Abstract
Episodic angioedema with eosinophilia (Gleich syndrome) is a rare disorder characterized by episodes of angioedema and eosinophilia that occur at monthly intervals and resolve spontaneously without therapy. Despite the striking periodicity of this disorder, its similarity to other cyclic hematopoietic disorders with multilineage involvement has not been assessed. To characterize the involvement of cell lineages in the etiology and pathogenesis of episodic angioedema with eosinophilia, four subjects were evaluated by blood counts and other analyses over the course of 1–2 months. Surface marker expression was assessed on T cells by flow cytometry and clonality by polymerase chain reaction. Intracellular cytokine evaluation, bone marrow and skin biopsies were performed during different parts of the cycle. Cycling of multiple cell lineages, including neutrophils, lymphocytes and eosinophils, was observed in the four subjects with the disorder with a periodicity of 25–35 days. An aberrant CD3−CD4+ T-cell population was detected in all four subjects, and T-cell receptor rearrangement studies showed a clonal pattern in three subjects. A peak of type II cytokines was detected in the serum of subjects prior to the onset of symptoms and eosinophil cycling and corresponded to ex-vivo type II cytokines detected intracellularly in CD3+CD4+CD154+ T cells. Although the etiology of episodic angioedema with eosinophilia is not yet known, multiple lineages, including lymphocytes, neutrophils and mast cells, are involved and may be related to disease pathogenesis. Whether these cells act directly or promote eosinophilia and eosinophil activation remains to be elucidated. All subjects gave informed consent and were evaluated under an Institutional Review Board-approved protocol (NCT00001406).
Introduction
Episodic angioedema with eosinophilia (EAE), also known as Gleich syndrome, is a rare disorder characterized by recurrent episodes of urticaria, fever, angioedema, weight gain and dramatic eosinophilia that occur at 3–4 week intervals and resolve with spontaneous diuresis in the absence of therapy.1 Although the syndrome is often classified in the broad category of idiopathic hypereosinophilic syndromes (HES),2 EAE is a distinct eosinophilic syndrome that is remarkably homogenous in clinical presentation, suggesting a common etiology in affected subjects. Early studies described cyclic elevations of serum interleukin (IL)-5 preceding the rise in eosinophilia, increased numbers of activated T cells and eosinophilic degranulation in the dermis during symptomatic episodes,1,3 supporting the hypothesis that activation of blood and tissue eosinophils by T lymphocytes drives EAE. More recently, cycling of additional serum cytokines, including IL-3, IL-6, IL-1 and soluble IL-2 receptor (sIL-2R), has been described in isolated case reports,4,5 although the sources of these cytokines have not been determined, and some cytokines, such as IL-6, reach maximum levels only after the peak of eosinophilia.5 The presence of a clonal T-cell population with an aberrant CD3−CD4+ surface phenotype, commonly present in the lymphocytic variant of HES, has been reported in three patients with EAE.6–8
Whereas patients with the lymphocytic variant of HES can present with intermittent angioedema and/or urticaria,9 the regular periodicity of eosinophilia and symptoms in EAE is a distinguishing feature that is reminiscent of other cyclic hematologic disorders, such as cyclic neutropenia10,11 and cyclic thrombocytopenia.12,13 Of note, cycling of multiple additional cell lineages, including eosinophils, platelets, monocytes and lymphocytes, has been reported in patients with these disorders.10,13 Furthermore, populations of abnormal lymphocytes, specifically large granular lymphocytes, are often present in patients with the adult onset form of cyclic neutropenia11 and have been described in cyclic thrombocytopenia.12 Although mutations in neutrophil elastase (ELANE)14 have been identified in many patients with cyclic neutropenia, the role of these mutations in neutrophil cycling remains controversial.
Unlike patients with cyclic neutropenia, who require therapy once the diagnosis is made to prevent life-threatening complications of neutropenia, patients with EAE can often be followed without therapy providing a unique opportunity to investigate the etiology and pathogenesis of this disorder. In the present study, we identified four patients with definite EAE and monitored their clinical and laboratory characteristics, including absolute cell counts and cytokine responses, over the course of an entire cycle. Our data confirm that eosinophils are likely the primary cell responsible for the pathogenesis of clinical symptoms in EAE; however, cyclic variation in the numbers of peripheral blood cells other than eosinophils, including neutrophils and lymphocytes, was observed in all four patients, suggesting that EAE is a multilineage cell cycling disorder.
