Abstract
Objective
Over a quarter million individuals in the US have Multiple Sclerosis (MS). Chronic pain and depression are disproportionately high in this population. The purpose of this study was to examine the relationship between chronic pain and depression in MS and to examine potentially meditational effects of anxiety, fatigue and sleep.
Methods
Cross-sectional data from self-reported instruments measuring multiple symptoms and quality of life indicators were used in this study. Structural equation modeling (SEM) was utilized to model direct and indirect effects of pain on depression in a sample of 1245 community dwelling individuals with MS. Pain interference, depression, fatigue and sleep disturbance were modeled as latent variables with 2 to 3 indicators each. The model controlled for age, sex, disability status (EDSS) and social support.
Results
A model with indirect effects of pain on depression had adequate fit and accounted for nearly 80% of the variance in depression. The effects of chronic pain on depression were almost completely mediated by fatigue, anxiety, and sleep disturbance. Higher pain was associated with greater fatigue, anxiety, and sleep disturbance, which in turn were associated with higher levels of depression. The largest mediating effect was through fatigue. Additional analyses excluded items with common content and suggested that the meditational effects observed were not attributable to content overlap across scales.
Conclusions
Individuals living with MS who report high levels of chronic pain and depressive symptoms may benefit from treatment approaches that can address sleep, fatigue, and anxiety.
Keywords: Multiple Sclerosis, pain, depression, sleep, structural equation model
Over a quarter million people in the US live with multiple sclerosis (MS), a disease characterized by physical, cognitive, and emotional symptoms (Anderson et al., 1992; Hirtz et al., 2007). The most common symptoms are fatigue, numbness, gait problems, bowel and bladder dysfunction, vision problems, dizziness and vertigo, sexual dysfunction, pain, cognitive function, emotional changes, depression, and spasticity. Chronic pain is common among persons living with MS; the prevalence has been estimated to be as high as 50% (Archibald et al., 1994; Ehde et al., 2003; O'Connor, Schwid, Herrmann, Markman, & Dworkin, 2008). Individuals who experience pain report poorer mental health, social functioning, and participation in valued activities. Pain has also been reported to negatively affect quality of life (Archibald et al., 1994) and be associated with increased psychological distress, such as depression and anxiety. Reports of lifetime risk of major depressive disorder (MDD) in MS range from 23 to 54%, and the 12-month prevalence of MDD is thought to be more than twice that of the general population (15.7% versus 7.4%, respectively) (Patten, Beck, Williams, Barbui, & Metz, 2003). Higher pain interference with daily activities has been associated with greater depression severity, (Ehde et al., 2003) however depression is also related to other symptoms in MS such as health distress (White, White, & Russell, 2008). Anxiety in MS has been less studied than depression, but it has been reported to be associated with pain severity (Kalia & O'Connor, 2005). Prevalence estimates of anxiety in MS have been reported as high as 41% (Korostil & Feinstein, 2007). These symptoms often co-occur leading to a particularly high symptom burden for some patients (Alschuler, Ehde, & Jensen, 2013; Krupp, 2003). It is important to note that depression exacerbates other MS symptoms, (Patten et al., 2003) and treatment of depression improves adherence rates for disease modifying therapies (Mohr et al., 1997).
Fatigue occurs in over 80% of people living with MS (L. A. Chwastiak et al., 2005). MS-related fatigue is qualitatively distinct from other forms of fatigue in that onset may occur more quickly, frequently, and severely than it does for other populations (Krupp, 2003). Several studies have found that a substantial portion of individuals living with MS consider fatigue the worst or most disabling symptom (Fisk, Pontefract, Ritvo, Archibald, & Murray, 1994; Krupp, 2003; Krupp, Alvarez, LaRocca, & Scheinberg, 1988). Fatigue can compound other related MS symptoms (Krupp et al., 1988), and it has also been reported to be associated with affective disorders, including depression (Mills & Young, 2011). In addition to fatigue, sleep is an independent predictor of health-related quality of life (Merlino et al., 2009). Approximately 50% of individuals with MS report problems related to sleep (Bamer, Johnson, Amtmann, & Kraft, 2008), and sleep difficulties can also exacerbate other MS-related symptoms (Manocchia, Keller, & Ware, 2001).
MS symptoms such as pain, fatigue, and depression frequently co-occur and are often referred to as symptom clusters. The symptom cluster of pain, fatigue and depression has been linked to decreased physical activity, self-efficacy, and functional ability in MS (Motl & McAuley, 2009). A symptom cluster of pain, depression, fatigue and perceived cognitive decline was related to decreased quality of life (Motl, Suh, & Weikert, 2010) and a symptom cluster of pain, depression, fatigue, sleep disturbance and perceived cognitive deficits was associated with an increased likelihood of being unemployed (Newland, Fearing, Riley, & Neath, 2012). The majority of the studies on symptom clusters in MS, however only examine whether symptoms are related to each other and whether they predict a particular outcome of interest (e.g. physical activity).
While prevalence rates of symptom clusters are clinically informative, greater insight into the mechanisms that drive these interrelationships could prove helpful in treatment planning (Ehde, Osborne, & Jensen, 2005). For example, if increased depression in chronic pain patients is partly attributable to inadequate sleep (i.e. increased pain leads to difficulties in sleeping, which in turn lead to increased depression), then additional relief from depression could be expected by adjunctive treatments specifically targeting sleep. Because of the high symptom burden people with MS often experience, targeted treatments could impact multiple related domains and may better explain the relationships observed between symptom clusters, quality of life, and critical outcomes like employment status and disability. To this end, this study aimed to explore potential mechanistic pathways between chronic pain and depression. Because these symptoms commonly oc-occur with related symtpoms of sleep disturbance, fatigue, and anxiety, we evaluated a model that could explain the increased prevalence of depression observed in MS patients living with pain that is theoretically plausible and statistically testable.
