Abstract
We prospectively evaluated the activity and tolerance of lenalidomide-dexamethasone in 35 patients with acute light chain-induced renal failure. The lenalidomide dose was adapted to the estimated glomerular filtration rate and dexamethasone was given at high dose in cycle one and at low dose thereafter. Four patients died within the first two cycles, and five discontinued therapy leaving 26 patients for the per-protocol analysis. Responses were observed in 24/35 (68.6%) patients of the intent-to-treat population. Complete response was noted in seven patients (20%), very good partial response in three patients (8.6%), partial response in 14 patients (40%), and minimal response in one patient (2.9%). Renal response was observed in 16 (45.7%) patients: five (14.2%) achieved complete, four (11.4%) partial and seven (20%) minor renal responses. Five of 13 patients who were dialysis dependent at baseline became dialysis independent. The median time to myeloma and to renal response was 28 days for both parameters, while the median time to best myeloma and best renal response was 92 and 157 days, respectively. The median estimated glomerular filtration rate increased significantly in patients with partial response or better from 17.1 mL/min at baseline to 39.1 mL/min at best response (P=0.001). The median progression-free and overall survival was 5.5 and 21.8 months, respectively, in the intent-to-treat population and 12.1 and 31.4 months, respectively, in the per-protocol group. Infections, cardiotoxicity, anemia and thrombocytopenia were the most frequent toxicities. In conclusion, the lenalidomide-dexamethasone regimen achieved rapid and substantial myeloma and renal responses. The trial was registered under EUDRACT number 2008-006497-15.
Introduction
Renal failure is a frequent complication of multiple myeloma and can be elicited by various factors such as infections, non-steroidal anti-inflammatory drugs, nephrotoxic antibiotics, iodinated contrast media, hypercalcemia, tumor lysis syndrome, myeloma cell infiltration of the kidney, renal vein or artery thrombosis and, frequently, by clonotypic light chains. Renal impairment induced by dehydration, hypercalcemia, anti-inflammatory drugs and infections is often less severe and usually reversible.1 This is in contrast to several forms of light chain-induced renal injuries such as glomerular and vascular amyloidosis, light chain deposit disease, light chain proximal tubulopathy with/without Fanconi syndrome, or cast nephropathy, which is the most frequent sequel of kidney-pathogenic light chains and typically associated with much faster deterioration of renal function.2 In cast nephropathy excessive glomerular filtration of free light chains saturates the clearance mechanism of the proximal tubule3 and the resulting overflow of light chains into the distal tubule precipitates with Tamm-Horsefall protein, particularly in the case of high binding affinity of both protein types4 and altered osmolality. This process results in tubular damage and interstitial inflammation.5
Light chain-induced acute kidney impairment may be the salient symptom leading to diagnosis of myeloma or, more frequently, a consequence of progressive disease in patients with an established diagnosis. The occurrence of this complication has substantial impact on the prognosis with a significantly higher risk of life-threatening infections and mortality. In fact, recent surveys from Ireland6 and the UK7 showed that survival of myeloma patients requiring dialysis during the first weeks after diagnosis of multiple myeloma is in the range of 6 to 11 months and has not improved substantially during recent years despite the availability of novel drugs. In patients with less severe renal impairment survival has improved, but highly active anti-myeloma therapy is required to exploit the window of opportunity for reversing renal function.8
Here we evaluate lenalidomide-dexamethasone as treatment for patients with acute light chain-induced renal failure. The primary objectives of the study were to determine the rates of recovery of renal function and myeloma response. Progression-free survival, overall survival and toxicity were evaluated as secondary goals.