Methods
Baseline clinical assessment of study subjects
All subjects signed informed consent, and research was performed under an Institutional Review Board-approved protocol (NCT00001406) to evaluate subjects with eosinophilia. Four subjects with suspected EAE underwent a thorough history-taking and physical examination with attention paid to timing of the cycling and exclusion of iatrogenic causes of episodic eosinophilia, including intermittent administration of corticosteroids. Baseline diagnostic testing included complete blood count with differential, routine blood chemistry, creatine kinase, lactate dehydrogenase, C-reactive protein, erythrocyte sedimentation rate, total serum immunoglobulin, vitamin B12 and tryptase levels, assessment for the FIP1L1/PDGFRA mutation by reverse-transcriptase polymerase chain reaction (RT-PCR), electrocardiography, echocardiography, spirometry with lung volumes and compute tomography scans of the chest, abdomen and pelvis. T-cell clonality was determined in peripheral blood by PCR of T-cell receptor (TCR)-γ rearrangement patterns.15 Additional studies to assess end organ involvement were performed as clinically indicated.
Longitudinal assessment of clinical and laboratory parameters
Complete blood counts with differential were performed and serum collected every 2–3 days over the course of 1–2 months in four subjects. During this time, subjects maintained a symptom diary. Serum was stored at −80°C.
Serological assessments
Serum IL-5, IL-8, IL-9, IL-13, sIL-2Rα, eotaxin-1, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), interferon (IFN)-γ, macrophage inflammatory protein (MIP)-1-β, tumor necrosis factor (TNF-α) and IL-1-β levels (limits of detection listed in the Online Supplementary Material) were assessed by suspension array technology in multiplex (Millipore). Serum CCL17/TARC (R&D) and total serum tryptase were quantified as previously described.15,16 Stem cell factor (SCF) was assessed by an enzyme-linked immunosorbent assay according to the manufacturer’s (R&D) instructions; the limit of detection was 9 pg/mL. Total serum IgE levels were determined by ImmunoCAP (Phadia).
Whole blood flow cytometry
Whole blood was collected in EDTA tubes, diluted with phosphate-buffered saline and spun at 1000 rpm at room temperature. Following aspiration of the supernatant, 100 μL of the well-mixed pellet were added to polystyrene tubes, pre-wetted with 0.5 mL of 5% fetal calf serum, and stained with Blue-O LSA-1 and Blue-O LSA-2 (Blue Ocean Medical, FL, USA) at 4°C for 30 min. Red blood cells were lysed with FACS lysing solution (BD Biosciences). Cells were washed and fixed with 1.0% formaldehyde prior to analysis within 2 h on a Blue Ocean CR 300 automated instrument.
Intracellular cytokine analysis
Peripheral blood mononuclear cells were isolated using density gradient separation (Ficoll-Paque Plus, GE Healthcare) and cryo-preserved in liquid nitrogen. The thawed peripheral blood mononuclear cells were stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin and stained with a panel of antibodies to detect intracellular cytokines (IL-4, IL-13, IL-5, and IFN-γ) in CD4+ cells. Detailed methods are provided in the Online Supplement.
Immunohistochemistry
Skin and bone marrow core biopsies were stained with hematoxylin & eosin for morphological examination. Immunohistochemical staining for eosinophil peroxidase and tryptase, CD3 and CD4 were performed and are further described in the Online Supplement.
Sequencing of the gene for neutrophil elastase, ELANE
DNA was purified from frozen peripheral blood mononuclear cells using the Gentra Puregene kit (Qiagen) and three fragments of DNA spanning all five exons of ELANE were amplified using published primers.17 PCR products were eluted from bands cut out of 2% agarose gels and sequenced using standard capillary electrophoresis (Macrogen, Inc.).
Results
Clinical and laboratory characteristics of the study subjects
Four subjects (subjects 1, 3 and 4 off therapy, subject 2 on stable, low-dose prednisone) were determined to have definite EAE based on differential blood counts over a period of 42–70 days (Figure 1). A fifth subject with probable EAE had clearly documented episodic cycling in the past during a serial collection, but took a burst of corticosteroids to treat a flare during evaluation at our institution, precluding analysis of his data.
Figure 1.
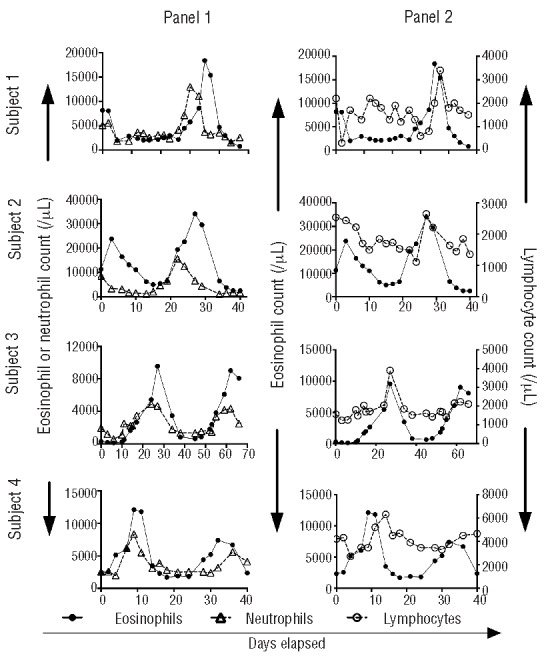
Cyclic variation in eosinophils, neutrophils and lymphocytes in subjects with EAE. Absolute numbers of eosinophils, neutrophils and lymphocytes are shown as a function of time during an entire cycle for four subjects.
