Abstract
Refractory cytopenia of childhood is the most common type of childhood myelodysplastic syndrome. Because the majority of children with refractory cytopenia have a normal karyotype and a hypocellular bone marrow, differentiating refractory cytopenia from the immune-mediated bone marrow failure syndrome (very) severe aplastic anemia can be challenging. Flow cytometric immunophenotyping of bone marrow has been shown to be a valuable diagnostic tool in differentiating myelodysplastic syndrome from non-clonal cytopenias in adults. Here, we performed the first comprehensive flow cytometric analysis of immature myeloid, lymphoid cells and erythroid cells, and granulocytes, monocytes, and lymphoid cells in bone marrow obtained from a large prospective cohort of 81 children with refractory cytopenia. Children with refractory cyotopenia had a strongly reduced myeloid compartment, but not as severe as children with aplastic anemia. Furthermore, the number of flow cytometric abnormalities was significantly higher in children with refractory cytopenia than in healthy controls and in children with aplastic anemia, but lower than in advanced myelodysplastic syndrome. We conclude that flow cytometric immunophenotyping could be a relevant addition to histopathology in the diagnosis of refractory cytopenia of childhood. (The multi-center studies EWOG-MDS RC06 and EWOG-MDS 2006 are registered at clinicaltrials.gov identifiers 00499070 and 00662090, respectively).
Introduction
Myelodysplastic syndrome (MDS) in childhood is rare and has an annual incidence of 0.8 to 1.8 per million children aged 0 to 14 years.1–3 Refractory cytopenia of childhood (RCC), defined as myelodysplasia without an increased blast count, is the most common variant of pediatric MDS. Based on the 2008 WHO criteria,4 a distinction is made between RCC and the immune-mediated bone marrow (BM) failure syndrome (very) severe aplastic anemia ((v)SAA), with presence or absence of patchy erythropoiesis, respectively, as main differentiating parameter.5 Nonetheless, because the majority of children with RCC have a normal karyotype and 80% of patients have a hypocellular BM, differentiating RCC from (v)SAA can be challenging.4,6 Similar challenges are encountered in distinguishing (v)SAA and other non-clonal cytopenias from low-grade MDS in adults, especially in cases without specific morphological or cytogenetic aberrations. Flow cytometric immunophenotyping is a valuable addition to morphology in the diagnosis of MDS in adults.7 Abnormalities detected by flow cytometry in myelomonocytic, erythroid and/or myeloid blast cells8–17 can be of diagnostic and prognostic relevance in adult MDS.18–20
In pediatric MDS, only a limited number of flow cytometric immunophenotyping studies have been reported. In advanced pediatric MDS, CD7 expression on myeloid blast cells was described to correlate with dismal survival.21 We recently showed that, in RCC, a simple and reproducible flow cytometric scoring system, described by Ogata and others as a diagnostic tool in adult low-grade MDS,15,17 cannot be applied due to its low sensitivity.22
In the present study, we performed a comprehensive flow cytometric analysis of the maturing granulocytic, monocytic, and erythroid lineages in BM aspirates of 81 RCC patients, collected prospectively by the European Working Group of MDS in Childhood (EWOG-MDS), and in BM aspirates of healthy controls, advanced MDS and (v)SAA patients. Our aims were three-fold. First, to describe the immunophenotypic characteristics of RCC compared to healthy and pathological controls. Second, to correlate flow cytometric findings with clinical characteristics of RCC patients. And third, to assess whether flow cytometry can be of value in differentiating RCC from (v)SAA.
Methods
Patients and controls
BM samples for flow cytometric immunophenotyping were obtained from 81 primary RCC patients aged 18 years of age or under, who had not previously been treated with immunosuppressive therapy (IST). Consecutive patients, diagnosed between June 2005 and December 2011, were included from the prospective, multi-center studies EWOG-MDS RC06 and EWOG-MDS 2006. Institutional review boards of participating institutions approved the studies and patients and/or parents or legal guardians of patients provided written informed consent for study participation in accordance with local laws and regulations. RCC was diagnosed according to WHO criteria4 and confirmed by central review of BM morphology and histology in participating national study centers by reference pathologists of EWOG-MDS. BM samples were obtained from 17 pediatric (v)SAA patients (median age 9.6 years; range 1.6–18.1 years), 7 pediatric patients with advanced MDS [refractory anemia with excess blasts (RAEB) or refractory anemia with excess blasts in transformation (RAEB-t)] (median age 14.3; range 3.9–17.7 years) diagnosed according to WHO criteria4 by reference pathologists of EWOG-MDS, and from 9 healthy adult stem cell donors who served as controls. The histopathological differentiation between RCC and (v)SAA is mainly based on the presence of patchy erythropoiesis with defective maturation and/or the presence of micromegakaryocytes in RCC, and the absence of erythropoiesis and megakaryopoiesis in (v)SAA.5 It was recently shown that a reproducible distinction between RCC and (v)SAA can be made based on these criteria.5 In the RCC cohort, PNH clones and T-cell receptor Vβ skewing were detected, as described previously.23,24 Further details may be found in the Online Supplementary Appendix.
Bone marrow immunophenotyping by flow cytometry
BM samples were collected in heparinized tubes, sent to the Erasmus MC, and generally analyzed within 24 h of collection. A minimum of 500 μL BM was lysed with NH4Cl, washed, re-suspended in 350 μL PBS/BSA or to a maximum concentration of 20×106 cells/mL, and 50 μL aliquots were stained for 15 min at room temperature with a monoclonal antibody panel as described in Online Supplementary Table S1. Cells were acquired on a FACSCanto II flow cytometer (BD Biosciences), and data were analyzed using Infinicyt software (Cytognos, Salamanca, Spain) or BD FACSDiva Software, v.6.1.2 (BD Biosciences). Gating strategies and cell population definitions are described in the Online Supplementary Appendix.