Methods
Thirty-five patients with acute light chain-induced renal failure were enrolled. The diagnosis was established by documentation of light chain proteinuria and urine electrophoresis patterns typical of cast nephropathy9 in conjunction with increased or increasing serum levels of free light chains, highly abnormal free light chain ratio, progressive myeloma and exclusion of other frequent causes of renal impairment in myeloma, such as severe infection or sepsis, recent use of non-steroidal anit-inflammatory drugs and/or nephrotoxic antibiotics, contrast media, or hypercalcemia. Patients with established amyloidosis or light or heavy chain deposit disease were excluded. Cast nephropathy as the leading cause of renal failure was confirmed in all 15 patients who had a renal biopsy. Both patients presenting with previously unknown multiple myeloma and patients with a previously established diagnosis of multiple myeloma were included. The former group of patients had to have presented with an estimated glomerular filtration rate (eGFR) <50 mL/min and a serum creatinine ≥2.0 mg/dL, and light chaininduced renal injury as the underlying cause of renal impairment while the latter group had to have had documented eGFR ≥60 mL/min and serum creatinine ≤1.2 mg/dL within 6 weeks before deterioration of eGFR to <50 mL/min and of creatinine to ≥2.0 mg/dL due to light chain-induced kidney injury. According to a recent recommendation for cancer patients,10 eGFR was estimated using the Cockcroft-Gault formula.11
A total of nine cycles of lenalidomide and dexamethasone were planned. Lenalidomide was administered from day 1–21 of each 28-day cycle with dose adaptation according to eGFR (eGFR 30–50 mL/min: 10 mg/day; eGFR <30 mL/min without requiring dialysis: 15 mg/48 h; eGFR <30 mL/min requiring dialysis: 5 mg daily following each dialysis). Dexamethasone was administered at a dose of 40 mg on days 1–4, 9–12 and 17–21 during the first cycle and thereafter at a dose of 40 mg once weekly. Treatment after the end of the nine cycles was not predefined and was left to the discretion of the individual investigator.
Renal response was defined as complete (CRrenal, with an eGFR ≥60 mL/min), partial (PRrenal with an eGFR increase >100%, from <15 mL/min to 30-<60 mL/min), or minor (MRrenal with an eGFR increase >50%, either from <15 mL/min to 15-<30 mL/min or from 15-<30 mL/min to 30-<60 mL/min), as previously described.12,13
Myeloma response was assessed according to the International Uniform Response Criteria for Multiple Myeloma14 and defined as complete (CR), very good partial (VGPR), and partial (PR) with the addition of a minor response (MR).15
The sample size was calculated based on the assumption that the lenalidomide-dexamethasone combination would show substantial activity and induce reversal of renal impairment in ≥50% of patients at the end of the therapy. Assuming the rate of reversal with other, conventional therapies to be 20%, by accrual of 50 eligible patients, the predicted rate of reversal of renal failure would be verified with a power of 95% and significance level of 5% by a two-sided, one-grouped chi square test.
Response rates, progression-free survival, overall survival and toxicities were calculated by intent-to-treat analysis, but progression-free and overall survival were also investigated in the patients treated pre-protocol and the kinetics of the eGFR and 24-hour proteinuria were studied in the group with a myeloma response. Overall survival and progression-free survival were estimated by the product limit method.16 Due to the limited sample size multivariate analysis could not be performed. The Kruskal-Wallis test17 was used to compare groups of patients. The level of statistical significance for all analyses was set at 0.05 and all values reported are two-sided. SPSS, version 17 was used for all analyses.
The following risk factors were tested for possible correlations with myeloma and renal responses (CRrenal, ≥PRrenal), progression-free survival and overall survival in univariate analyses: age >65 years, International Staging System stage III, Eastern Cooperative Oncology Group score <2, β-2 microglobulin >5.5 mg/mL, albumin <3.5 g/L, hemoglobin <8.0 g/dL, lactate dehydrogenase >226 U/L, calcium >2.24 mmol/L, C-reactive protein >5 mg/L, C-reactive protein >13.2 mg/L, eGFR <15 mL/min, eGFR <30 mL/min, dialysis, baseline platelets <150×109/L, cytogenetic risk factors [t(4;14) ± del 17p] and 1q21 ± t(4;14) ± del 17p, reduction of baseline involved free light chains >95%.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 3.0, of the National Cancer Institute.18
Patients were enrolled by eight participating centers in Austria, Germany and the Czech Republic between July 2009 and January 2013, and were followed until April 2014. All patients gave written informed consent before entering the study, which was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2008. The study was approved by the ethical committees responsible for all participating study centers and was registered under EUDRACT number 2008-006497-15.