Baseline demographic, clinical and laboratory characteristics of the four subjects with definite EAE are given in Table 1. Symptoms at the time of cycling were similar in all subjects and included angioedema, urticaria, fatigue, weight gain, and fever. The location and extent of angioedema varied with symptom severity, but most subjects complained of worse symptoms in the face and upper body. Inter-individual cycling time and magnitude of eosinophilia varied between the subjects, but was consistent for each subject (Table 1). All subjects, with the exception of subject 3, had persistent eosinophilia above the normal range even at the nadir of cycles. One subject experienced complete resolution of symptoms on low-dose (<5 mg/day) corticosteroid therapy, and one subject reported moderate improvement in symptoms on chronic, moderate-dose corticosteroids (10–20 mg/day). None of the subjects reported a family history of EAE. The reported onset of symptoms and eosinophilia was between 10 and 34 years of age, and despite disease durations ranging from 9–16 years, none of the subjects had evidence of chronic eosinophil-related, end-organ pathology at the time of presentation.
Table 1.
Demographic, clinical and laboratory characteristics of the four subjects with EAE.
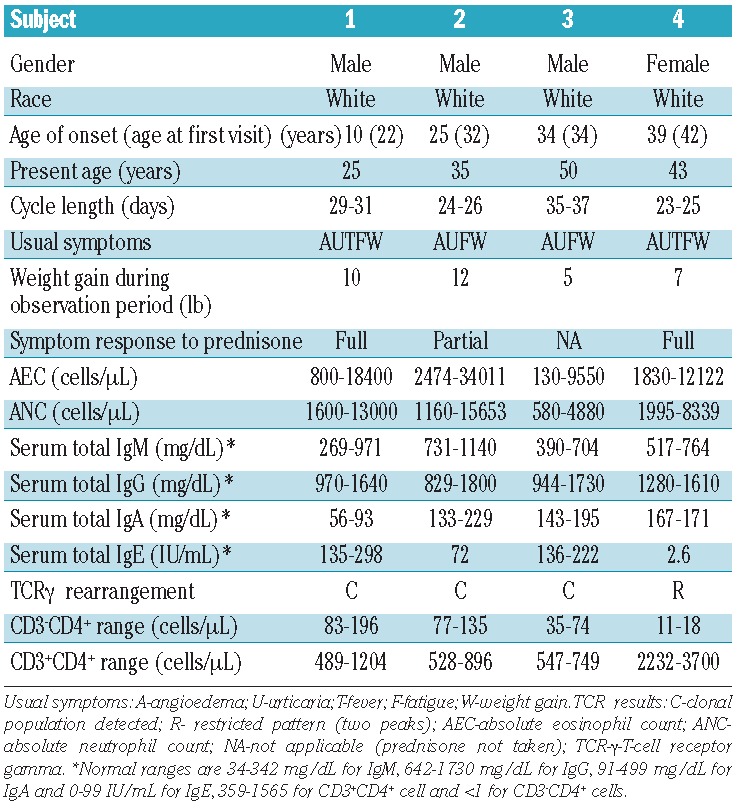
As has been previously reported in EAE,3,18 all subjects had elevated serum IgM levels on initial assessment. Three subjects also had elevated serum IgE levels. Aberrant CD3− CD4+ lymphocyte populations were detected in all four EAE subjects and clonal lymphocyte populations were detected by PCR in three of the four subjects (Table 1). Myeloproliferative features, including anemia, thrombocytopenia, elevated serum B12 and tryptase levels, were absent, and FIP1L1/PDGFRA testing was negative in all four subjects. Bone marrow aspirates from all four subjects demonstrated increased eosinophils, but no increase in blasts or cytogenetic abnormalities.
Multilineage cell cycling in episodic angioedema with eosinophilia
Serial complete blood counts demonstrated not only cyclic eosinophilia, but also cycling of other cell populations (Figure 1). The absolute neutrophil count showed a cyclic pattern in all four subjects with the peak neutrophil count preceding the peak eosinophil count in two subjects (Figure 1). Neutropenia was not observed with the exception of a single low absolute neutrophil count of 580/mL in subject 3 during one cycle. Absolute lymphocyte counts showed a cyclic pattern, peaking either with the absolute eosinophil count (subjects 2 and 3) or slightly after the cycle (subjects 1 and 4) with an approximate two-fold increase in absolute lymphocyte count around the time of the peak in all four subjects. Subject four had a milder flare during the second peak of the sample collection, which is reflected in the less impressive changes noted in cellular and cytokine changes during the second peak. The absolute monocyte and platelet counts and hemoglobin levels did not cycle, and there was no apparent relationship between absolute eosinophil count and absolute counts of other cell lineages in four hypereosinophilic subjects without EAE or in one normal control subject tested serially (2–3 times/week) over a 1-month period (data not shown).