Parameters that were evaluated in myeloid and lymphoid progenitor cells, maturing granulocytes, monocytes and erythrocytes are described in Table 1, and are largely in line with European LeukemiaNet (ELN) recommendations for flow cytometry in MDS.7 Maturation patterns of the myelomonocytic lineage, and expression of CD markers on myeloid blast cells, granulocytes and monocytes were compared with reference images (Infinicyt settings: resolution 128, maximum density increment, linear) that were generated, depending on the combination of antibodies used, by merging flow cytometric data from 6–8 normal BM samples (100,000 events per sample, merged into 100,000 events). Maturation patterns and expression of CD markers were scored as aberrant when the expression of the analyzed population deviated more than 0.5 log decade from the expression of the reference population. Granularity of myeloid blasts (CD117+), granulocytes (CD33dim), and monocytes (CD64+) was expressed as the relative SSC compared to lymphocytes. The relative granularity of myeloid blasts (CD117+), granulocytes (CD33dim), and monocytes (CD64+) of individual RCC patients or controls was scored as abnormal when the relative granularity was less than 2 SD of the mean of the relative granularity of healthy controls. When less than 50 myeloid blasts, less than 1000 granulocytes, or less than 50 immature erythroid cells were present, percentages were reported, but cell populations were not further characterized.
Table 1.
Analyzed parameters per cell population.
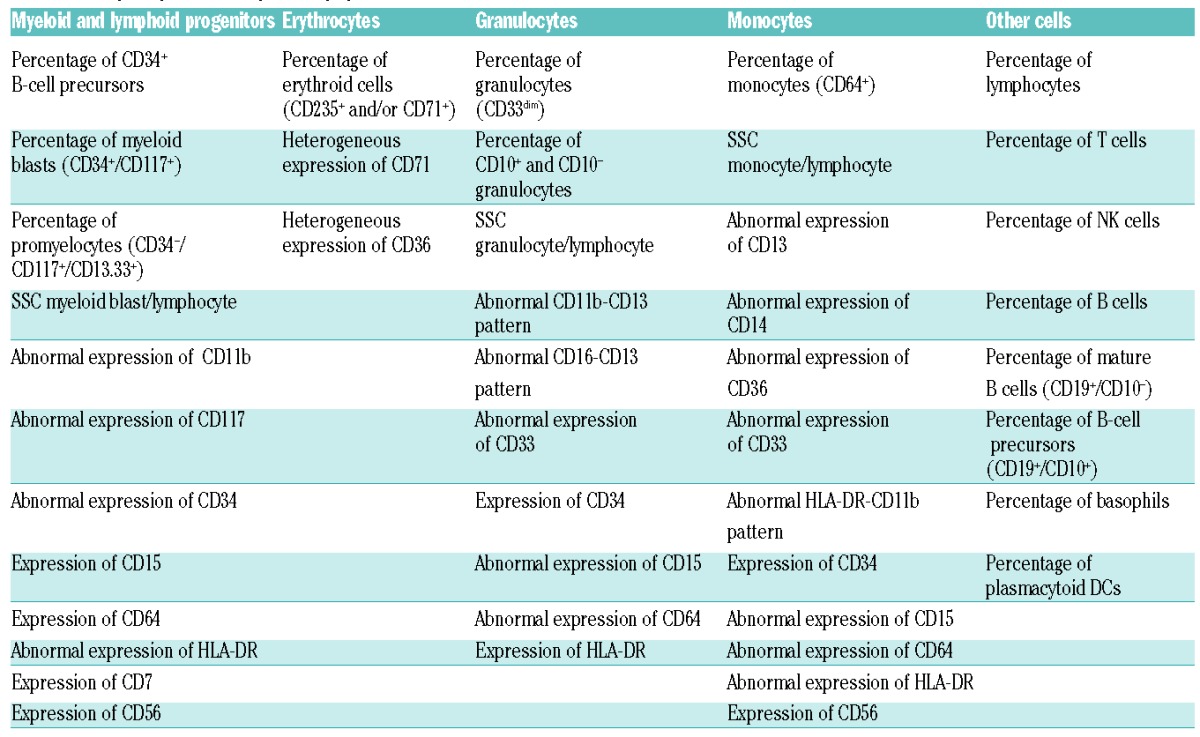
The total number of flow cytometric abnormalities was calculated by adding up all flow cytometric abnormalities described in Table 1.
Statistical analyses
Statistical analyses are described in the Online Supplementary Appendix.
Results
Patients’ characteristics
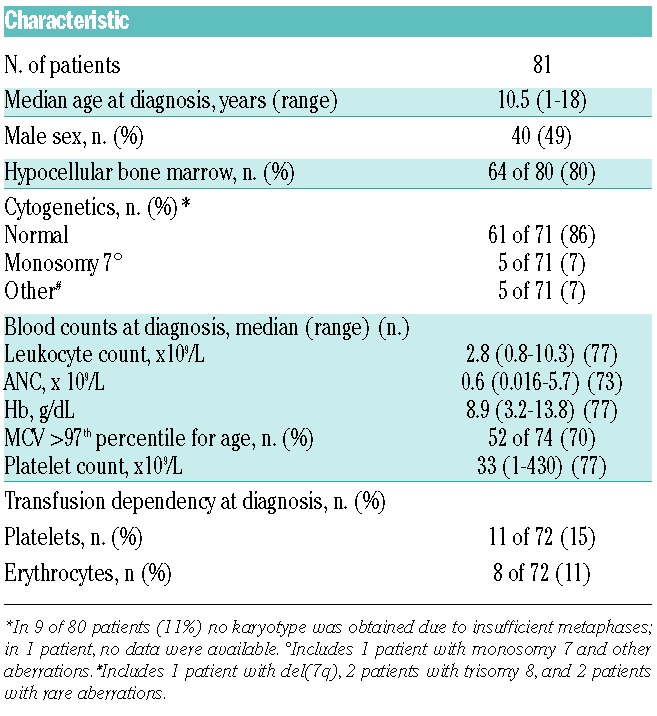
A total of 81 treatment-naïve RCC patients (40 male, 41 female), with a median age at diagnosis of 10.5 years (range 1–18 years), were analyzed. Median time from diagnosis to analysis was 1.5 months. Clinical characteristics of the patients included in the study are summarized in Table 2. Hypocellular BM was reported in 80% of patients, similar to the previously reported frequency of 81% of primary RCC patients in an interim analysis of studies EWOG-MDS 1998 and 2006.6 Conventional cytogenetics displayed a normal karyotype in 61 of 71 evaluable patients (86%, which is slightly higher than the previously reported frequency of 77%),6 monosomy 7 in 5 of 71 patients (including one patient with monosomy 7 and additional aberrations) (7%), and other aberrations in 5 of 71 patients (trisomy 8 in 2 patients, rare aberrations in 2 patients, and del(7q) in one patient) (7%). In 9 patients, no karyotype was obtained due to insufficient metaphases; in one patient, no information was available.