Directionality of effects between pain in depression (i.e., whether pain causes depression or depression causes pain or both) remains an active area of investigation in the literature. It has been thought that depression is a consequence of living with chronic pain (Fishbain, Cutler, Rosomoff, & Rosomoff, 1997). However, a recent longitudinal study of chronic pain in primary care patients suggested a bidirectional relationship between pain intensity and depression over the course of one year (K. Kroenke et al., 2011). Tang and colleagues provide evidence supporting directional effects from depressed mood to pain, with pain being measured with a self-reported 0–100 numerical rating of “overall level of pain” and pain-tolerance defined as length of time a participant could physically persist in holding a heavy shopping bag. Their findings support a theoretically plausible and compelling case that depressed mood may affect arousal leading to increased pain perception. However, the degree to which pain inteferes with carrying out daily activities and responsibilities (i.e. pain interference) is a qualitatively and quantitatively distinct construct from pain intensity, and directionality of effects may vary depending on construct measured. Moreover, seemingly contradictory evidence in this literature, (Brown, 1990; Davis, Reeves, Hastie, Graff-Radford, & Naliboff, 2000; Gerrits et al., 2012; Tang et al., 2008) may also be attributable, in part, to the variety of methodologies employed.
Compelling evidence supporting directionality from pain to depression comes from a study using cross-lagged structural equation models, a statistical technique that capitalizes on longitudinal assessment in statistical hypothesis testing (Brown, 1990). This technique allows for simultaneous estimation of directional effects, and the study findings that a uni-directional model from pain to depression fit the data better are persuasive. Moreover, a substantial and targeted review from 1997 of the issue of directionality in pain and depression concludes that there is greater evidence supporting depression as consequence of chronic pain rather than as an antecedent (Fishbain et al., 1997). Lastly, a recent 12-year longitudinal cohort study examining directionality using covariate-adjusted Cox regression models similarly concluded that the risk of developing depressive symptoms was significant in non-depressed participants with elevated pain at baseline, while the inverse effect of depression at baseline in pain-free participants was not statistically predictive of higher pain levels over time (Hilderink, Burger, Deeg, Beekman, & Oude Voshaar, 2012). Building on this evidence, we aimed to explore the mechanism through which chronic pain may exert its effect on depressive symptoms.
In order to examine the impact of pain interference on depression, we examined three theoretical mediators: anxiety, sleep problems, and fatigue. As outlined above, all of these symptoms are higher in people with MS and are associated with other symptoms of MS. In order to include these variables as mediators, a temporal relationship from the predictor to the mediator and from the mediator to the outcome has to be suggested by previous literature. Pain can lead to increased anxiety, fatigue and sleep problems supporting the use of these three variables as mediators with pain as the predictor. In a sample of people with fibromyalgia, pain predicted sleep quality the subsequent night and sleep quality mediated the relationship between pain and fatigue the next day (Nicassio, Moxham, Schuman, & Gevirtz, 2002). Although numerous studies have found that anxiety can affect pain sensitivity (Rhudy & Meagher, 2000), research from animal studies suggests that pain also increases anxiety (Narita et al., 2006; Roeska, Doods, Arndt, Treede, & Ceci, 2008). The first set of relationships in our mediation model, from pain to the mediators, is therefore supported by prior work.
Previous research from both the general population and other medical populations also suggests that anxiety, sleep problems and fatigue are related to subsequent depression, supporting the second half of the mediation model (from mediator to outcome). Studies from the general population suggest that anxiety can precede depression, although in some cases depression may precede anxiety (Moffitt et al., 2007). Problems with sleep including insomnia and hypersomnia have long been known to precede the development of major depressive episodes (Ford & Kamerow, 1989). Considering the literature on pain leading to increased anxiety, sleep disturbance and fatigue and the literature linking these mediators with subsequent depression, we utilized structural equation modeling (SEM) to test the directional relationships of these symptoms that commonly cluster together.
SEM is particularly well suited to the aims of this study, as it facilitates testing of theoretical models by simultaneously estimating effects between multiple related factors. SEM is an advanced statistical technique employing both factor and path analyses that is increasingly used to test empirically complex and sophisticated models that include multiple biopsychosocial determinants of health. SEM also allows for inspection and testing of direct and indirect pathways of effects and is appropriate for studies examining the structural inter-relationships among multiple correlated symptoms and conditions such as the aim of the current study. We proposed and tested a SEM that explains the commonly observed relationship between PI and depression that evaluates direct and indirect (mediated) effects of anxiety, sleep disturbance and fatigue.
Methods
Participants
Data for this study were collected as part of an ongoing longitudinal study exploring the impact of MS on symptoms and quality of life indicators. Letters of invitation were sent to 7,806 people from the Greater Northwest chapter of the National Multiple Sclerosis Society (NMSS). NMSS, and it’s local chapters, are non-profit charitable organization that provide programs and services for people with MS and their families. The NMSS funds research, conducts advocacy and education program and collaborates with MS organization around the world with the aim of achieving a world free of MS. From this mailing by NMSS, 1,629 (20.9%) individuals returned a response card or contacted research staff directly to indicate interest. Eligibility criteria included a definitive diagnosis of MS and being at least 18 years of age. In total, 1,597 people who were eligible and interested in participating were either mailed a self-report paper survey (n=1,368) or directed to an online version of the survey (n=229), with reminder letters sent to non-responders 3–6 weeks later. The goal was to collect a completed survey from the first 1,350 consecutively-enrolled individuals who met eligibility criteria. A total of 1,271 participants with MS returned surveys. The participation rate of 79.6% of all individuals who responded to recruitment efforts is similar or higher than reports by other large-scale MS studies (Anens, Emtner, Zetterberg, & Hellstrom, 2014; Broadley, Deans, Sawcer, Clayton, & Compston, 2000; L. Chwastiak et al., 2002; Ploughman et al., 2014). Any surveys with missing data were followed up by phone. We were unable to collect missing data for approximately 2% (n=26) of participants, and these were excluded from analysis. A short, anonymous demographics survey was sent one month later to non-responders of the original NMSS mailing list to assess possible recruitment bias. Responses received from 1,046 non-responders indicated that 13% did not have MS despite being listed as persons with MS on the mailing list, and 34% did not recall receiving the initial survey invitation. Overall, the 1,271 individuals who completed the study survey were similar on demographic variables to the non-responders except they were more educated (84% reported some college or more education compared to 72% of non-responders; chi2=30.7, p<0.001), slightly younger (53% of responders were 51 or older compared to 71% of non-responders; chi2=70.3, p<0.001), and had shorter mean disease duration (M=13, SD=10) than non-responders (M=17, SD=12) [t(2041)=8.00, p<0.0001]. The Human Subjects Division of the University of Washington approved all study procedures, and all participants provided informed consent.