Flow cytometric analysis was performed on whole blood from subject 1 when he was asymptomatic and at three time points surrounding the peak of eosinophilia during a cycle and from subject 3 at four separate times over the course of two cycles (Online Supplementary Figure S1). The absolute numbers of CD3+CD4+ and CD3+CD8+ T cells, CD19+ B cells and natural killer cells increased during the flare in subject 1, peaking simultaneously with the absolute eosinophil count. Although less dramatic, increased numbers of CD3+CD4+ and CD3+CD8+ T cells and CD19+ B cells were also associated with peak eosinophilia in subject 3. The absolute number of CD3− CD4+ cells was assessed in subject 1 and demonstrated a similar pattern, with the number of aberrant cells increasing from 83/μL to 196/μL at the peak of the flare (data not shown).
Bone marrow biopsies were performed at the peak and nadir of symptoms in subject 1. The aspirate smear cell counts paralleled the findings in peripheral blood with 63% eosinophils (53% of them immature) at the peak and only 12% eosinophils (48% of them immature) at the nadir (Figure 2A,B). The core biopsy was hypercellular (70%) with prominent eosinophilia at the peak, and hypocellular (35%) with mild eosinophilia at the nadir (Figure 2C,D). Immunostaining showed mild increases in CD117 and tryptase-positive mast cells (Figure 2E,F), but no increases in CD34+ blasts, CD20+ or CD5+ cells, or reticulin fibrosis at either time point.
Figure 2.
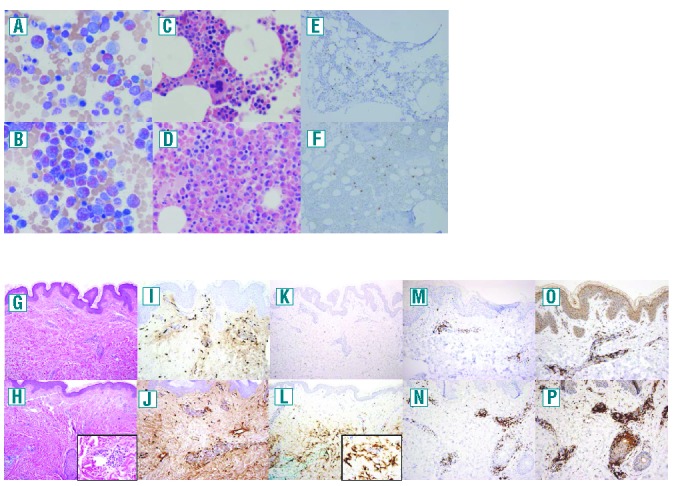
Cyclic variation in bone marrow and skin eosinophils and skin lymphocytes, but not mast cells in the skin of a subject. (A) and (B) bone marrow aspirates (500× magnification), (C)–(F) bone marrow biopsies (500× magnification) and (G)–(P) skin biopsies [(G)–(H), (K)–(L); 100× original magnification, insets 600× magnification, (I)–(J), (M)–(N), (O)–(P); 200× original magnification] during the asymptomatic (top) and symptomatic (bottom) phases of the cycle. (A)–(D), (G) and (H) are stained with hematoxylin and eosin, panels (E), (F), (I) and (J) with tryptase, and panels (K) and (I) with anti-eosinophil peroxidase antibody, (M) and (N) with anti-CD3, and (O) and (P) with anti-CD4.
Mutations in the gene encoding neutrophil elastase (ELANE) are common in patients with cyclic neutropenia and have been implicated in the pathogenesis of the disorder.19 Neutrophil elastase is also a potent activator of eosinophils.20 Since neutrophil cycling was observed in all four subjects with definite EAE and preceded the eosinophilia in two subjects, the coding regions of ELANE were sequenced using DNA from three subjects and two normal controls. No polymorphisms were identified in the DNA from the EAE subjects compared to normal controls or the published reference sequence (NCBI Accession AC_Y00477).