Table 2.
Clinical and laboratory characteristics of refractory cytopenia of childhood patients included in the study.
Cellular composition of RCC versus healthy control bone marrow
Patients with RCC showed a strong decrease in the myeloid and lymphoid progenitor compartment compared to healthy controls. The median percentage of myeloid blast cells (CD117+) was only 0.1% in RCC, compared to 0.9% in controls; similarly, the percentages of promyelocytes and immature erythroid cells were decreased. As previously observed in adult MDS patients,19 the percentage of CD34+ B-cell precursors was significantly decreased in RCC, with a median percentage of 0.0% in RCC versus 0.3% in controls (Table 3).
Table 3.
Bone marrow cellular composition in refractory cytopenia of childhood patients and controls.
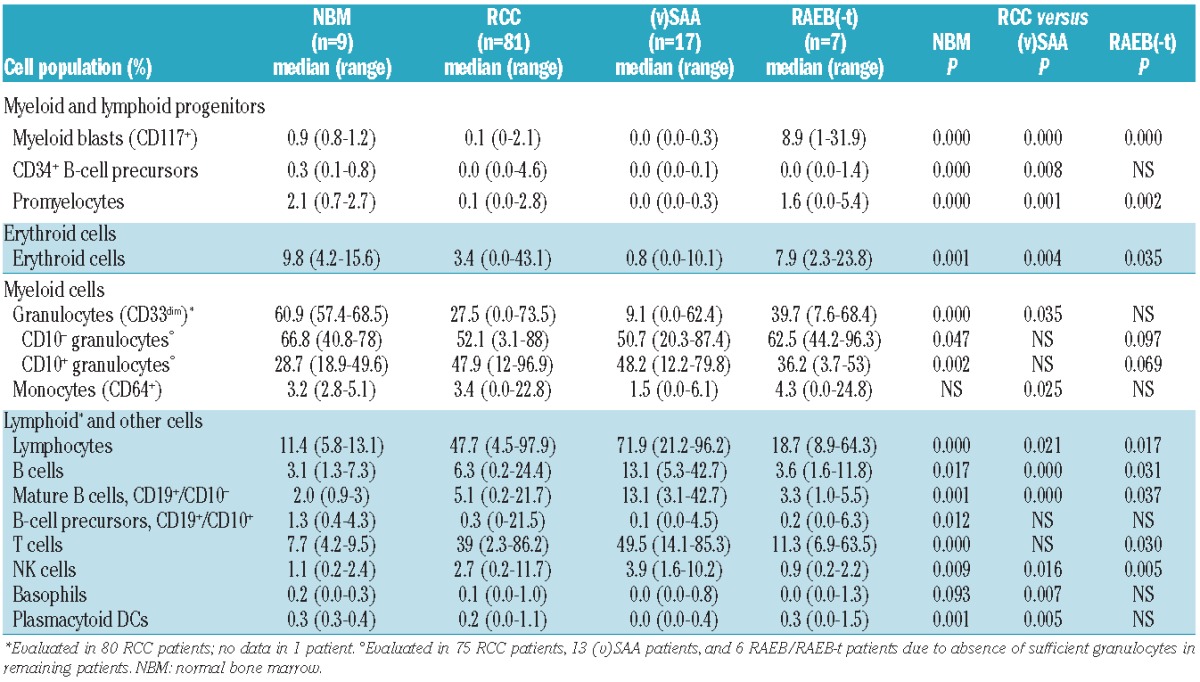
In parallel with a reduction in immature myeloid cells, the percentage of granulocytes (CD33dim) was strongly decreased in RCC (median: 27.5% in RCC vs. 60.9% in controls), with a relative increase in mature CD10+ granulocytes in RCC (median: 47.9% in RCC vs. 28.7% in controls). The percentage of monocytes (CD64+) was similar in RCC patients and controls, although monocytes were low or absent in some RCC patients (Table 3). Remarkably, in one RCC patient, 23% of all nucleated cells were monocytes.
Likely in part due to the reduction in myeloid cells, the proportion of lymphocytes in RCC was strongly increased, with a median of 47.7% in RCC versus 11.4% in controls. The median percentage of T cells was 39% in RCC and 7.7% in controls. The percentages of NK and total B cells were also increased in RCC, but the proportion of B-cell precursors was reduced. There was no difference in the percentage of basophils in RCC to those of healthy controls, while the percentage of plasmacytoid DCs was reduced. Results are summarized in Table 3 and Figure 1.
Figure 1.
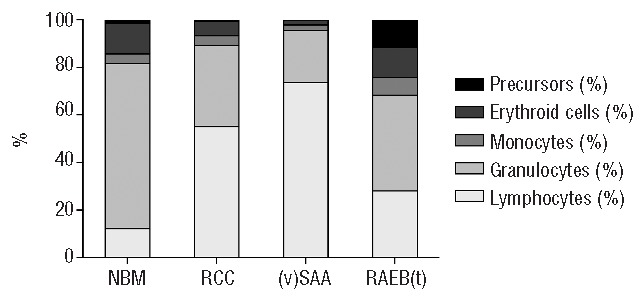
Cellular composition of bone marrow in refractory cytopenia of childhood patients and controls. For graphical representation, means of the main cell populations were calculated per patient and control group, and scaled to 100%. Precursors consist of myeloid blast cells and CD34+ B-cell precursors. NBM: normal bone marrow.