Results
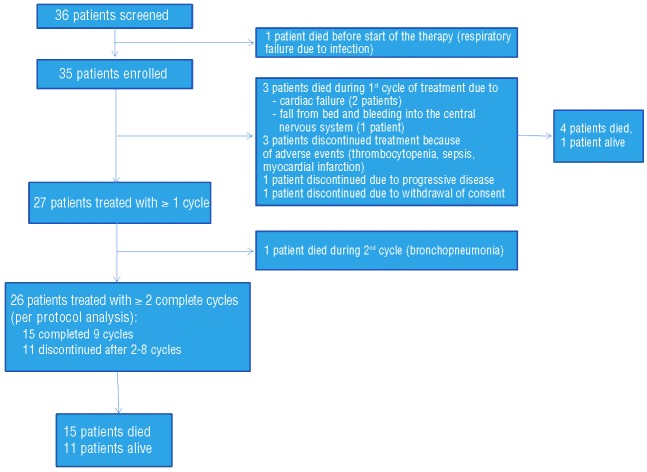
The patients’ characteristics are shown in Table 1 and the patients’ flow through the trial is depicted in the consort diagram (Figure 1). The trial was discontinued after inclusion of 35 patients because of slow enrollment. The 35 patients comprise the intent-to-treat population (ITT). Four of these patients died within the first two cycles of treatment and five discontinued therapy (3 due to adverse events, 1 due to progressive disease and 1 withdrew consent), leaving 26 patients for the per protocol (PP) analysis. After a median follow up of 17.7 months, myeloma response to therapy was noted in 24/35 (68.6%) of the ITT population. Seven patients (20%) had a CR, three (8.6%) had a VGPR, 14 (40%) had a PR, and one (2.9%) had a MR. The median time to first myeloma response was 28 days, and time to best myeloma response was 92 days. The median baseline concentration of involved free light chains in the ITT population was 5,465 mg/L (range, 147–42,700 mg/L) and 8,350 mg/L (range, 234–35,500 mg/L) in patients reaching ≥PR and ≤MR, respectively, and decreased significantly to a median of 95,75 mg/L (range, 11.3–5,630 mg/L, P<0.001) in the former, but only to a median of 4500 mg/L (234–18,705 mg/L, P=0.5) in the latter group (Figure 2A).
Table 1.
Patients’ characteristics.
Figure 1.
Consort diagram of the patients’ flow through the study.
Figure 2.
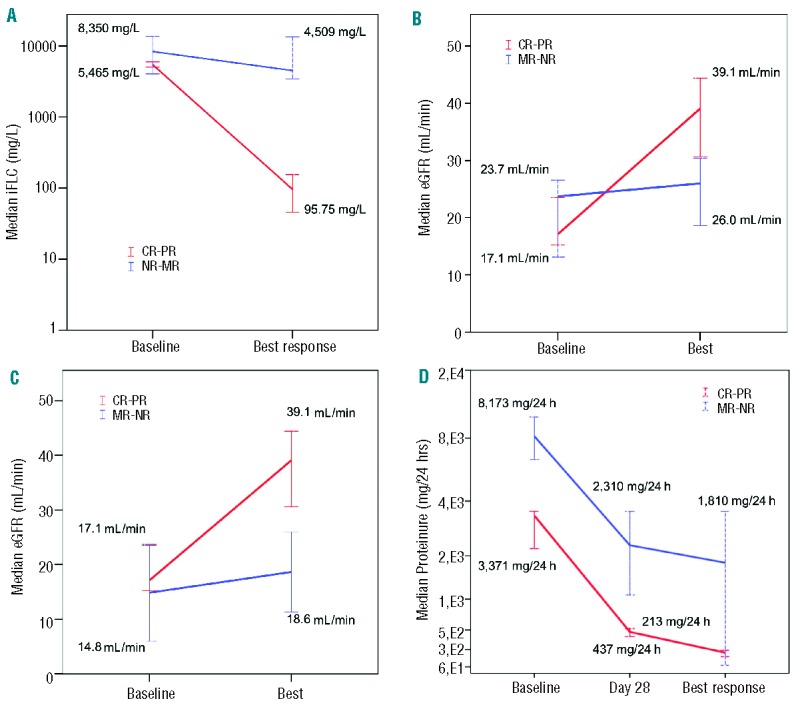
(A) ITT population; median (range) of involved free light chain (iFLC) concentrations at baseline and at best response in patients with CR-PR compared to the no response (NR)-MR population. (B) ITT population; median (range) of eGFR in patients with CR-PR or MR-NR at baseline and at best response. (C) PP population; median eGFR in patients with CR-PR and those with MR-NR at baseline and at best response. (D) ITT population; median 24-h proteinuria in patients with CR-PR and those with MR-NR at baseline, day 28 and at best response
A renal response was observed in 16 (45.7%) of the 35 patients in the ITT group, with five (14.2%), four (11.4%) and seven (20%) achieving a CRrenal, PRrenal, and MRrenal, respectively. The median time to a renal response was 28 days and the median time to best renal response was 157 days. The median eGFR increased significantly in patients with ≥PR from 17.1 mL/min at baseline to 39.1 mL/min at best response (P<0.001), and from 23.7 mL/min to 26.0 mL/min in patients with ≤MR in the ITT population (P=0.469) (Figure 2B). The corresponding figures in the PP group were 17.1 mL/min and 39.1 mL/min in patients with ≥PR (P<0.001), and 14.85 mL/min and 18.65 mL/min, respectively, in those with ≤MR (Figure 2C).