Longitudinal analysis of serum and intracellular cytokine levels
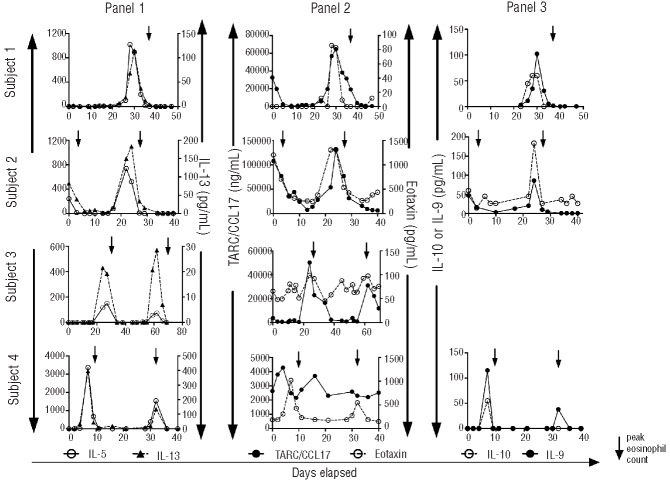
Serum levels of the type II cytokines, IL-5, IL-13, IL-9 and IL-10, showed cyclic variation and peaked prior to the peak of eosinophilia in all four subjects (Figure 3). A similar pattern was also seen with the eosinophil and CD4+ recruiting chemokines, eotaxin-1 in all four subjects and TARC/CCL17 in subjects 1–3 but not subject 4 (Figure 3). Despite occasional variation in levels of serum GM-CSF, G-CSF, IFN-γ, MIP-1-β, TNF-α and IL-1-β and IL-6, there was no clear relationship between serum levels of any of these cytokines or chemokines and the onset of symptoms or absolute eosinophil count (data not shown).
Figure 3.
Cyclic variation in serum cytokine levels in subjects with EAE. Serum levels of IL-5 and IL-13 (panel 1), CCl17/TARC and eotaxin (panel 2) and IL-9 and IL-10 (panel 3) are shown as a function of time during an entire cycle for four subjects. Symbols represent individual measurements, and an arrow indicates the peak eosinophil count during a cycle.
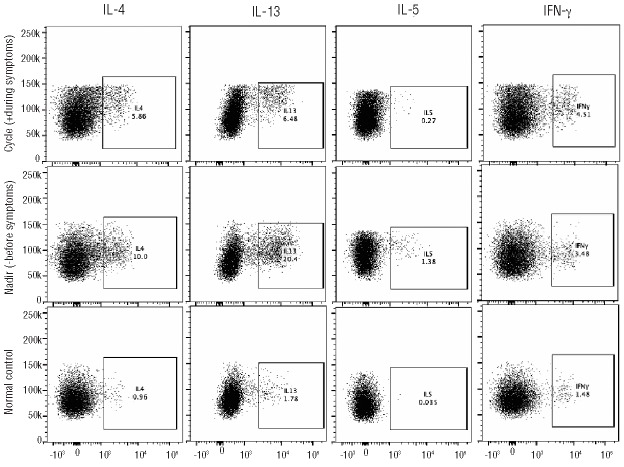
In order to explore the role of lymphocytes as a possible source of the type II cytokine variation, peripheral blood mononuclear cells were obtained from subject 1 near the peak (8 days after onset of symptoms), and nadir (7 days prior to onset of symptoms, and 14 days prior to the peak of the next cycle) of eosinophilia, stimulated with PMA/ionomycin and assessed for cytokine production using intracellular flow cytometry. Elevated levels of IL-4, IL-13, and IL-5 were detected in the CD3+CD4+CD154+ (stimulated21) population at the nadir of eosinophilia with a decline in intracellular Th2 cytokines at the peak of eosinophilia, consistent with exhaustion of intracellular cytokine stores (Figure 4). The numbers of CD3− CD4+CD154+ cells were inadequate in the frozen samples to perform intracellular staining. IL-5 mRNA expression was detected in purified CD4+ cells from each of the three definite EAE subjects tested (1/(2ΔCT)*105 from 80 to 30,000), but not in two normal controls. Although mRNA levels varied dramatically (>100-fold) in the two patients for whom samples were available from two time points during the cycle, the pattern of change was inconsistent (data not shown).
Figure 4.
Variation in intracellular production of IL-4, IL-5, IL-13 and IFN-γ by activated CD4+ lymphocytes in a subject with EAE. After gating on viable CD3+CD4+CD154+ cells, side scatter versus intracellular cytokine (% cells positive) dot plots were generated. Intracellular IL-4, IL-5, IL-13 and IFN-γ levels are shown for subject 1 before and during symptoms (nadir and cycle, respectively) and for a normal control.