Measures
Pain Interference (PI)
PI was measured by 2 scales. The Brief Pain Inventory (BPI) includes a 7-item PI subscale that has been widely used in research on people with many kinds of disabilities (Cleeland, 1989). Scores for the BPI range from 0 (pain does not interfere) to 10 (completely interferes) and are calculated as the average of the 7 item responses. The Pain Impact Questionnaire 6 (PIQ-6) is a 6-item scale designed to measure the impact of pain on an individual’s well-being and ability to perform usual activities, including work and leisure activities (Becker, Schwartz, Saris-Baglama, Kosinski, & Bjorner, 2007). PIQ-6 was developed using item response theory (IRT) and has demonstrated good internal consistency, reliability, and construct validity (Becker et al., 2007). Weighted scores range from 40 to 78, with higher scores reflecting greater pain impact.
Fatigue
Fatigue was measured by three scales. The Modified Fatigue Impact Scale (MFIS) contains a subset of the 21-item Fatigue Impact Scale (Fisk et al., 1994) and is recommended for measuring fatigue in people living with MS, because it measures the physical, cognitive and psychosocial aspects of fatigue. Participants completed the MFIS using a five-point Likert scale ranging from 0 (never) to 4 (almost always), with higher summary scores reflecting increased fatigue. The Fatigue Severity Scale (FSS) is comprised of items selected for their ability to identify common features of fatigue in patients with multiple sclerosis and systemic lupus erythematosus (Krupp, Larocca, Muirnash, & Steinberg, 1989). The FSS includes nine items pertaining to fatigue in the last week, and each is scored from 1 to 7 (1 = completely disagree, 7 = completely agree). The scores are averaged to create a total score, with higher scores indicating greater fatigue severity. The Multidimensional Assessment of Fatigue (MAF) measures fatigue severity, distress, degree of interference in activities of daily living, and frequency (Tack, 1990). Scores for each dimension contribute to a Global Fatigue Index that ranges from 0 (no fatigue) to 50 (severe fatigue). While originally developed for patients with rheumatoid arthritis, the MAF has been tested in populations with other chronic conditions such as HIV, multiple sclerosis, and cancer.
Depression
Depression was measured using a 10-item short form of the Center for Epidemiologic Studies Depression Scale (CES-D) and the 9-item Patient Health Questionnaire (PHQ-9). The CES-D was developed to screen for depressive symptoms, and sum scores have demonstrated adequate reliability and validity (Andresen, Malmgren, Carter, & Patrick, 1994). Scores on the CES-D range from 0 to 30, and higher scores indicate more depressive symptoms. The PHQ-9 has been used as a depression measure in a variety of settings and clinical populations and has also been administered to assess depressive symptoms and associated functional impairment.(K. Kroenke, Spitzer, R.L., Williams, J.B., 2001) Respondents rate how often over the last 2 weeks they had been bothered by each of the 9 symptoms that comprise the DSM-IV major depression criteria; summary scores range from 0 to 27, and higher scores indicate more depressive symptoms.
Anxiety
Anxiety was measured using the Hospital Anxiety and Depression Scale (HADS). The HADS has been shown to be a reliable measure for screening clinically significant anxiety in patients (Zigmond & Snaith, 1983). A total of 7 items from the HADS pertain to anxiety, with higher sum scores reflecting greater anxiety.
Sleep
The 5-item Women’s Health Initiative Insomnia Rating Scale (WHIIRS) is a self-report measure of sleep disturbance developed through a large sample survey of postmenopausal women. In addition to completing an overall assessment of sleep quality, respondents indicated how often they experienced each of four sleep problems during the previous 4 weeks. Total scores range from 0 to 20, with higher scores indicating greater sleep disturbance. The WHIIRS was found to have excellent short-term test-retest reliability and is correlated with other measures of sleep quality (Levine et al., 2003). Study participants were also administered the sleep adequacy items of the Medical Outcomes Survey (MOS-SAD).(Hays, Martin, Sesti, & Spritzer, 2005) The MOS-SAD was developed using item response theory (IRT) to evaluate perceived adequacy of sleep - that is, whether a person gets the amount of sleep needed to feel rested. The IRT-derived scores are transformed on to a 100-point scale with a mean of 50 and a standard deviation of 10. Higher scores reflect greater sleep adequacy. The MOS sleep scale has been validated in multiple populations, including those with neuropathic pain (Hays et al., 2005; Manocchia et al., 2001).
Analysis
SEM was used to examine the direct and indirect effects of PI on depression in this cross-sectional study. Direct effects assess the effects of independent variables on a dependent variable directly; indirect effects represent the effects of independent variables on a dependent variable through other mediating variables. Potential mediators of the relationship between PI and depression considered in this study included commonly co-occuring symptoms of anxiety, fatigue, and sleep disturbance. SEM allows for multiple indicators (i.e., measures) of the constructs of interest and reduces the overall effect of measurement error on the accuracy of the estimates (Kline, 1998). Furthermore, SEM allows researchers to estimate and test effects for complex models with many related variables simultaneously, making estimation more efficient.