Cellular composition of bone marrow of RCC versus (v)SAA and RAEB(-t)
Although the proportions of progenitor and mature myeloid cells were strongly reduced in RCC patients compared to healthy controls, in (v)SAA this reduction was even more severe. The median percentage of myeloid blasts, promyelocytes, and CD34+ B-cell precursors was 0.0% in (v)SAA; the proportion of immature erythroid cells was 0.8%. The percentage of granulocytes (CD33dim), already reduced in RCC compared to healthy controls, was further decreased in (v)SAA (median 9.1%) compared to RCC, as was the percentage of monocytes (CD64+). The proportion of lymphocytes was strongly increased in (v)SAA compared to RCC, with a median of 71.9%, while the percentage of T cells was similar in (v)SAA and RCC. Percentages of B and NK cells were higher in (v)SAA, while basophils and plasmacytoid DCs were reduced in (v)SAA compared to RCC (Table 3).
Not unexpectedly, compared to patients with RCC, pediatric patients with RAEB(-t) had increased percentages of myeloid blasts (CD117+), promyelocytes, and erythroid cells, while the percentage of CD34+ B-cell precursors, granulocytes and monocytes were equally low. The proportion of lymphocytes, T, NK, and B cells were lower in RAEB(-t) than in RCC patients; the percentages of basophils and plasmacytoid DCs were similar. Results are summarized in Table 3 and Figure 1. A graphic presentation of the BM cellular composition of RCC patients and controls is provided in Online Supplementary Figure S1.
Immunophenotype of myeloid blast cells in RCC and controls
In adult MDS patients, the granularity of myeloid blast cells is frequently decreased, and lineage infidelity markers are often detected.19 In our RCC series, the granularity of myeloid blast cells (CD117+), expressed as ratio to the SSC of lymphocytes, was slightly increased compared to healthy controls, but did not differ from that of (v)SAA patients (median 2.15, 1.76, and 2, respectively; P=0.000 and P=NS). A decreased granularity (less than 2 SD of the mean of healthy controls) was observed in only one of 45 RCC patients with sufficient evaluable myeloid blast cells (CD117+) and none of the 2 evaluable (v)SAA patients. Expression of the lineage infidelity marker CD7 on myeloid blast cells was detected in 6 of 45 evaluable RCC patients (13%), and in none of the healthy controls or (v)SAA patients; expression of CD56 was also detected in 6 of 45 RCC patients, and in none of the healthy controls or (v)SAA patients. In RAEB(-t) patients, multiple abnormalities were detected in myeloid blast cells (Table 4).
Table 4.
Flow cytometric abnormalities in refractory cytopenia of childhood patients and controls.
Immunophenotype of erythroid cells in RCC and controls
The most frequently observed abnormality in immature erythroid cells in RCC was heterogeneous expression of CD71 (transferrin receptor) and CD36 (thrombospondin receptor), which was detected in 42 of 72 RCC patients (58%) with evaluable erythroid cells (Figure 2A–C). In healthy controls, one patient showed heterogeneous expression of CD36; 2 of 11 evaluable (v)SAA patients (18%) and 3 of 7 RAEB(-t) patients (43%) showed heterogeneous expression of CD36 and CD71 (Table 4). In the RCC, (v)SAA, and RAEB(-t) patients with heterogeneous expression of CD71 and CD36, this always co-occurred.
Figure 2.
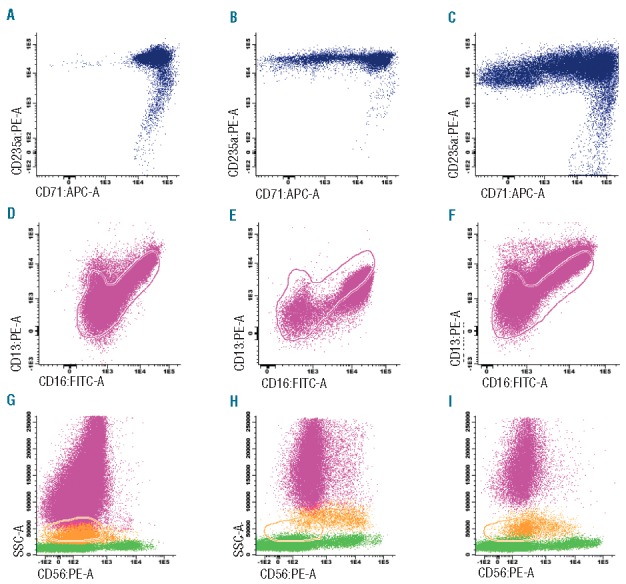
Immunophenotype of immature erythroid cell, granulocytes, and monocytes in refractory cytopenia of childhood patients and controls. (A) Normal expression of CD71 in healthy control bone marrow, ID NBM 023. (B) Heterogeneous CD71 expression in RCC patient, ID CH028. (C) Heterogeneous CD71 expression in RCC patient, ID I 220. (D) Normal pattern of CD16-CD13 expression in healthy control bone marrow, ID NBM 023. (E) Abnormal pattern of CD16-CD13 expression in RCC patient, ID D 663. (F) Abnormal pattern of CD16-CD13 expression in RCC patient, ID D 555. (G) Absence or low level of CD56 expression on monocytes, indicated in orange, in healthy control bone marrow, ID NBM 023. Granulocytes and lymphocytes are indicated in pink and green, respectively. (H) Aberrant expression (>20%) of CD56 on monocytes in RCC patient, ID CZ078. (I) Aberrant expression (>20%) of CD56 on monocytes in RCC patient, ID SC126. Pink and orange lines indicate reference image of normal granulocytes and monocytes, respectively.
Immunophenotype of granulocytes in RCC and controls
No significant differences in the relative SSC of granulocytes (CD33dim) were observed between RCC and healthy controls, or between RCC and (v)SAA patients (data not shown). However, of the 75 RCC patients with sufficient granulocytes (CD33dim) for reliable evaluation, 6 (8%) had a relative SSC that was below the normal range (mean +/−2SD) of the relative SSC in healthy controls. Such low relative SSC was also observed in 3 out of 13 evaluable (v)SAA patients (23%) but in none of the healthy controls. An abnormal pattern of CD16 and CD13 expression on maturing granulocytes was detected in 14% of evaluable RCC patients, comparable to 15% detected in (v)SAA; none of the healthy controls showed an abnormal CD16-CD13 pattern (Figure 2D–F). Of note, the mere absence of a granulocyte subset was not considered an abnormal pattern. Less frequently occurring abnormalities in RCC and (v)SAA, and abnormalities detected in RAEB(-t) patients are described in Table 4.