Assessment of cellular involvement in skin manifestations of episodic angioedema with eosinophilia
To assess the role of different cell lineages in the pathophysiology of EAE, two skin biopsies were taken from the upper arm of subject 1, the first at the site of angioedema during symptoms, and the second, near the same site as the first biopsy, after resolution of the symptoms. Although neither skin biopsy showed an eosinophilic cellular infiltrate on routine staining (Figure 2G,H), immunohistochemical staining with antibody to eosinophil peroxidase revealed a marked increase in eosinophil granule protein deposition at the time of symptoms (Figure 2K,L) compared to the asymptomatic period. Anti-CD4 antibodies revealed an increased infiltrate during the symptomatic period (Figure 2P) with less dramatic increases in anti-CD3 (Figure 2N). In addition, tryptase immunostaining demonstrated a mild increase in mast cells [39–44/high power field (hpf)] in both biopsies (Figure 2I,J). Of note, tryptase immunostaining of bone marrow examinations performed at the same two time points also revealed a mild, but comparable, increase in mast cell numbers (above the normal range of <1 mast cell/hpf at our institution) with an average of 5.6 mast cells/hpf and 8.6 mast cells/hpf at the peak and nadir, respectively (Figure 2E,F). Too few mast cells were present in the bone marrow aspirate to quantify CD2 and CD25 expression by flow cytometry at either time point.
Total serum tryptase and SCF levels were measured at all time points in two subjects (1 and 2). Despite the relative stability of mast cell numbers in the skin (Figure 2I,J) and bone marrow (Figure 2E,F) observed in subject 1, total serum tryptase levels exhibited a cyclic pattern in all three subjects tested, varying two-fold over the course of the cycle in all three subjects tested, albeit remaining in the normal range (1–11.4 ng/mL). Serum SCF levels showed a similar pattern (Figure 5). SCF peaked before the first cycle in subject 4 and was not detected during the second eosinophil peak during a milder flare of symptoms. SCF was detectable in serum at only one time point in the normal subject who had blood drawn three times per week for 4 weeks. Mature tryptase was not detected in any of the three subjects tested.
Figure 5.
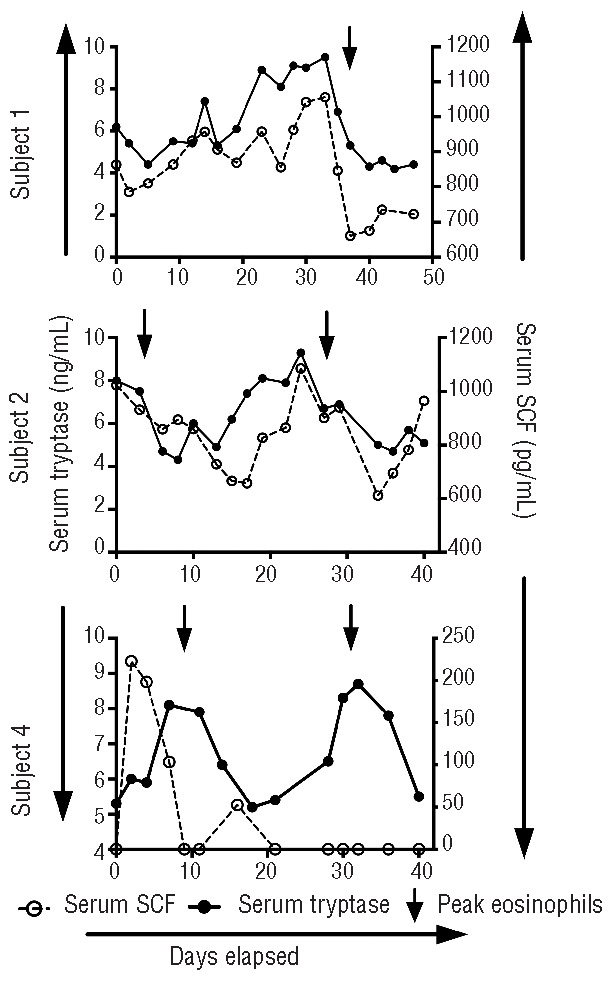
Cyclic variation of serum tryptase and SCF (limit of detection 9 pg/mL) levels in EAE. Serum levels of tryptase and SCF are shown as a function of time during an entire cycle for three subjects. Symbols represent individual measurements, and an arrow indicates the peak eosinophil count during a cycle.
Discussion
EAE is an exceedingly rare disorder with less than 50 cases reported in the literature to date. Consistent with prior case reports, the four subjects reported in the present series had cyclic eosinophilia and symptoms, including angioedema, with a defined periodicity that was preceded by a rise in serum IL-5. The age of onset (10–32 years of age) was typical and despite prolonged and dramatic eosinophilia, none of the subjects experienced chronic, end-organ manifestations typical of HES. Finally, as has been reported previously,1 all four subjects with definite EAE had elevated serum IgM levels, an uncommon laboratory abnormality in patients with HES. In fact, on review of laboratory data from 224 subjects with HES, elevated serum IgM was documented on more than one occasion in only 12 (5%) subjects in whom two or more serum IgM levels were available for analysis (unpublished data).