Except for anxiety, each variable had multiple indicators. Fatigue was modeled as a latent factor with 3 indicators: the MFIS, FSS, and the MAF. The PIQ-6 and BPI represented PI as a latent factor, and the factor for depression was represented by the CES-D and PHQ-9. Sleep disturbance was represented by the WHIIRS and the MOS-SAD, and anxiety was measured as a manifest (i.e. directly measured) variable, because only one measure of anxiety was available. Additional terms were added to model paths to control for the effects of age, sex, disability status (EDSS), and level of social support. Model fit was evaluated through examination of standard errors, residual correlations, and model-fit criteria. Goodness of fit statistics included χ2, but as χ2 is known to be an increasingly conservative measure of fit as sample sizes increase (Bentler, 1980), Comparative Fit Index (CFI) (Bentler, 1990), Tucker-Lewis Index (TLI) (Tucker & Lewis, 1973), Root Mean Square Error of Approximation (RMSEA), and Standardized Root Mean Square Residual (SRMR) were also considered when evaluating model fit. CFI and TLI values above 0.95 are preferable; RMSEA values near 0.06 indicate adequate fit; and SRMR above 0.08 indicate poor fit (Browne & Ceduk, 1993; Hu & Bentler, 1999; Marsh, 2004). Data preparation and descriptive statistics were calculated using STATA X1 (StataCorp, 1985–2009). SEM was carried out using Mplus 6.1 (Muthén & Muthén, 1998–2010).
Three sets of analyses were carried out estimate the effect of PI on depression and to evaluate stability of model estimates. The first model (model one) included a direct path from PI to depression as well as mediating pathways through sleep disturbance, anxiety and fatigue. Sleep disturbance was also expected to increase fatigue, and this expectation was modeled as a direct effect. Essentially, the same model was run again with the same mediation pathways but without the direct effect pathway from PI to depression (model two). Measures of fit and effect estimates were compared between models one and two. The final set of analyses involved re-calculating scores of the CES-D and PHQ-9 without the items that ask about fatigue, sleep, and anxiety (3 items from the PHQ-9 and 3 items from the CES-D). While it would be difficult to support the clinical utility of a depression measure without these important symptoms, we conducted this analysis to evaluate whether the effects observed could be attributed to the content overlap among the measures. In other words, we wanted to examine whether removing items that ask about depression symptoms that could actually be symptoms of MS and not necessarily of depression, results in substantial changes in model parameters.
Results
Table 1 summarizes the demographic characteristics of the sample (n=1245). Participants in the study were predominantly women (80%), white (91%), employed (41%), and/or married or living with a partner (70%). The percentages of women, caucasians, and those employed are very similar to those of a large national MS registry (NARCOMS) with over 35,000 participants(Kobelt, Berg, Atherly, & Hadjimichael, 2006). A percentage of participants with relapsing remitting type of MS in our sample (56%) was slightly higher than that reported by NARCOMS (48%) suggesting perhaps lower participation by people with progressive types of MS. Over half of study participants attended at least some college, and income levels were relatively evenly distributed. The study sample reported mild to moderate fatigue, pain intensity, sleep problems, anxiety, PI, and depression.
Table 1.
Demographic and clinical profile of study participants
| Mean | SD | n=1245 | % | |
|---|---|---|---|---|
| Age | 50.7 | 11.6 | ||
| Years since MS diagnosis | 13.2 | 10.1 | ||
| MS Subtype | ||||
| Relapsing Remitting | 700 | 56.2 | ||
| Other | 497 | 40.0 | ||
| Missing | 48 | 3.9 | ||
| Gender | ||||
| Male | 245 | 19.7 | ||
| Female | 991 | 79.6 | ||
| Missing | 9 | 0.7 | ||
| Ethnicity | ||||
| White | 1138 | 91.4 | ||
| Non-white | 107 | 8.6 | ||
| Marriage Status | ||||
| Never-married | 110 | 8.8 | ||
| Married / Living with partner in committed relationship | 867 | 69.6 | ||
| Separated/Divorced/Widowed | 257 | 20.7 | ||
| Missing | 11 | 0.9 | ||
| Education | ||||
| Less than high school | 19 | 1.5 | ||
| High school graduate/GED | 155 | 12.5 | ||
| Vocational or technical school | 98 | 7.9 | ||
| Some college/technical degree/AA | 366 | 29.4 | ||
| College degree (BA/BS) | 374 | 30.0 | ||
| Advanced degree (MA, PHD, MD) | 224 | 18.0 | ||
| Missing | 9 | 0.7 | ||
| Employment | ||||
| Employed | 508 | 40.8 | ||