Twenty-four-hour proteinuria in the PP group at baseline was lower, albeit not significantly so (P=0.052), in patients with subsequent myeloma response than in non-responders [3,371 mg/24 h (410–13,872 mg/24 h) versus 8,173 mg/24 h (6,374–9,972 mg/24 h)] (Figure 2D). The protein excretion decreased substantially during the first treatment cycle in both groups, but the decline was more pronounced in patients with a myeloma response, both at day 28 [473 mg/24 h (60–1,604 mg/24 h) versus 2,310 mg/24 h (1.080–3,540 mg/24 h); P=0.034] and at the time of the best renal response [213 mg/24 h (40–850 mg/24 h) versus 1,810 mg/24 h (79–3,540 mg/24 h); P=0.676]. All seven patients who had a CR had normalization of 24-h proteinuria [median 119 mg/24 h (59–192 mg/24 h)]. The greatest decline in 24-h proteinuria was noted in patients with a renal response [baseline: median, 3,360 mg/24 h (range, 1,000–6,350 mg/24 h); at day 28, median 480 mg/24 h (60–1,600 mg/24 h); at best response, median 192 mg/24 h (40–588 mg/24 h)]. Thirteen patients were dialysis dependent at baseline and five of them became dialysis independent. The maximal decline in 24-h proteinuria did not differ between patients who became dialysis independent [260 mg/24 h (130–420 mg/24 h) or not (270 mg/24 h (60–3,540 mg/24 h); P=0.831].
Median progression-free survival and overall survival were 5.5 and 21.8 months, respectively, in the ITT group and 12.1 and 31.4 months, respectively, in the PP group (Figure 3).
Figure 3.
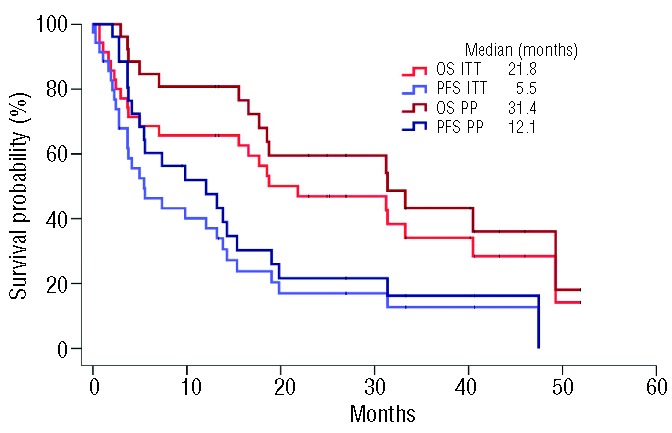
Progression-free survival (PFS) and overall survival (OS) in the ITT and PP populations.