A novel finding in the present study was cycling of hematopoietic cell lineages other than eosinophils in all four subjects with definite EAE. This finding is similar to that in other cyclic hematopoietic disorders, including cyclic neutropenia10 and cyclic thrombocytopenia,13 and could be consistent with isolated involvement of a single lineage with secretion of cytokines or chemokines leading to secondary cycling of other cell types or with primary involvement of a hematopoietic precursor. In order to explore the etiology of the cycling in EAE, serum and intracellular cytokine and chemokine levels were examined every 2–3 days over the course of an entire cycle in four subjects on no treatment (n=3) or stable, low-dose prednisone therapy (n=1). Serum levels of type II cytokines, including the primary eosinophilopoietic cytokine, IL-5, were consistently elevated prior to the eosinophilia in all subjects and intracellular staining of mitogen-stimulated lymphocytes at the peak and nadir of the cycle demonstrated an increased capacity for production of these cytokines by CD3+CD4+ lymphocytes prior to the development of eosinophilia in two subjects, consistent with a primary role for lymphocytes in driving the eosinophilia in EAE. Since activated eosinophils are known to secrete a wide variety of mediators and cytokines,22 including the neutrophil chemoattractant IL-8,23 cyclic changes in eosinophil activation could, in turn, explain the variation in neutrophil counts. Although a primary stem cell disorder could also explain multilineage involvement in EAE, the bone marrow did not show changes in CD34+ cell numbers. Moreover, lymphocytes, but not monocytes, appear to be involved. In addition, human androgen receptor (HUMARA) assay analysis performed using purified eosinophils, neutrophils and lymphocytes from the one female subject failed to demonstrate clonality in any of the lineages tested (Online Supplementary Figure S2).
Lymphocyte-driven eosinophilia and dermatological manifestations, including urticaria and angioedema, are characteristic of the lymphocytic variant of HES. Aberrant and/or clonal lymphocytes, most commonly CD3−CD4+, have been shown to produce increased amounts of type II cytokines, driving the eosinophilia in these patients. All four of the subjects with EAE in the present study had detectable CD3−CD4+ aberrant T cells that were increased in number at the peak of eosinophilia in those patients seen at the time of symptoms. In three subjects, clonality could be demonstrated by TCR rearrangement studies. Unfortunately, there were insufficient numbers of aberrant CD3−CD4+ cells to assess intracellular cytokine production in frozen samples.
Angioedema and urticaria are typically associated with mast cell and basophil release. Despite the presence of multilineage cycling in the peripheral blood, the skin biopsy findings in the current study are most consistent with a primary role for eosinophils in the angioedema of EAE. The presence of eosinophil granules in the skin of patients with EAE has been previously reported.1 Not only can intact eosinophil granules release pre-formed cytokines, including IL-4,24 which could lead to mast cell activation, but eosinophil granule proteins have been shown to activate mast cells directly in vitro,25–26 to increase vasopermeability,27,28 and to produce erythematous, indurated palpable lesions when injected intradermally in rabbits and guinea pigs.27 Although eosinophil granule proteins can cause vasopermeability independently of histamine,28 the association with cycling serum tryptase and SCF, as well as mild non-cyclic increases in skin and bone marrow mast cell numbers in these subjects, suggests that mast cells play a role in the clinical manifestations of EAE.
In summary, the present study provides evidence that EAE is a multilineage cell cycling disorder associated with the presence of aberrant and/or clonal T-cell populations and eosinophil-driven pathology. Although the intracellular cytokine profile in T lymphocytes suggests that the dramatic eosinophilia and eosinophil activation may be lymphocyte-driven, the etiology of the cycling in EAE, as well as in the other cyclic hematologic disorders, remains to be elucidated.