| Unemployed | 728 | 58.5 | ||
| Missing | 9 | 0.7 | ||
| Household Income | ||||
| Less than $25,000 | 218 | 17.5 | ||
| $25,000–$40,000 | 179 | 14.4 | ||
| $41,000–$55,000 | 173 | 13.9 | ||
| $56,000–$70,000 | 145 | 11.7 | ||
| $71,000–$85,000 | 122 | 9.8 | ||
| $86,000–$100,000 | 115 | 9.2 | ||
| Greater than $100,000 | 216 | 17.3 | ||
| Missing | 77 | 6.2 | ||
| Self-reported EDSS score | ||||
| Mild (≤ 4) | 401 | 32.2 | ||
| Moderate (4.5 – 6.5) | 592 | 47.6 | ||
| Severe (≥ 7) | 243 | 19.5 | ||
| Missing | 9 | 0.7 | ||
| Fatigue | ||||
| FSS | 5.1 | 1.5 | ||
| MFIS | 44.2 | 18.2 | ||
| MAF | 25.8 | 9.3 | ||
| Pain Intensity (NPS) | 4.8 | 2.2 | ||
| Pain-Interference | ||||
| PIQ-6 | 59.1 | 7.6 | ||
| BPI-PI | 3.7 | 2.5 | ||
| Depression | ||||
| CES-D | 10.0 | 6.7 | ||
| PHQ-9 | 8.3 | 6.1 | ||
| Problematic sleep | ||||
| WHIIRS | 9.8 | 5.3 | ||
| MOS-SAD | 48.6 | 26.6 | ||
| Anxiety (HADS) | 5.9 | 4.2 | ||
| Social Support (MSPSS) | 5.5 | 1.4 |
SD, Standard deviation; FSS, Fatigue Severity Scale; MFIS, Modified Fatigue Impact Scale; MAF, Multidimensional Assessment of Fatigue; NPS, Numerical Pain Scale; PIQ-6, Pain Impact Questionnaire-6; BPI-PI, Brief Pain Inventory; CES-D, Center for Epidemiological Studies Depression Scale; PHQ-9, Patient Health Questionnaire-9; WHIIRS, Women's Health Initiative Insomnia Rating Scale; MOS-SAD, Medical Outcomes Survey Sleep Adequacy; HADS, Hospital Anxiety and Depression Scale; MSPSS, Multidimensional Scale of Perceived Social Support
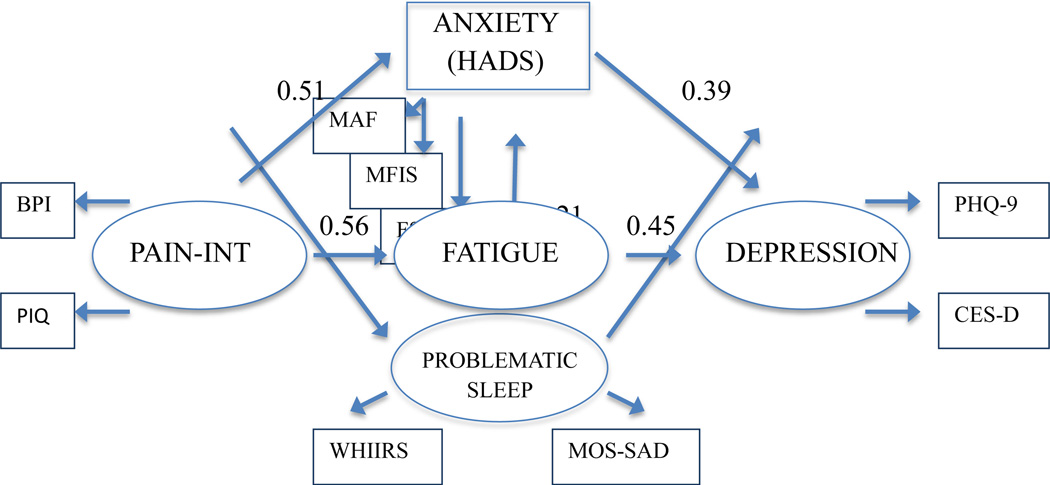
The goodness-of-fit criteria indicated that model one fit the data adequately (Table 2). While the chi-square test statistic suggested inadequate model fit (X2 = 343, df = 48, p<0.01), other fit indices indicated that the model adequately accounted for the covariance structure observed in the data: CFI = .97 and SRMR =.03, TLI = .94 and RMSEA = .07. Factor loadings for each indicator of Fatigue, PI, and Depression are presented in Table 3. All standardized factor loadings were moderately high to high in magnitude and were statistically significant. Table 4 outlines estimated direct and indirect effects of both models controlling for age, sex, disability status, and social support, and all estimates were statistically significant. Standardized direct effects of PI on anxiety, fatigue, and problematic sleep were comparatively strong (0.51 to 0.63), suggesting that as PI increases, anxiety, fatigue, and problems with sleep also increase. Direct effects of anxiety (0.39) and fatigue (0.44) on depression were of moderate strength, suggesting that higher anxiety and fatigue are associated with greater depression. Direct effect estimates were smaller for the effects of problematic sleep on fatigue and depression (0.22 each). The standardized direct effect for PI on depression was 0.02 and was not statistically significant. The standardized indirect effects of PI on depression were 0.63, for a combined total effect of 0.65.
Table 2.
Model fit criteria and estimates of variance explained for both models
| Model one | Model two | |||
|---|---|---|---|---|
| Estimate | R2 | Estimate | R2 | |
| X2 (df) | 343.04 (48) | 343.26 (49) | ||
| RMSEA (90%CI) | 0.07 (0.06 – 0.08) | 0.07 (0.06 – 0.08) | ||
| CFI | 0.97 | 0.97 | ||
| TLI | 0.94 | 0.94 | ||
| SRMR | 0.03 | 0.03 | ||
| Anxiety | 0.33 | 0.33 | ||
| Fatigue | 0.57 | 0.57 | ||
| Problematic sleep | 0.40 | 0.40 | ||
| Depression | 0.79 | 0.79 | ||
RMSEA, Root mean square error of approximation; CI, Confidence interval; CFI, Comparative fit index; TLI, Tucker Lewis index; SRMR, Standardized root mean square residual
Table 3.
Standardized factor loadings and standard errors for latent constructs
| Model one | Model two | |||
|---|---|---|---|---|
| Factor | λ | λSE | λ | λSE |
| Fatigue | ||||
| FSS | 0.83 | 0.01 | 0.83 | 0.01 |
| MFIS | 0.90 | 0.01 | 0.90 | 0.01 |
| MAF | 0.86 | 0.01 | 0.86 | 0.01 |
| Problematic sleep | ||||
| WHIIRS | 0.66 | 0.03 | 0.66 | 0.03 |
| MOS-SAD | −0.73 | 0.03 | −0.73 | 0.02 |
| Pain interference | ||||
| PIQ-6 | 0.88 | 0.01 | 0.88 | 0.01 |
| BPI | 0.92 | 0.01 | 0.92 | 0.01 |
| Depression | ||||
| CESD | 0.94 | 0.01 | 0.94 | 0.01 |
| PHQ-9 | 0.91 | 0.01 | 0.91 | 0.01 |
SE, Standard error; FSS, Fatigue Severity Scale; MFIS, Modified Fatigue Impact Scale; MAF, Multidimensional Assessment of Fatigue; WHIIRS, Women's Health Initiative Insomnia Rating Scale; MOS-SAD, Medical Outcomes Survey Sleep Adequacy; PIQ-6, Pain Impact Questionnaire-6; BPI, Brief Pain Inventory; CES-D, Center for Epidemiological Studies Depression Scale; PHQ-9, Patient Health Questionnaire-9
Table 4.