Immunophenotype of monocytes in RCC and controls
The relative SSC of monocytes (CD64+) in RCC was increased compared to healthy controls, but did not differ from (v)SAA patients (median 3.1, 2.8, and 3, respectively; P=0.006 and P=NS). Three of 74 RCC patients (4%) with sufficient cells to evaluate, none of the 13 evaluable (v)SAA patients, and one healthy control had a decreased granularity. Expression of CD56 on more than 20% of monocytes occurred in 15 of 74 RCC patients (20%), and in none of the healthy controls and (v)SAA patients (Figure 2G–I). Abnormalities detected in RCC patients and controls, including RAEB(-t), are described in Table 4.
Total number of flow cytometric abnormalities in RCC compared to controls
Of the 81 RCC patients, 57 (70%) displayed one or more flow cytometric abnormalities, and 49 (60%) two or more. In one healthy control BM sample, flow cytometric abnormalities were present. This control sample displayed a decreased granularity of the monocytes, and heterogeneous expression of CD71 on immature erythroid cells. Of the (v)SAA patients, 6 of 17 (35%) had one or more, and 2 of 17 (12%) had two or more abnormalities. In the RAEB(-t) group, all patients displayed two or more abnormalities. Using a cutoff of 2 abnormalities, flow cytometry has a sensitivity for the recognition of RCC of 60%, and a specificity, using (v)SAA patients as control group, of 88%.
The median number of abnormalities was 2 (range 0–6) in the RCC patients, which is higher than the number of abnormalities in healthy control BM (median 0, range 0–2; P=0.0018), and higher than in the (v)SAA patients (median 0, range 0–3; P=0.0012), but lower than in RAEB(-t) patients (median 4, range 2–9; P=0.0017) (Figure 3).
Figure 3.
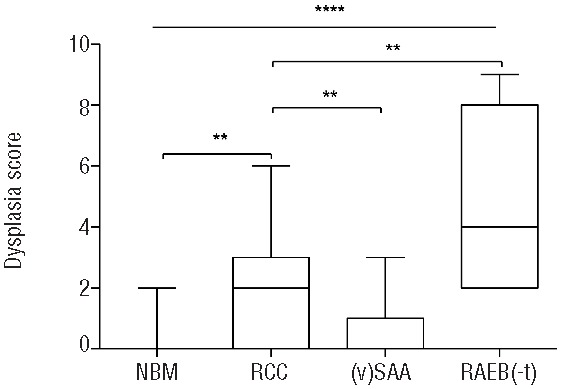
Number of flow cytometric abnormalities in refractory cytopenia of childhood patients and controls. Lines indicate medians. Boxes extend from the 25th to 75th percentile; lines in the boxes indicate medians, whiskers indicate minimum and maximum values. **P<0.01; ****P<0.0001. NBM: normal bone marrow.
It is worthy of note that one patient with MDS-RAEB had no detectable CD34+ B-cell precursors, very few CD19+/CD10+ B-cell precursors and NK cells, a large proportion of large granular lymphocyte-type cells (CD3+CD16.56+), no monocytes, and an increased percentage of myeloid blast cells, that were phenotypically normal. Granulocytes showed an abnormal CD11b-CD13 and CD16-CD13 expression (Online Supplementary Figure S2). This immunophenotype was previously described in patients with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome who developed MDS,25,26 in whom germ-line mutations in GATA2 were later identified.27–31 Indeed, in the present MDS-RAEB patient, a GATA2 mutation was detected.
Flow cytometric abnormalities and clinical characteristics in RCC
No significant associations were detected between the presence of flow cytometric abnormalities (defined as 2 or more abnormalities) in RCC patients and age or sex, the presence of human leukocyte antigen (HLA)-DR15 (found in an increased frequency in adult low-grade MDS and aplastic anemia patients32,33 and associated with a better response to IST34), BM cellularity, transfusion dependency at diagnosis, the presence of a PNH clone, or skewing of the T-cell receptor Vβ chain (data not shown). Interestly, however, all 5 RCC patients carrying the cytogenetic abnormality monosomy 7, associated with a high risk of progression to AML, had at least two immunophenotypic abnormalities. Heterogeneous expression of CD71 and CD36 on erythroid cells, also the most frequently occurring abnormality in the total RCC cohort, was present in 4 of 5 patients carrying monosomy 7; the other abnormalities were non-recurrent or recurrent in only 2 of 5 patients (Online Supplementary Table S2). No relation was detected between the presence of flow cytometric abnormalities and white blood cell count, absolute neutrophil count, hemoglobin level, MCV, or thrombocyte count (data not shown). Only 22 RCC patients with a hypocellular bone marrow included in this study were treated with IST. In this limited number of patients, no significant association was observed between the presence of flow cytometric abnormalities and IST response (data not shown).
Combination of cytopenia and flow cytometric abnormalities to identify RCC and (v)SAA patients
In the majority of RCC patients, immature myeloid and/or lymphoid cells were reduced in numbers, but still detectable, while in the vast majority of (v)SAA patients, myeloid blast cells and CD34+ B-cell precursors were absent: both cell types were absent in 27 of 81 RCC patients (33%) and in 15 of 17 (v)SAA patients (88%). Two or more immunophenotypic abnormalities were detected in 49 of 81 RCC patients (60%), and in 2 of 17 (v)SAA patients (12%). If a diagnosis of RCC was considered when myeloid blast cells and/or CD34+ B-cell precursors were present, or if two or more immunophenotypic abnormalities were detected, 61 of 81 RCC patients (84%) could be correctly classified, whereas the specificity of this combination, using (v)SAA as a control group, was 76% (13 of 17 (v)SAA patients) (using conventional diagnostics including histopathology as the gold standard). The positive and negative predictive values were 94% (68 of 72 patients) and 50% (13 of 26 patients), respectively. In 55 RCC patients with a hypocellular BM, and a normal karyotype or insufficient metaphases to obtain a karyotype, 45 patients were correctly identified by flow cytometry as RCC, resulting in a sensitivity of the combined score in this group of patients of 82%.