Acknowledgments
We acknowledge clinical providers who provided support and care for the patients, including Cheryl Talar-Williams and JeanAnne Ware, Jodie Keary for assistance with the HUMARA assay, as well as the patients who donated a significant amount of their time, blood, and tissue samples.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
Division of Intramural Research, NIAID, National Institutes of Health; Clinical Trials: NCT00001406. This project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract N. HHSN2610080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the National Institute of Allergy and Infectious Diseases.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Gleich GJ, Schroeter AL, Marcoux JP, et al. Episodic angioedema associated with eosinophilia. N Engl J Med. 1984;310(25): 1621–1626. [DOI] [PubMed] [Google Scholar]
- 2.Klion AD, Bochner BS, Gleich GJ, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117(6):1292–1302. [DOI] [PubMed] [Google Scholar]
- 3.Butterfield JH, Leiferman KM, Abrams J, et al. Elevated serum levels of interleukin-5 in patients with the syndrome of episodic angioedema and eosinophilia. Blood. 1992; 79(3):688–692. [PubMed] [Google Scholar]
- 4.Putterman C, Barak V, Caraco Y, et al. Episodic angioedema with eosinophilia: a case associated with T cell activation and cytokine production. Ann Allergy. 1993;70(3):243–248. [PubMed] [Google Scholar]
- 5.Tillie-Leblond I, Gosset P, Janin A, et al. Increased interleukin-6 production during the acute phase of the syndrome of episodic angioedema and hypereosinophilia. Clin Exp Allergy. 1998;28(4):491–496. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez Delgado P, de la Sen Fernandez ML, Soriano Gomis V, et al. Cyclical hypereosinophilia with skin manifestations and a clonal T cell population. J Investig Allergol Clin Immunol. 2008;18(5):401–403. [PubMed] [Google Scholar]
- 7.Morgan SJ, Prince HM, Westerman DA, et al. Clonal T-helper lymphocytes and elevated IL-5 levels in episodic angioedema and eosinophilia (Gleich’s syndrome). Leuk Lymphoma. 2003;44(9):1623–1625. [DOI] [PubMed] [Google Scholar]
- 8.Novembre E, Mori F, Arcangeli F, et al. High intracytoplasmatic levels of Il-4 and Il-5 in a patient with Gleich’s syndrome: case report. Int J Immunopathol Pharmacol. 2006;19(4): 935–938. [DOI] [PubMed] [Google Scholar]
- 9.Roufosse F, Cogan E, Goldman M. The hypereosinophilic syndrome revisited. Annu Rev Med. 2003;54:169–184. [DOI] [PubMed] [Google Scholar]
- 10.Engelhard D, Landreth KS, Kapoor N, et al. Cycling of peripheral blood and marrow lymphocytes in cyclic neutropenia. Proc Natl Acad Sci USA. 1983;80(18):5734–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loughran TP, Hammond WP. Adult-onset cyclic neutropenia is a benign neoplasm associated with clonal proliferation of large granular lymphocytes. J Exp Med. 1986;164(6):2089–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty PF, Stetler-Stevenson M, Pereira A, et al. Large granular lymphocytic proliferation-associated cyclic thrombocytopenia. Am J Hematol. 2005;79(4):334–336. [DOI] [PubMed] [Google Scholar]
- 13.Fureder W, Mitterbauer G, Thalhammer R, et al. Clonal T cell-mediated cyclic thrombocytopenia. Br J Hematol. 2002;119(4):1059–1061. [DOI] [PubMed] [Google Scholar]
- 14.Dale DC, Person RE, Bolyard AA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96(7):2317–2322. [PubMed] [Google Scholar]
- 15.Roufosse F, de Lavareille A, Schandene L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(4):828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz LB, Min HK, Ren S, et al. Tryptase precursors are preferentially and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, Mono-Mac-6 cells, and human skin-derived mast cells. J Immunol. 2003;170(11):5667–5673. [DOI] [PubMed] [Google Scholar]
- 17.Ancliff PJ, Gale RE, Liesner R, et al. Mutations in the ELA2 gene encoding neutrophil elastase are present in most patients with sporadic severe congenital neutropenia but only in some patients with the familial form of the disease. Blood. 2001;98(9):2645–2650. [DOI] [PubMed] [Google Scholar]
- 18.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008; 38(5):709–750. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz MS, Corey SJ, Grimes HL, et al. ELANE mutations in cyclic and severe congenital neutropenia: genetics and pathophysiology. Hematol Oncol Clin North Am. 2013;27(1):19–41, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Lazarus SC, Caughey GH, et al. Neutrophil elastase and elastase-rich cystic fibrosis sputum degranulate human eosinophils in vitro. Am J Physiol. 1999; 276(1 Pt 1):L28–34. [DOI] [PubMed] [Google Scholar]
- 21.Shipkova M, Wieland E. Surface markers of lymphocyte activation and markers of cell proliferation. Clin Chim Acta. 2012;413(17–18):1338–1349. [DOI] [PubMed] [Google Scholar]
- 22.Spencer LA, Szela CT, Perez SA, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85(1):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousefi S, Hemmann S, Weber M, et al. IL-8 is expressed by human peripheral blood eosinophils. Evidence for increased secretion in asthma. J Immunol. 1995;154(10):5481–5490. [PubMed] [Google Scholar]
- 24.Neves JS, Perez SAC, Spencer LA, et al. Eosinophil granules function extracellularly as receptor mediated secretory organelles. PNAS. 2008;105(47):18478–18483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell MC, Ackerman SJ, Gleich GJ, et al. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J Exp Med, 1983;157(6):1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheutlin LM, Ackerman SJ, Gleich GJ, et al. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. J Immunol. 1984;133(4):2180–2185 [PubMed] [Google Scholar]
- 27.Plager DA, Davis MD, Andrews AG, et al. Eosinophil ribonucleases and their cutaneous lesion-forming activity. J Immunol. 2009;183(6):4013–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minnicozzi M, Duran WN, Gleich GJ, et al. Eosinophil granule proteins increase microvascular macromolecular transport in the hamster cheek pouch. J Immunol. 1994;153(6):2664–2670. [PubMed] [Google Scholar]