Standardized effects controlling for age, sex, disability status, and social support for both models
| Model one | Model two | |||
|---|---|---|---|---|
| Direct effects | Direct effects | |||
| Predictor Outcome | β | β SE | β | β SE |
| Pain Interference | ||||
| Anxiety | 0.51 | 0.03 | 0.51 | 0.03 |
| Fatigue | 0.55 | 0.04 | 0.56 | 0.04 |
| Problematic sleep | 0.63 | 0.04 | 0.63 | 0.04 |
| Depression | 0.02 | 0.04 | - | |
| Anxiety | ||||
| Depression | 0.39 | 0.02 | 0.39 | 0.02 |
| Problematic sleep | ||||
| Fatigue | 0.22 | 0.04 | 0.21 | 0.04 |
| Depression | 0.22 | 0.03 | 0.22 | 0.03 |
| Fatigue | ||||
| Depression | 0.44 | 0.03 | 0.45 | 0.03 |
| Indirect effects | Indirect effects | |||
| Mediators Pain -> Depression | β | β SE | β | β SE |
| Anxiety | 0.20 | 0.02 | 0.20 | 0.01 |
| Fatigue | 0.24 | 0.03 | 0.25 | 0.03 |
| Problematic sleep | 0.14 | 0.02 | 0.14 | 0.02 |
| Problematic sleep / Fatigue | 0.06 | 0.01 | 0.06 | 0.01 |
| Total Indirect Effect | 0.63 | 0.03 | 0.65 | 0.02 |
SE, Standard error
Because of the small direct effect of PI on depression in model one, we fit model 2 eliminating the direct pathway between PI and depression. All mediating pathways remained in model two, including the pathway from sleep disturbance to fatigue. See Tables 3 and 4 for model fit statistics and direct and indirect effects. Standardized effects are presented graphically in Figure 1 for model 2. While the chi-square test statistic suggested inadequate model fit (X2 = 343.3, df = 49, p < 0.01), other fit indices indicated that the model adequately accounted for the covariance structure observed in the data: CFI = .97 and SRMR =.03, TLI = .94 and RMSEA = .07. Model 2 accounted for a large proportion of the variance observed in depression (R2 = 0.79), and over half the variance in fatigue (R2 = 0.57) was explained by predictor variables. Approximately 33% and 40% of the variance was accounted for in the remaining factors of anxiety and problematic sleep, respectively. Model two resulted in a large standardized indirect effect of PI on depression of 0.65.
Figure 1.
Standardized direct effect estimates from structural equation model controlled for age, gender, disability status and social support
All effect estimates were statistically significant at p < 0.05.
PAIN-INT, Pain Interference; BPI, Brief Pain Inventory; PIQ-6, Pain Impact Questionnaire-6; HADS, Hospital Anxiety and Depression Scale; MAF, Multidimensional Assessment of Fatigue; MFIS, Modified Fatigue Impact Scale; FSS, Fatigue Severity Scale; WHIIRS, Women's Health Initiative Insomnia Rating Scale; MOS-SAD, Medical Outcomes Survey Sleep Adequacy; PHQ-9, Patient Health Questionnaire-9; CES-D, Center for Epidemiological Studies Depression Scale
In comparing model one and model two, the model without a direct path from PI to depression (model two) was preferred. The direct path from PI to depression only increased the total effects by 0.005, and the measure of direct effect was very weak and statistically non-significant. Estimates for indirect paths changed little in the presence of the direct path, and the largest change (0.006) was observed for the indirect path through fatigue. The difference in fit between model one and two was not statistically significant when assessed by X2, (Table 3) and there was no difference between the models when examining the estimates and confidence intervals of RMSEA.
Furthermore, there was little difference in fit when comparing CFI, TLI, or SRMR, and the proportions of variance explained were nearly identical for all variables. As a result the more parsimonious model (model two) is preferred.
When model two was re-evaluated using modified depression scores (i.e. scores on the CES-D and PHQ-9 without items related to sleep, anxiety, and fatigue) in the third set of analyses, the majority of the observed effects were maintained (R2: Anxiety = 0.33, Fatigue = 0.57, Problematic sleep = 0.40, Depression = 0.71). Indirect effects totaled 0.56 (vs. 0.63 using full CES-D and PHQ-9 scores). Likewise, model fit did not degrade substantially (X2 = 346.05, df = 49, p<0.01; CFI = 0.96; TLI = 0.94; RMSEA = 0.07, SRMR = 0.03).
Discussion
Results of the study suggest that the primary impact of PI on depression in MS is indirect. Higher PI appears to increase fatigue, anxiety, and problematic sleep, and these in turn appear to increase depression. The relationships among the constructs remain similar when depression is scored without the items that measure fatigue, anxiety and sleep, suggesting that the model effects cannot be attributed to content overlap across different measures.
The findings of this study have important implications for clinical research and practice in the treatment of chronic pain. Patients who present with problematic sleep and/or fatigue (with or without anxiety) may be more likely to experience higher depressive symptoms. The results are also consistent with previously published research that suggested that a transdiagnostic approach to symptoms may be more effective than targeting each symptom separately, such as depression treatment or pain treatment alone. Transdiagnostic models explain how multiple comorbid symptoms or disorders develop rather than creating disorder or symptom specific models (Nolen-Hoeksema & Watkins, 2011). A trans-diagnostic treatment is an intervention that targets a range of diagnoses or problems through the use of treatment strategies that target psychological processes that are common across disorders (Clark, 2009). This is consistent with the literature on symptom clusters (Motl & McAuley, 2009; Motl, Suh, et al., 2010; Motl, Weikert, Suh, & Dlugonski, 2010) that suggests common factors in the development and maintenance of pain, depression and fatigue. In the psychotherapy literature, trans-diagnostic treatments have been used to treat vulnerabilities or behaviors hypothesized to be central to the etiology of a variety of disorders such as anxiety and depression. When feasible and applicable, it may be useful to consider all five factors-pain, depression, anxiety, sleep, and fatigue-in developing a treatment plan. While it may be premature to test any specific trans-diagnostic interventions in MS, the results do suggest further exploration and development of trans-diagnostic theories of and treatments for the constellation of biopsychosocial concerns affecting many people living with MS. In particular, these results suggest that targeting chronic pain with an intervention that can be applied to the other symptoms (anxiety, sleep disturbance, depression and fatigue) such as cognitive behavioral therapy (CBT) may be particularly useful. Preliminary evidence regarding beneficial effects on depression of CBT targeting insomnia, further support this transdiagnostic approach (Manber et al., 2008). At a minimum, the findings highlight a need for comprehensive assessment of multiple concerns such as depression, anxiety, sleep problems, or fatigue when treating people with MS who report higher levels of pain.