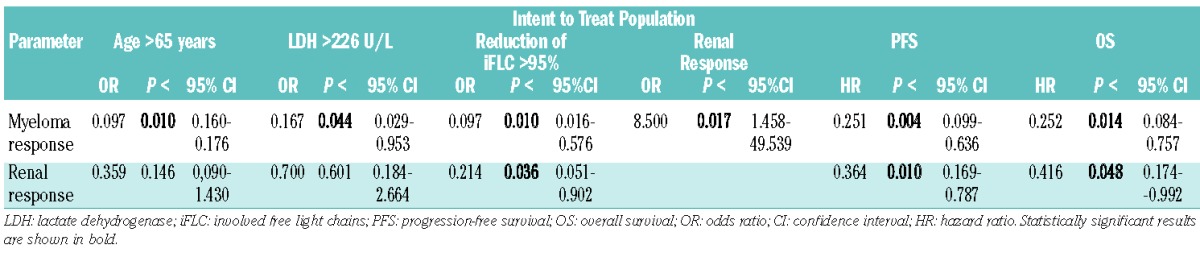
Univariate analysis in the ITT group revealed significant correlations between myeloma response (≥PR) and renal response (P<0.017), age >65 years (P<0.01), lactate dehydrogenase (P<0.044), progression-free survival (P<0.004), and overall survival (P<0.014). Renal response was, in addition, associated with progression-free survival (P<0.01), and overall survival (P<0.048), and expectedly with >95% reduction in levels of involved free light chains (P<0.036, Table 2). High C-reactive protein levels (>13.2 mg/L) were associated with shorter overall survival (P<0.045), and Eastern Cooperative Oncology Group status ≤1 correlated with progression-free survival (P<0.029).
Table 2.
Univariate analysis of correlations between myeloma and renal response and clinical parameters.
Patients who discontinued the trial within the first two cycles of treatment tended to be slightly older (median age 71 versus 66 years; P=0.186), and had higher baseline C-reactive protein levels (23.6 versus 12.63 mg/L; P=0.462). They also showed a tendency to have more aggressive disease, manifested by higher lactate dehydrogenase levels (267 versus 222 U/L; P=0.157)
Of the four patients who died during the first two cycles of treatment, three died within cycle 1. Two of them died of heart failure, which likely was a consequence of the cardiotoxicity of high-dose dexamethasone, and one of cerebral bleeding. Overall, 23 patients died, 14 (40%) due to progressive disease, three each due to infection (n=3, 9%), cardiac toxicity (n=3, 9%) or complications of the disease or therapy (n=3, 9%). Grade 3/4 anemia was the most frequent hematologic toxicity, occurring in 15 (43%) patients, followed by thrombocytopenia in eight (23%) patients and neutropenia in five (15%). Other non-hematologic adverse events consisted mainly of grade 3/4 infection in 13 (38%) patients followed by cardiac toxicity in four (11%) patients. Grade 3 diarrhea and vomiting were noted in one (3%) patient each.
Discussion
Acute light chain-induced renal failure usually presents as cast nephropathy19 and is a medical emergency in multiple myeloma, necessitating fast diagnostic work up and rapid initiation of effective therapy.20 Here we show that lenalidomide-dexamethasone administered with high-dose dexamethasone during the first cycle and thereafter at a reduced once weekly dose exerted substantial activity and induced an overall myeloma response rate of 68.6% in the ITT population with 20% achieving CR and 8.6% VGPR. Importantly, a myeloma response was achieved after 1 month of therapy, and the best myeloma response, after 3 months of therapy. The median serum levels of involved free light chains decreased by >99% from 6,303 mg/L to 42,85 mg/L in patients with ≥VGPR, creating the required precondition for recovery of renal function.21 Accordingly, renal response was achieved in 45.7% of the patients of the ITT group (14.2%, 11.4%, and 20% achieved CRrenal, PRrenal, and MRrenal, respectively). First renal response occurred concomitantly with first myeloma response, but the best renal response was reached 2 months after the best myeloma response. Comparison of these data with those from our previous phase 2 trial12 employing a triple regimen of bortezomib plus doxorubicin plus dexamethasone revealed a similar overall myeloma response rate, but the rates of CR/nearCR (38%) and renal response (62%) were higher with the triple combination. Nevertheless, the lenalidomide-dexamethasone regimen employed in the present study resulted in a significant increase in eGFR from 17.1 mL/min at baseline to 39.1 mL/min at best response in patients in the PP group achieving ≥PR. Patients with a renal response had marked decreases in involved circulating light chains and in 24-h urine proteinuria, the latter reaching normal levels in all responding patients. Leung et al. previously showed that in cases with cast nephropathy the overwhelming amount of secreted protein is Bence-Jones protein with less than 7% being due to albumin.9 A significant reduction in 24-h proteinuria was also seen in the five patients who came off hemodialysis but this was also true for five of the eight patients who needed chronic kidney replacement therapy in spite of a significant decrease in pathogenic light chains. The latter finding highlights the need for early diagnosis and effective therapy in order to prevent irreversible kidney damage as the chance of reversing renal impairment seems to decrease with increasing duration of the complication, with 3 weeks having been mentioned as an approximate, critical time point for no return of renal function.22
The importance of a significant myeloma response as a prerequisite for a renal response is also substantiated by results from the univariate analysis which revealed a statistically significant correlation between myeloma response and renal response (P<0.017). Renal response also correlated with progression-free survival (P<0.01) and overall survival (P<0.048) a finding which may be expected23 but which has not been uniformly observed in other studies.24 Such correlations likely depend on the nature and severity of the underlying renal disease and on other patients’ characteristics as well as on the treatment employed.
