Abstract
Primordial germ cells (PGCs) are the earliest population of germ cells established during embryonic development and constitute the beginning of the totipotent state. A recent study provides a new protocol for the efficient generation of PGC-like cells from human embryonic stem cells, providing an in vitro platform to study human PGC differentiation and specification.
Germ cells (i.e., sperm cells and oocytes) are unique cells that, upon fertilization, give rise to the totipotent zygote. They are the most important cells in the body for the generation of an organism as they transmit the necessary genetic information to form a new human being. The precursors of sperm cells and oocytes, known as primordial germ cells (PGCs), are specified around the time of gastrulation by ongoing signals between the extra-embryonic tissues and the developing epiblast. Most of the studies on PGC specification, and therefore our knowledge of gametogenesis, have involved the use of mouse embryos as a model system, partly because of the lack of a human system that would allow for the study of gametogenesis. However, several critical differences between early mouse and human embryonic development exist which limits the translatability of these findings1. In a study recently published in Cell, the groups of Azim Surani at Cambridge University and Jacob Hanna at The Weizmann Institute reported the establishment of a robust, reproducible, and efficient protocol for the generation of human PGC-like cells in vitro from human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs)2 (Figure 1).
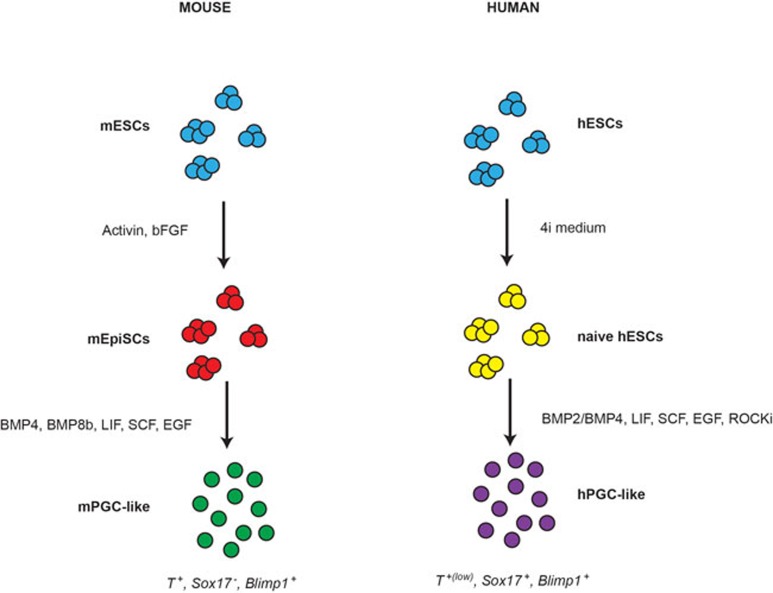
Figure 1.
Schematic representation of two protocols used for the in vitro generation of PGC-like cells in mice and humans. Mouse embryonic stem cells (mESCs) are first converted to Epiblast-like cells (mEpiSCs) via activin and bFGF treatment, then mEpiSCs are differentiated to PGC-like cells using a combination of small molecules (BMP4, BMP8b, LIF, SCF, EGF). Human ESCs (hESCs) are converted to naïve hESCs using 4i medium and then differentiated to PGC-like cells using BMP2 or BMP4 in addition to LIF, SCF, EGF, and ROCK inhibitor. mPGC-like cells express high levels of T (Brachyury) and no Sox17, while hPGC-like cells express Sox17 and low levels of T (Brachyury).
In the study, TALEN technology was first utilized to introduce the coding sequence for mCherry in the genomic locus of the PGC-specific gene Nanos3 in two male and one female hESC lines, thus creating reporters for PGC differentiation. Leveraging previous knowledge from mouse studies suggesting a higher potential of germ cell fate differentiation for cells derived from naïve mouse ESCs3,4, hESCs were converted to a more naïve state using a protocol developed by Hanna's group5. Embryoid bodies were generated from these cells and grown in medium containing BMP2 or BMP4, LIF, stem cell factor (SCF), epidermal growth factor (EGF), and Rho-kinase (ROCK) inhibitor. Within 4-5 days, about a third of the cells revealed expression of the PGC reporter. To confirm the successful generation of PGC-like cells, RNA-seq was used to compare the induced cells to human PGCs from a 7-week-old male embryo as well as to a human seminoma originating from the germline in vivo. The comparison showed that these cells had significant transcriptome similarities, although some differences in gene expression still remained. Surprisingly, SOX17, a known marker and determinant of endodermal differentiation, was identified as the key regulator and earliest marker of PGC fate specification, acting upstream of the early PGC marker BLIMP1. SOX17 was both necessary and sufficient to induce PGC-like cells as demonstrated by studies using SOX17 knockout (KO) and BLIMP1 KO hESCs, leading to the conclusion that SOX17 induces germ cell and endoderm fate while BLIMP1 acts downstream of SOX17 to suppress endoderm (and mesoderm) fate. This result is in contrast to what had been previously observed in mice, where SOX17 is not expressed in PGCs and the mesodermal determinant T (Brachyury) is required for activation of PGC determinants6,7. This suggests important differences between mouse and human PGC specification and thus highlights the need for a human-specific system to study gametogenesis.
The discovery of a robust and efficient protocol to generate hPGC-like cells opens up numerous lines of investigation into how germ cells are specified in humans and will allow for in vitro modeling human diseases linked to defective germline development. While it is not clear why non-naïve hESCs cannot be induced as efficiently as naïve hESCs to become PGC-like cells, the development of this new protocol sets the stage for the exploration of germline differentiation and maturation. Additionally, these cells constitute a novel system to study epigenetic changes in humans, as hPGC-like cells displayed early signs of DNA demethylation. Finally, these new findings have both research and clinical implications, as they represent a first step in the establishment of a way to obtain mature germ cells in vitro. Human oocytes are a limiting factor in the context of in vitro fertilization procedures as well as for basic research in the context of somatic cell nuclear transfer. Thus, the ability to differentiate mature oocytes from pluripotent stem cells will become a game changer and provide fertile grounds for the advancements of stem cell science.
References
- Irie N, Tang WW, Surani MA. Reprod Med Biol. 2014. pp. 203–215. [DOI] [PMC free article] [PubMed]
- Irie N, Weinberger L, Tang WW, et al. Cell. 2014. pp. 253–268. [DOI] [PMC free article] [PubMed]
- Hayashi K, Ogushi S, Kurimoto K, et al. Science. 2012. pp. 971–975. [DOI] [PubMed]
- Hayashi K, Ohta H, Kurimoto K, et al. Cell. 2011. pp. 519–532. [DOI] [PubMed]
- Gafni O, Weinberger L, Mansour AA, et al. Nature. 2013. pp. 282–286. [DOI] [PubMed]
- Aramaki S, Hayashi K, Kurimoto K, et al. Dev Cell. 2013. pp. 516–529. [DOI] [PubMed]
- Bernardo AS, Faial T, Gardner L, et al. Cell Stem Cell. 2011. pp. 144–155. [DOI] [PMC free article] [PubMed]