Discussion
Flow cytometric immunophenotyping has been shown to be of value in distinguishing MDS from non-clonal cytopenias in adult patients.7 In the present study, we evaluated whether flow cytometry can help distinguish RCC from healthy controls, and from (v)SAA and advanced MDS. We analyzed percentages and/or dysplastic features of the immature myeloid and lymphoid, immature erythroid, maturing granulocytic, monocytic and lymphocyte compartments.
In RCC, the myeloid compartment, both mature and immature, appeared severely compromised in comparison to healthy controls. This finding is not unexpected because BM is hypocellular in the majority of RCC cases. In comparison with (v)SAA, however, the reduction in myeloid cells was less severe in RCC, similar to what has been shown previously by Reiterova and others, although in a smaller cohort of pediatric RCC and (v)SAA patients.35 An important difference was the complete absence of myeloid blast cells and CD34+ B-cell precursors in the majority of (v)SAA patients, while in most RCC patients some progenitor cells were still detectable. Interestingly, in 5 pediatric patients with other cytopenias than RCC or (v)SAA (3 with Fanconi anemia, one with transient erythroblastopenia of childhood, one with immune thrombocytopenic purpura), the percentages of CD34+ B-cell precursors, myeloid blast cells, and promyelocytes were significantly higher than in RCC (data not shown). We compared the BM composition of pediatric patients with healthy adult controls because healthy pediatric controls are rarely available. In comparison with healthy pediatric controls, the reduction in myeloid blast cells and CD34+ B-cell precursors in RCC patients would probably be even more severe because the precursor compartment in children is relatively larger than in adults.22,36
In comparison with studies in adult low-grade MDS, we detected relatively few flow cytometric abnormalities in RCC, apart from the severe reduction of myeloid cells. Furthermore, there was a difference in the type of immunophenotypic abnormalities between RCC and adult low-grade MDS. One of the commonly occurring abnormalities in adult MDS is an abnormally decreased SSC of granulocytes, which is reflective of hypogranularity. The relative SSC of granulocytes in RCC did not differ from that of healthy controls or (v)SAA patients. Expression of lineage infidelity markers on myeloid blast cells, another common finding in adult MDS, was also not observed frequently in RCC. In adult MDS, lineage infidelity marker expression correlates with an increased blast percentage and a more advanced disease stage.19,37 That RCC is a low-grade MDS subtype may partly explain the low frequency of lineage infidelity marker expression on myeloid blast cells. In RCC, the most frequently occurring immunophenotypic abnormality was heterogeneous expression of CD71 and CD36 on erythrocytes in 58% of cases (see below), followed by aberrant expression of CD56 on monocytes in 20% of cases, which might reflect stressed hematopoiesis rather than true dysplasia. All other abnormalities occurred in a lower frequency in RCC. Of interest, although only 5 patients with monosomy 7 were included in the present study, all of them displayed at least 2 flow cytometric abnormalities. RCC with monosomy 7 confers a high risk of progression to acute myeloid leukemia, but histopathologically no differences can be detected between RCC cases with or without monosomy 7.6 A possible explanation for this difference might be that flow cytometry can detect subtle dysplastic changes with a greater sensitivity than histopathology, partly because more cells can be examined by flow cytometry (generally ≥100,000 cells) than by morphology (generally ≤500 cells).
The histopathological differentiation between RCC and (v)SAA is mainly based on the presence of patchy erythropoiesis with defective maturation and/or the presence of micromegakaryocytes in RCC, and the absence of erythropoiesis and megakaryopoiesis in (v)SAA.5 These histopathological differences are reflected by the differences we detected by flow cytometry between RCC and (v)SAA. In our flow cytometric analyses, the percentage of immature erythroid cells in RCC was higher than in (v)SAA patients. Furthermore, we detected heterogeneous expression of CD71 and CD36 in a large proportion of RCC patients. The exact meaning of this heterogeneous expression is unclear, but flow cytometric abnormalities in CD71 and/or CD36 expression on immature erythroid cells are specific and recurrent findings in adult MDS patients.8,10,11,38–40 Flow cytometric evaluation of megakaryopoieis still has its limitations, but it was recently shown that abnormalities detected in peripheral blood platelets by flow cytometry are of diagnostic significance in adult MDS.41 Future studies will be needed to reveal whether immunophenotyping of platelets and/or megakaryocytes can be of additional value in distinguishing RCC from (v)SAA as well. Apart from flow cytometric immunophenotyping, deep-sequencing approaches, which have now become readily available, but have not yet been systematically applied in RCC and (v)SAA, might be of value in identifying differences between the two groups.
Distinguishing RCC from (v)SAA remains of clinical relevance, even though the pathophysiology of RCC seems, similar to (v)SAA, at least in part immune-mediated, also given the good response in selected RCC patients to IST.23,42 The rate of clonal evolution after IST in RCC patients, ranging from 4% to 14% (in rabbit and horse ATG, respectively),42 might be higher than in (v)SAA patients, in whom the probability of clonal evolution had dropped to 3% in a recent interim analysis (type of ATG not reported).5 Furthermore, while the conditioning regimen before hematopoietic stem cell transplantation is myeloablative (reduced intensity in selected patients) in RCC, this is not the case in (v)SAA patients.43,44
One of the limitations of this study is the possibility of peripheral blood contamination of BM aspirates. Future studies might choose to determine the purity of BM or to correct for peripheral blood contamination by methods that have been described previously.45–47 A certain degree of peripheral blood contamination seems unavoidable given the hypocellularity of BM in the majority of RCC patients. Another limitation of our study and of most other studies evaluating the value of flow cytometry as a diagnostic tool in MDS, is that, although determining cell numbers by flow cytometry is fairly objective, scoring of dysplasia remains subjective. Therefore, future studies assessing the value of flow cytometry as a means to differentiate RCC from non-clonal cytopenias should employ more standardized protocols and antibody panels, and novel software tools that might be able to more objectively evaluate myeloid dysplasia.48,49 When our results can be reproduced in an independent cohort of RCC and (v)SAA patients, flow cytometric immunophenotyping could be included in the diagnostic workup of childhood cytopenias also in laboratories with a more limited expertise in flow cytometry.