Several studies have assessed the role of treatment combinations based on bortezomib, thalidomide, and lenalidomide in newly diagnosed young and elderly patients as well as in those with relapsed/refractory disease25–31 with different degrees of renal impairment. Almost all of these studies were post hoc, retrospective analyses, usually not discriminating between renal pathologies caused by light chains, and other much more frequent and trivial causes, such as hypovolemia, infection, nephrotoxic drugs, and contrast media, which are mostly rapidly reversible.32 In these studies no attempts were made to rule out hypertension-associated glomerular sclerosis or to perform a renal biopsy in cases with uncertain causes of renal impairment.
Results of large clinical trials show improvement of renal function particularly with bortezomib-based regimens. In the VISTA trial, a CRrenal was achieved in 37% of patients with eGFR <30 mL/min who were treated with bortezomib, melphalan and prednisone and in 7% of those treated with melphalan and prednisone.26 In the more recent HOVON/95 - GMMG-HD4 trial, more patients with baseline creatinine level of ≥2 mg/mL achieved a renal response with bortezomib, adriamycin and dexamethasone (PAD) induction therapy compared to those receiving vincristine, adriamycin and dexamethasone (VAD) induction therapy (81% versus 63%; P=0.31).25 Overall survival at 3 years was similar in patients with and without increased creatinine levels in the PAD-treated patients (74% versus 79%; P=0.68) but was clearly inferior in patients with renal impairment treated with VAD (34% in those with creatinine ≥2 mg/mL versus 76% in patients with creatinine <2 mg/mL, P<0.001, across all groups) showing higher efficacy of the bortezomib-based regimen in overcoming the negative impact of decreased renal function. Of note, screening in both trials took longer than in our study, thus excluding patients with renal emergencies and requirement for immediate initiation of therapy and limiting, to some extent, direct comparison of the results. An analysis of 133 consecutive patients with eGFR ≤60 mL/min treated between 2001 and 2011 with regimens based on one of the three first-generation novel agents showed similar renal response rates (thalidomide-based: 74%, lenalidomide-based: 64%, bortezomib-based: 81%; P=0.153), but bortezomib-containing regimens induced deeper renal responses (P<0.011).33 When interpreting these findings it should be kept in mind that lenalidomide-treated patients were significantly older than patients treated with bortezomib-based therapy (76 versus 65 years), which per se is associated with lower GFR and mandates less aggressive therapy, limitations that likely contributed to the differences in outcome. Nonetheless, when treatment has to be selected for patients not participating in clinical trials a three-drug combination including bortezomib, an immunomodulatory drug or another suitable active substance and dexamethasone seems to be an optimal choice, if the patient is fit enough to tolerate such a regimen. When feasible, high-dose dexamethasone should be used during the first cycle in order to maximize the reduction of pathogenic light chains but infectious prophylaxis and careful monitoring of the patient are essential in order to be able to intervene immediately in the case of imminent or actual complications. Lenalidomide-dexamethasone may be a particularly suitable option in patients with severe preexisting neuropathy and in those in whom bortezomib-based regimens have failed. Although not formally tested in a prospective trial, addition of cyclophosphamide or another suitable third drug may be considered in fit patients with the aforementioned conditions.
