiogenesis factor bFGF, inflammatory factors IL-1RA and IL-10, and chemokine MIP-1α in CLL patients after 9 cycles of therapy. The results further substantiate the immunomodulatory effects of lenalidomide in CLL patients. To date, there have been limited attempts to reverse immune defects in CLL patients. A recent clinical trial among CLL patients with high-risk cytogenetics treated with lenalidomide showed a 38% overall response rate, with 19% of patients achieving a complete response.33 We previously reported that treatment with lenalidomide was associated with an overall response rate of 31% in CLL patients with 11q or 17p deletion, of 24% in patients with unmutated V(H), and of 25% in patients with fludarabine-refractory disease.31 These studies suggest that the restoration of immune function have the potential to effectively eliminate B-CLL cells. Despite the small sample size due to the limited number of participants for this optional immunologic study, herein, we have provided evidence that treatment with lenalidomide modulates T-cell immunity profiles of CLL patients and that changes in the proportion of functional T-cell subsets may reverse CLL-related immune defects and contribute to a favorable clinical response.
Acknowledgments
CONFLICT OF INTEREST DISCLOSURES
This study was supported in part by research funding from Celgene Corporation to Alessandra Ferrajoli.
Bang-Ning Lee, Alessandra Ferrajoli, and James M. Reuben conceived of design for the immunology studies and are responsible for preparation of the manuscript. Hui Gao and Evan N. Cohen were responsible for quantifying the T-cell subsets and TR cells, the functional studies of TCR-activated PBMC assays, and statistical analysis. Xavier Badoux, William G. Wierda, Zeev Estrov, Stefan H. Faderl, Alessandra Ferrajoli, and Michael J. Keating conceived and conducted the clinical trial, obtained informed consent from the patients, and reviewed the manuscript.
The authors acknowledge Maude Veech and Diane Hackett for their help in editing the manuscript, and Sanda Tin, Ying-Dong Li, and Matthew Galland for processing the blood samples for functional studies.
REFERENCES
- 1.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. http://content.nejm.org/cgi/content/abstract/352/6/549. [DOI] [PubMed] [Google Scholar]
- 2.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. http://bloodjournal.hematologylibrary.org/cgi/content/abstract/bloodjournal;108/10/3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 4.Pellagatti A, Jadersten M, Forsblom AM, et al. Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proc Natl Acad Sci U S A. 2007;104:11406–11411. doi: 10.1073/pnas.0610477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;12:36–46. doi: 10.1186/1756-8722-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallek M, Pflug N. Chronic lymphocytic leukemia. Ann Oncol. 2010;21(suppl 7):vii154–vii164. doi: 10.1093/annonc/mdq373. [DOI] [PubMed] [Google Scholar]
- 7.Brown JR. Immunomodulators in chronic lymphocytic leukemia: where does lenalidomide belong? Leuk Lymphoma. 2010;51:1382–1385. doi: 10.3109/10428194.2010.496017. [DOI] [PubMed] [Google Scholar]
- 8.Saloura V, Grivas PD. Lenalidomide: a synthetic compound with an evolving role in cancer management. Hematology. 2010;15:318–331. doi: 10.1179/102453310X12647083620921. [DOI] [PubMed] [Google Scholar]
- 9.Ferrajoli A, Badoux XC, O’Brien S, et al. Combination therapy with lenalidomide and rituximab in patients with relapsed chronic lymphocytic leukemia(CLL) [abstract] Blood. 2009;114(suppl 1):89. Abstract 206. [Google Scholar]
- 10.Brown JR, Abramson J, Hochberg E, et al. A phase I study of lenalidomide in combination with fludarabine and rituximab in previously untreated CLL/SLL. Leukemia. 2010;24:1972–1975. doi: 10.1038/leu.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapalombella R, Andritsos L, Liu Q, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115:2619–2629. doi: 10.1182/blood-2009-09-242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorgun G, Ramsay AG, Holderried TA, et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106:6250–6255. doi: 10.1073/pnas.0901166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84:553–559. doi: 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]
- 14.Binet JL, Caligaris-Cappio F, Catovsky D, et al. Perspectives on the use of new diagnostic tools in the treatment of chronic lymphocytic leukemia. Blood. 2006;107:859–861. doi: 10.1182/blood-2005-04-1677. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Work Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 16.Reuben JM, Lee BN, Johnson H, Fritsche H, Kantarjian HM, Talpaz M. Restoration of Th1 cytokine synthesis by T cells of patients with chronic myelogenous leukemia in cytogenetic and hematologic remission with interferon-alpha. Clin Cancer Res. 2000;6:1671–1677. [PubMed] [Google Scholar]
- 17.Marson A, Kretschmer K, Frampton GM, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BN, Duvic M, Tang CK, Bueso-Ramos C, Estrov Z, Reuben JM. Dysregulated synthesis of intracellular type 1 and type 2 cytokines by T cells of patients with cutaneous T-cell lymphoma. Clin Diagn Lab Immunol. 1999;6:79–84. doi: 10.1128/cdli.6.1.79-84.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44:383–389. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 20.Jak M, Mous R, Remmerswaal EB, et al. Enhanced formation and survival of CD4+ CD25hi Foxp3+ T-cells in chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:788–801. doi: 10.1080/10428190902803677. [DOI] [PubMed] [Google Scholar]
- 21.Kiaii S, Choudhury A, Mozaffari F, Kimby E, Osterborg A, Mellstedt H. Signaling molecules and cytokine production in T cells of patients with B-cell chronic lymphocytic leukemia (B-CLL): comparison of indolent and progressive disease. Med Oncol. 2005;22:291–302. doi: 10.1385/MO:22:3:291. [DOI] [PubMed] [Google Scholar]
- 22.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fayad L, Keating MJ, Reuben JM, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97:256–263. doi: 10.1182/blood.v97.1.256. [DOI] [PubMed] [Google Scholar]
- 24.Yen Chong S, Lin YC, Czarneski J, et al. Cell cycle effects of IL-10 on malignant B-1 cells. Genes Immun. 2001;2:239–247. doi: 10.1038/sj.gene.6363773. [DOI] [PubMed] [Google Scholar]
- 25.Djurdjevic P, Zelen I, Ristic P, Baskic D, Popovic S, Arsenijevic N. Role of decreased production of interleukin-10 and interferon-gamma in spontaneous apoptosis of B-chronic lymphocytic leukemia lymphocytes in vitro. Arch Med Res. 2009;40:357–363. doi: 10.1016/j.arcmed.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 27.Vukmanovic-Stejic M, Zhang Y, Cook JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 29.Giannopoulos K, Schmitt M, Własiuk P, et al. The high frequency of T regulatory cells in patients with B-cell chronic lymphocytic leukemia is diminished through treatment with thalidomide. Leukemia. 2008;22:222–224. doi: 10.1038/sj.leu.2404869. [DOI] [PubMed] [Google Scholar]
- 30.Quesada JR, Reuben JM, Scouros MA, Murphy SG. Autologous mixed lymphocyte reaction in lymphoid malignancies. Lack of correlation with disease activity or clinical remission. Cancer Immunol Immunother. 1982;12:231–239. [Google Scholar]
- 31.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. http://bloodjournal.hematologylibrary.org/cgi/content/abstract/bloodjournal;111/11/5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sher T, Miller KC, Lawrence D, et al. Efficacy of lenalidomide in patients with chronic lymphocytic leukemia with high-risk cytogenetics. Leuk Lymphoma. 2010;51:85–88. doi: 10.3109/10428190903406806. http://informahealthcare.com/doi/abs/10.3109/10428190903406806. [DOI] [PubMed] [Google Scholar]