In summary, RCC patients have a strongly reduced myeloid compartment in comparison to healthy controls, but the reduction is not as severe as in (v)SAA patients. We also report that the number of abnormalities detected by flow cytometry is significantly higher in RCC patients than in healthy controls and in pediatric patients with (v)SAA, but lower than in advanced MDS. Our results indicate that, although flow cytometric abnormalities in RCC patients are present at a relatively low frequency, flow cytometric immunophenotyping might be a relevant addition to histopathology and cytogenetic analysis in the diagnosis of RCC. Particularly the combined use of cellular composition and flow cytometric abnormalities may support the distinction between RCC and SAA with good specificity (84%) and sensitivity (76%).
Acknowledgments
The authors acknowledge excellent technical support by research technicians from the Leukemia and Lymphoma Diagnostics group from the Department of Immunology and logistic support from the Department of Pediatric Oncology/Hematology, Erasmus MC.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
AMA and this research were supported by the KiKa Foundation, Amstelveen, the Netherlands. In the Czech Republic RCC diagnosis and treatment were supported by a grant of the Ministry of Health for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic) and grant NT/14534 (Ministry of Health).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hasle H, Kerndrup G, Jacobsen BB. Childhood myelodysplastic syndrome in Denmark: incidence and predisposing conditions. Leukemia. 1995;9(9):1569–1572. [PubMed] [Google Scholar]
- 2.Hasle H, Wadsworth LD, Massing BG, McBride M, Schultz KR. A population-based study of childhood myelodysplastic syndrome in British Columbia, Canada. Br J Haematol. 1999;106(4):1027–1032. [DOI] [PubMed] [Google Scholar]
- 3.Passmore SJ, Chessells JM, Kempski H, Hann IM, Brownbill PA, Stiller CA. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia in the UK: a population-based study of incidence and survival. Br J Haematol. 2003; 121(5):758–767. [DOI] [PubMed] [Google Scholar]
- 4.Baumann I, Niemeyer CM, Bennett JM, Shannon K. Childhood myelodysplastic syndromes. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008:104–107. [Google Scholar]
- 5.Baumann I, Fuhrer M, Behrendt S, et al. Morphological differentiation of severe aplastic anaemia from hypocellular refractory cytopenia of childhood: reproducibility of histopathological diagnostic criteria. Histopathology. 2012;61(1):10–17. [DOI] [PubMed] [Google Scholar]
- 6.Niemeyer CM, Baumann I. Classification of childhood aplastic anemia and myelodysplastic syndrome. Hematology Am Soc Hematol Educ Program. 2011;84–89. [DOI] [PubMed] [Google Scholar]
- 7.Westers TM, Ireland R, Kern W, et al. Standardization of flow cytometry in myelodysplastic syndromes: a report from an international consortium and the European LeukemiaNet Working Group. Leukemia. 2012;26(7):1730–1741. [DOI] [PubMed] [Google Scholar]
- 8.Stetler-Stevenson M, Arthur DC, Jabbour N, Xie XY, et al. Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood. 2001; 98(4):979–987. [DOI] [PubMed] [Google Scholar]
- 9.Maynadie M, Picard F, Husson B, et al. Immunophenotypic clustering of myelodysplastic syndromes. Blood. 2002; 100(7):2349–2356. [DOI] [PubMed] [Google Scholar]
- 10.Della Porta MG, Malcovati L, Invernizzi R, et al. Flow cytometry evaluation of erythroid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2006; 20(4):549–555. [DOI] [PubMed] [Google Scholar]
- 11.Mathis S, Chapuis N, Debord C, et al. Flow cytometric detection of dyserythropoiesis: a sensitive and powerful diagnostic tool for myelodysplastic syndromes. Leukemia. 2013;27(10):1981–1987. [DOI] [PubMed] [Google Scholar]
- 12.Ogata K, Kishikawa Y, Satoh C, Tamura H, Dan K, Hayashi A. Diagnostic application of flow cytometric characteristics of CD34+ cells in low-grade myelodysplastic syndromes. Blood. 2006;108(3):1037–1044. [DOI] [PubMed] [Google Scholar]
- 13.Matarraz S, Lopez A, Barrena S, et al. The immunophenotype of different immature, myeloid and B-cell lineage-committed CD34+ hematopoietic cells allows discrimination between normal/reactive and myelodysplastic syndrome precursors. Leukemia. 2008;22(6):1175–1183. [DOI] [PubMed] [Google Scholar]
- 14.Matarraz S, Lopez A, Barrena S, et al. Bone marrow cells from myelodysplastic syndromes show altered immunophenotypic profiles that may contribute to the diagnosis and prognostic stratification of the disease: a pilot study on a series of 56 patients. Cytometry B Clin Cytom. 2010; 78(3):154–168. [DOI] [PubMed] [Google Scholar]
- 15.Ogata K, Della Porta MG, et al. Diagnostic utility of flow cytometry in low-grade myelodysplastic syndromes: a prospective validation study. Haematologica. 2009; 94(8):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kern W, Haferlach C, Schnittger S, Haferlach T. Clinical utility of multiparameter flow cytometry in the diagnosis of 1013 patients with suspected myelodysplastic syndrome: correlation to cytomorphology, cytogenetics, and clinical data. Cancer. 2010;116(19):4549–4563. [DOI] [PubMed] [Google Scholar]
- 17.Della Porta MG, Picone C, Pascutto C, et al. Multicenter validation of a reproducible flow cytometric score for the diagnosis of low-grade myelodysplastic syndromes: results of a European LeukemiaNET study. Haematologica. 2012;97(8):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells DA, Benesch M, Loken MR, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102(1):394–403. [DOI] [PubMed] [Google Scholar]