Table 3.
Hematologic and non-hematologic toxicities in the ITT population.
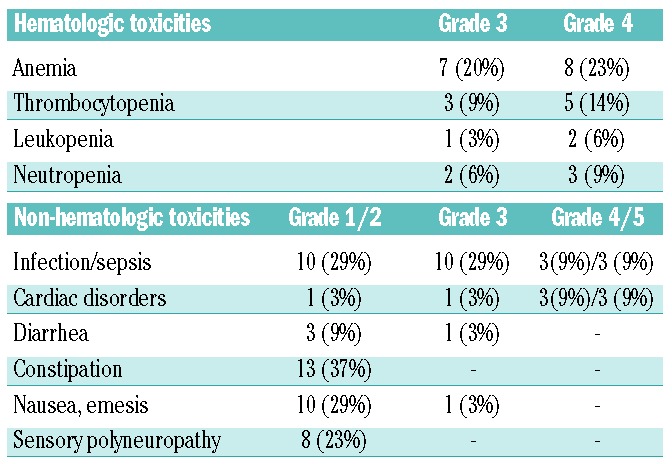
We consider the prospective nature and the careful monitoring of our patients as strengths of this trial. The inability to obtain a kidney biopsy in every patient but only in 43% of patients may be seen as limitation although full-blown cast nephropathy can be reliably diagnosed by careful clinical and laboratory investigation.9 The limited number of patients may be considered as a further weakness but the data provide the proof of principle that lenalidomide, at a dose adjusted according to renal function, can safely be given to patients with severe renal failure and results in very rapid clearance of involved free light chains when combined with high-dose dexamethasone in the first cycle of treatment. This combination can be associated with clinically relevant toxicity, particularly in patients with higher baseline C-reactive protein levels, more aggressive disease, as indicated by higher lactate dehydrogenase levels, and higher age. Patients with acute renal impairment pose a dilemma as high-dose dexamethasone is required to enhance the anti-myeloma effect and possibly also to suppress, non-specifically, immunoglobulin production, but this puts them at greater risk of side effects. Presently, it remains unclear whether lower doses, such as 20 mg, would be similarly effective with lower toxicity.1 Infections were the most frequent and severe non-hematologic toxicity and associated with a fatal outcome in six patients. This problem is well recognized in myeloma patients with renal impairment and is a major contributor to shortened survival,34 which was also seen in our cohort of patients. Dexamethasone, particularly at higher doses, is also cardiotoxic and this was recognized in this study too with three cardiac deaths and four cases of grade 3/4 toxicity. Other non-hematotoxic side effects were rare.
In conclusion, the combination of lenalidomide plus high-dose dexamethasone results in a rapid reduction of pathogenic lights chains with a greater than 95% reduction of involved free light chains within the first cycle of treatment. The overall myeloma response rate was 68.6% and renal responses were noted in 45.7% of patients with light chain-induced acute renal failure. Elderly patients with ongoing inflammation and/or infection were at greater risk of dexamethasone-related side effects, whereas lenalidomide-induced hematologic toxicity was readily manageable.