Limitations and future directions
There are multiple limitations to this study. While the directionality of effects from PI to depression tested in this study are plausible and supported by evidence from the literature, it is important to note that discussions on the issues of directionality in the broader literature are still ongoing. Experimentation or longitudinal models are more appropriate than cross-sectional models (used in this study) for testing reciprocal relations between variables because causes should precede effects (Cook & Campbell, 1979, p.43) Additional studies will need to be carried out to better understand directionality of the effects. Cross-lagged longitudinal study designs that simultaneously estimate directional effects in the same model may be a good way to further examine the issue. Also, while model fit was adequate, it was not optimal, and the authors recognize the potential that alternative explanatory models could fit the data better. Nevertheless, fit assessment must be balanced against competing concerns related to theory, adequacy and interpretability of parameter estimates, model complexity, and validity-related concerns (Hu & Bentler, 1999; Marsh, 2004) and these issues informed decisions regarding model re-specification. Replication in a different sample of individuals with MS is warranted before generalizing to the population of people with MS. Overall, the results of the study support assessment of depression, anxiety, sleep problems, and fatigue in people with MS who report elevated levels of pain and utilizing treatments that affect multiple symptoms or a combination of treatments.
IMPACT.
Previous research found that pain, fatigue, sleep, anxiety and depression frequently cluster together in Multiple Sclerosis and have complex relationships, but the indirect relationship between pain and depression as mediated by fatigue, sleep problems and anxiety has not been examined.
The study found that effects of pain on depression are almost completely explained by the indirect effects of pain on fatigue, problematic sleep and anxiety, which in turn affect depression.
Individuals undergoinging treatment for pain and depression in Multiple Sclerosis may benefit from expanded or adjunctive treatments targeting sleep, fatigue, and anxiety.
Acknowledgments
The contents of this paper were developed under grants from the Department of Education, NIDRR grant numbers H133B080025 (U.W. Rehabilitation Research and Training Center on Multiple Sclerosis), however those contents do not necessarily represent the policy of the Department of Education, and Federal Government endorsement should not be assumed.
This work was also funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant 5U01AR052171 to University of Washington. Information on the “Dynamic Assessment of Patient-Reported Chronic Disease Outcomes” can be found at http://nihroadmap.nih.gov/clinicalresearch/index.asp
Footnotes
The authors have no conflicts of interest.
References
- Alschuler KN, Ehde DM, Jensen MP. The co-occurrence of pain and depression in adults with multiple sclerosis. Rehabilitation psychology. 2013;58(2):217–221. doi: 10.1037/a0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW, Ellenberg JH, Leventhal CM, Reingold SC, Rodriguez M, Silberberg DH. Revised estimate of the prevalence of multiple sclerosis in the United States. Annals of Neurology. 1992;31(3):333–336. doi: 10.1002/ana.410310317. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Anens E, Emtner M, Zetterberg L, Hellstrom K. Physical activity in subjects with multiple sclerosis with focus on gender differences: a survey. BMC Neurol. 2014;14:47. doi: 10.1186/1471-2377-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald CJ, McGrath PJ, Ritvo PG, Fisk JD, Bhan V, Maxner CE, Murray TJ. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain. 1994;58(1):89–93. doi: 10.1016/0304-3959(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Multiple Sclerosis. 2008;14(8):1127–1130. doi: 10.1177/1352458508092807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Schwartz C, Saris-Baglama RN, Kosinski M, Bjorner JB. Using item response theory (IRT) for developing and evaluating the pain impact questionnaire (PIQ-6 (TM)) Pain Medicine. 2007;8:S129–S144. [Google Scholar]
- Bentler PM. Multivariate-Analysis with Latent-Variables - Causal-Modeling. Annual Review of Psychology. 1980;31:419–456. [Google Scholar]
- Bentler PM. Comparative Fit Indexes in Structural Models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Broadley SA, Deans J, Sawcer SJ, Clayton D, Compston DA. Autoimmune disease in first-degree relatives of patients with multiple sclerosis. A UK survey. Brain : a journal of neurology. 2000;123(Pt 6):1102–1111. doi: 10.1093/brain/123.6.1102. [DOI] [PubMed] [Google Scholar]
- Brown GK. A causal analysis of chronic pain and depression. Journal of abnormal psychology. 1990;99(2):127–137. doi: 10.1037//0021-843x.99.2.127. [DOI] [PubMed] [Google Scholar]
- Browne M, Ceduk R. Alternate ways of assessing model fit. In: Bollen K, Long J, editors. Testing structural equation models. London, England: Sage; 1993. pp. 136–162. [Google Scholar]
- Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862–1868. doi: 10.1176/appi.ajp.159.11.1862. [DOI] [PubMed] [Google Scholar]
- Chwastiak LA, Gibbons LE, Ehde DM, Sullivan M, Bowen JD, Bombardier CH, Kraft GH. Fatigue and psychiatric illness in a large community sample of persons with multiple sclerosis. J Psychosom Res. 2005;59(5):291–298. doi: 10.1016/j.jpsychores.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Clark DA. Cognitive Behavioral Therapy for Anxiety and Depression: Possibilities and Limitations of a Transdiagnostic Perspective. Cogn Behav Ther. 2009;1 [PubMed] [Google Scholar]
- Cleeland CS. Measurement of pain by subjective report. In: Chapman C, editor. Advances in Pain Research and Management. Vol. 12. New York, New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- Cook TD, Campbell DT. Quasi-experimentation: Design and analysis issues for field settings. Boston: Houghton Mifflin; 1979. [Google Scholar]