- 19.van de Loosdrecht AA, Westers TM, Westra AH, Drager AM, van der Velden VH, Ossenkoppele GJ. Identification of distinct prognostic subgroups in low- and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood. 2008; 111(3):1067–1077. [DOI] [PubMed] [Google Scholar]
- 20.Westers TM, Alhan C, Chamuleau ME, et al. Aberrant immunophenotype of blasts in myelodysplastic syndromes is a clinically relevant biomarker in predicting response to growth factor treatment. Blood. 2010; 115(9):1779–1784. [DOI] [PubMed] [Google Scholar]
- 21.Veltroni M, Sainati L, Zecca M, et al. Advanced pediatric myelodysplastic syndromes: can immunophenotypic characterization of blast cells be a diagnostic and prognostic tool? Pediatr Blood Cancer. 2009;52(3):357–363. [DOI] [PubMed] [Google Scholar]
- 22.Aalbers AM, van den Heuvel-Eibrink MM, de Haas V, et al. Applicability of a reproducible flow cytometry scoring system in the diagnosis of refractory cytopenia of childhood. Leukemia. 2013;27(9):1923–1925. [DOI] [PubMed] [Google Scholar]
- 23.Aalbers AM, van der Velden VH, Yoshimi A, et al. The clinical relevance of minor paroxysmal nocturnal hemoglobinuria clones in refractory cytopenia of childhood: a prospective study by EWOG-MDS. Leukemia. 2014;28(1):189–192. [DOI] [PubMed] [Google Scholar]
- 24.de Vries AC, Langerak AW, Verhaaf B, et al. T-cell receptor Vbeta CDR3 oligoclonality frequently occurs in childhood refractory cytopenia (MDS-RC) and severe aplastic anemia. Leukemia. 2008;22(6):1170–1174. [DOI] [PubMed] [Google Scholar]
- 25.Vinh DC, Patel SY, Uzel G, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvo KR, Vinh DC, Maric I, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica. 2011;96(8):1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43(10):1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43(10):929–931. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson RE, Griffin H, Bigley V, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118(10):2656–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasquet M, Bellanne-Chantelot C, Tavitian S, et al. High frequency of GATA2 muta tions in patients with mild chronic neu-tropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121(5):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maciejewski JP, Follmann D, Nakamura R, et al. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001;98(13):3513–3519. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood. 2002;100(12):3897–3902. [DOI] [PubMed] [Google Scholar]
- 34.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26(15):2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiterova M, Kramarzova K, Sukova M, et al. Changes Identified by Flow Cytometry and WT1 Expression in Consecutive Bone Marrow Samples in Refractory Cytopenia of Childhood and Aplastic Anemia Before Start of the Therapy. ASH Annual Meeting Abstracts. 2011;118(21):1342. [Google Scholar]
- 36.Bras AE, van den Heuvel-Eibrink MM, van der Sluijs-Gelling AJ, et al. No significant prognostic value of normal precursor B-cell regeneration in paediatric acute myeloid leukaemia after induction treatment. Br J Haematol. 2013;161(6):861–864. [DOI] [PubMed] [Google Scholar]
- 37.Ogata K, Kakumoto K, Matsuda A, et al. Differences in blast immunophenotypes among disease types in myelodysplastic syndromes: a multicenter validation study. Leuk Res. 2012;36(10):1229–1236. [DOI] [PubMed] [Google Scholar]
- 38.Malcovati L, Della Porta MG, Lunghi M, et al. Flow cytometry evaluation of erythroid and myeloid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2005;19(5):776–783. [DOI] [PubMed] [Google Scholar]
- 39.Kuiper-Kramer PA, Huisman CM, Van der Molen-Sinke J, Abbes A, Van Eijk HG. The expression of transferrin receptors on erythroblasts in anaemia of chronic disease, myelodysplastic syndromes and iron deficiency. Acta Haematol. 1997;97(3):127–131. [DOI] [PubMed] [Google Scholar]
- 40.Lorand-Metze I, Califani SM, Ribeiro E, Lima CS, Metze K. The prognostic value of maturation-associated phenotypic abnormalities in myelodysplastic syndromes. Leuk Res. 2008;32(2):211–213. [DOI] [PubMed] [Google Scholar]
- 41.Sandes AF, Yamamoto M, Matarraz S, et al. Altered immunophenotypic features of peripheral blood platelets in myelodysplastic syndromes. Haematologica. 2012; 97(6):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshimi A, van den Heuvel-Eibrink MM, Baumann I, et al. Comparison of horse and rabbit anti-thymocyte globulin in immunosuppressive therapy for refractory cytopenia of childhood. Haematologica. 2014; 99(4):656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strahm B, Locatelli F, Bader P, et al. Reduced intensity conditioning in unrelated donor transplantation for refractory cytopenia in childhood. Bone Marrow Transplant. 2007;40(4):329–333. [DOI] [PubMed] [Google Scholar]
- 44.Fuhrer M, Rampf U, Baumann I, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005; 106(6):2102–2104. [DOI] [PubMed] [Google Scholar]
- 45.Holdrinet RS, von Egmond J, Wessels JM, Haanen C. A method for quantification of peripheral blood admixture in bone marrow aspirates. Exp Hematol. 1980;8(1):103–107. [PubMed] [Google Scholar]
- 46.Brooimans RA, Kraan J, van Putten W, Cornelissen JJ, Lowenberg B, Gratama JW. Flow cytometric differential of leukocyte populations in normal bone marrow: influence of peripheral blood contamination. Cytometry B Clin Cytom. 2009;76(1):18–26. [DOI] [PubMed] [Google Scholar]
- 47.Loken MR, Chu SC, Fritschle W, Kalnoski M, Wells DA. Normalization of bone marrow aspirates for hemodilution in flow cytometric analyses. Cytometry B Clin Cytom. 2009;76(1):27–36. [DOI] [PubMed] [Google Scholar]
- 48.van Dongen JJ, Lhermitte L, Bottcher S, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalina T, Flores-Montero J, van der Velden VH, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