Acknowledgments
The authors thank Veronique Fritz for her secretarial and statistical assistance in finalizing this report. The study was supported in part by the Austrian Forum against Cancer and by a research grant from Celgene
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Haynes R, Leung N, Kyle R, Winearls CG. Myeloma kidney: improving clinical outcomes? Adv Chronic Kidney Dis. 2012; 19(5):342–351. [DOI] [PubMed] [Google Scholar]
- 2.Leung N, Bridoux F, Hutchison CA, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012; 120(22):4292–4295. [DOI] [PubMed] [Google Scholar]
- 3.Ying WZ, Sanders PW. Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein. Am J Pathol. 2001; 158(5):1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cserti C, Haspel R, Stowell C, Dzik W. Light-chain removal by plasmapheresis in myeloma-associated renal failure. Transfusion. 2007;47(3):511–514. [DOI] [PubMed] [Google Scholar]
- 5.Iggo N, Winearls CG, Davies DR. The development of cast nephropathy in multiple myeloma. QJM. 1997;90(11):653–656. [DOI] [PubMed] [Google Scholar]
- 6.Murphy PT, Baldeo C, O’Kelly P, et al. Dialysis-dependent renal failure at diagnosis continues to be associated with very poor outcome in multiple myeloma. Br J Haematol. 2014;165(6):890–891. [DOI] [PubMed] [Google Scholar]
- 7.Haynes RJ, Read S, Collins GP, Darby SC, Winearls CG. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single centre. Nephrol Dial Transplant. 2010;25(2):419–426. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25(1):195–200. [DOI] [PubMed] [Google Scholar]
- 9.Leung N, Gertz M, Kyle RA, et al. Urinary albumin excretion patterns of patients with cast nephropathy and other monoclonal gammopathy-related kidney diseases. Clin J Am Soc Nephrol. 2012;7(12): 1964–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ainsworth NL, Marshall A, Hatcher H, Whitehead L, Whitfield GA, Earl HM. Evaluation of glomerular filtration rate estimation by Cockcroft-Gault, Jelliffe, Wright and Modification of Diet in Renal Disease (MDRD) formulae in oncology patients. Ann Oncol. 2012;23(7):1845–1853. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol. 2010; 28(30):4635–4641. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009;9(4):302–306. [DOI] [PubMed] [Google Scholar]
- 14.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 15.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5): 1115–1123. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Ass. 1958;53:457–481. [Google Scholar]
- 17.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Ass. 1952;47:583–621. [Google Scholar]
- 18. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 19.Rota S, Mougenot B, Baudouin B, et al. Multiple myeloma and severe renal failure: a clinicopathologic study of outcome and prognosis in 34 patients. Medicine (Baltimore). 1987;66(2):126–137. [DOI] [PubMed] [Google Scholar]
- 20.Cockwell P, Hutchison CA. Management options for cast nephropathy in multiple myeloma. Curr Opin Nephrol Hypertens. 2010;19(6):550–555. [DOI] [PubMed] [Google Scholar]
- 21.Hutchison CA, Cockwell P, Stringer S, et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22(6):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung N, Nasr SH. Myeloma-related kidney disease. Adv Chronic Kidney Dis. 2014;21(1):36–47. [DOI] [PubMed] [Google Scholar]
- 23.Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000; 65(3):175–181. [DOI] [PubMed] [Google Scholar]
- 24.Eleftherakis-Papapiakovou E, Kastritis E, Roussou M, et al. Renal impairment is not an independent adverse prognostic factor in patients with multiple myeloma treated upfront with novel agent-based regimens. Leuk Lymphoma. 2011;52(12):2299–2303. [DOI] [PubMed] [Google Scholar]
- 25.Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014; 99(1):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27(36): 6086–6093. [DOI] [PubMed] [Google Scholar]
- 27.San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008;22(4):842–9. [DOI] [PubMed] [Google Scholar]
- 28.Tosi P, Zamagni E, Tacchetti P, et al. Thalidomide-dexamethasone as induction therapy before autologous stem cell transplantation in patients with newly diagnosed multiple myeloma and renal insufficiency. Biol Blood Marrow Transplant. 2010; 16(8):1115–1121. [DOI] [PubMed] [Google Scholar]
- 29.Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116(16):3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benboubker L, Dimopoulos MA, et al. Continuous lenalidomide and dexamethasone for newly diagnosed multiple myeloma. N Engl J Med 2014;371(10):906–917. [DOI] [PubMed] [Google Scholar]
- 31.Tosi P, Gamberi B, Castagnari B, et al. Lenalidomide in combination with dexamethasone in elderly patients with advanced, relapsed or refractory multiple myeloma and renal failure. Mediterr J Hematol Infect Dis. 2013;5(1):e2013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chanan-Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA. Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res. 2012; 18(8):2145–2163. [DOI] [PubMed] [Google Scholar]
- 33.Dimopoulos MA, Roussou M, Gkotzamanidou M, et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27(2):423–429. [DOI] [PubMed] [Google Scholar]
- 34.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukemia Working Party. J Clin Oncol. 2005;23(36): 9219–9226. [DOI] [PubMed] [Google Scholar]