- Davis PJ, Reeves JL, Hastie BA, Graff-Radford SB, Naliboff BD. Depression determines illness conviction and pain impact: a structural equation modeling analysis. Pain Medicine. 2000;1(3):238–246. doi: 10.1046/j.1526-4637.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Gibbons LE, Chwastiak L, Bombardier CH, Sullivan MD, Kraft GH. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler. 2003;9(6):605–611. doi: 10.1191/1352458503ms939oa. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Osborne TL, Jensen MP. Chronic pain in persons with multiple sclerosis. Physical medicine and rehabilitation clinics of North America. 2005;16(2):503–512. doi: 10.1016/j.pmr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. The Clinical journal of pain. 1997;13(2):116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1994;21(1):9–14. [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Gerrits MM, Vogelzangs N, van Oppen P, van Marwijk HW, van der Horst H, Penninx BW. Impact of pain on the course of depressive and anxiety disorders. Pain. 2012;153(2):429–436. doi: 10.1016/j.pain.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep medicine. 2005;6(1):41–44. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hilderink PH, Burger H, Deeg DJ, Beekman AT, Oude Voshaar RC. The temporal relation between pain and depression: results from the longitudinal aging study Amsterdam. Psychosomatic medicine. 2012;74(9):945–951. doi: 10.1097/PSY.0b013e3182733fdd. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- Kalia LV, O'Connor PW. Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Multiple Sclerosis. 2005;11(3):322–327. doi: 10.1191/1352458505ms1168oa. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York: The Guilford Press; 1998. [Google Scholar]
- Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006;66(11):1696–1702. doi: 10.1212/01.wnl.0000218309.01322.5c. [DOI] [PubMed] [Google Scholar]
- Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Multiple Sclerosis. 2007;13(1):67–72. doi: 10.1177/1352458506071161. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225–234. doi: 10.2165/00023210-200317040-00002. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435–437. doi: 10.1001/archneur.1988.00520280085020. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Larocca NG, Muirnash J, Steinberg AD. The Fatigue Severity Scale - Application to Patients with Multiple-Sclerosis and Systemic Lupus-Erythematosus. Archives of Neurology. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, Shumaker SA. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale. Psychological Assessment. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocchia M, Keller S, Ware JE. Sleep problems, health-related quality of life, work functioning and health care utilization among the chronically ill. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2001;10(4):331–345. doi: 10.1023/a:1012299519637. [DOI] [PubMed] [Google Scholar]
- Marsh HW. In search of golden rules: Comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler's (1999) findings. Structural Equation Modeling-a Multidisciplinary Journal. 2004;11(3):320–341. [Google Scholar]
- Merlino G, Fratticci L, Lenchig C, Valente M, Cargnelutti D, Picello M, Gigli GL. Prevalence of 'poor sleep' among patients with multiple sclerosis: an independent predictor of mental and physical status. Sleep medicine. 2009;10(1):26–34. doi: 10.1016/j.sleep.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Mills RJ, Young CA. The relationship between fatigue and other clinical features of multiple sclerosis. Mult Scler. 2011;17(5):604–612. doi: 10.1177/1352458510392262. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of general psychiatry. 2007;64(6):651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Goodkin DE, Likosky W, Gatto N, Baumann KA, Rudick RA. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol. 1997;54(5):531–533. doi: 10.1001/archneur.1997.00550170015009. [DOI] [PubMed] [Google Scholar]
- Motl RW, McAuley E. Symptom cluster as a predictor of physical activity in multiple sclerosis: preliminary evidence. J Pain Symptom Manage. 2009;38(2):270–280. doi: 10.1016/j.jpainsymman.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Motl RW, Suh Y, Weikert M. Symptom cluster and quality of life in multiple sclerosis. J Pain Symptom Manage. 2010;39(6):1025–1032. doi: 10.1016/j.jpainsymman.2009.11.312. [DOI] [PubMed] [Google Scholar]
- Motl RW, Weikert M, Suh Y, Dlugonski D. Symptom cluster and physical activity in relapsing-remitting multiple sclerosis. Res Nurs Health. 2010;33(5):398–412. doi: 10.1002/nur.20396. [DOI] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31(4):739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Newland PK, Fearing A, Riley M, Neath A. Symptom clusters in women with relapsing-remitting multiple sclerosis. J Neurosci Nurs. 2012;44(2):66–71. doi: 10.1097/JNN.0b013e3182478cba. [DOI] [PubMed] [Google Scholar]
- Nicassio PM, Moxham EG, Schuman CE, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain. 2002;100(3):271–279. doi: 10.1016/S0304-3959(02)00300-7. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A Heuristic for Developing Transdiagnostic Models of Psychopathology : Explaining Multifinality and Divergent Trajectories. Perspectives on Psychological Science. 2011;6:589. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- O'Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96–111. doi: 10.1016/j.pain.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Patten SB, Beck CA, Williams JV, Barbui C, Metz LM. Major depression in multiple sclerosis: a population-based perspective. Neurology. 2003;61(11):1524–1527. doi: 10.1212/01.wnl.0000095964.34294.b4. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Beaulieu S, Harris C, Hogan S, Manning OJ, Alderdice PW, Godwin M. The Canadian survey of health, lifestyle and ageing with multiple sclerosis: methodology and initial results. BMJ Open. 2014;4(7):e005718. doi: 10.1136/bmjopen-2014-005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84(1):65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139(2):349–357. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Tack B. A measure of fatigue in rheumatoid arthritis. Arthritis Care and Research. 1990;3(S13) [PubMed] [Google Scholar]
- Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008;138(2):392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Tucker LR, Lewis C. Reliability Coefficient for Maximum Likelihood Factor-Analysis. Psychometrika. 1973;38(1):1–10. [Google Scholar]
- White CP, White MB, Russell CS. Invisible and visible symptoms of multiple sclerosis: which are more predictive of health distress? The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses. 2008;40(2):85–95. 102. doi: 10.1097/01376517-200804000-00007. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]