Abstract
This study aimed to determine the impact of tyrosine kinase inhibitors given pre- and post-allogeneic stem cell transplantation on long-term outcome of patients allografted for Philadelphia chromosome-positive acute lymphoblastic leukemia. This retrospective analysis from the EBMT Acute Leukemia Working Party included 473 de novo Philadelphia chromosome-positive acute lymphoblastic leukemia patients in first complete remission who underwent an allogeneic stem cell transplantation using a human leukocyte antigen-identical sibling or human leukocyte antigen-matched unrelated donor between 2000 and 2010. Three hundred and ninety patients received tyrosine kinase inhibitors before transplant, 329 at induction and 274 at consolidation. Kaplan-Meier estimates of leukemia-free survival, overall survival, cumulative incidences of relapse incidence, and non-relapse mortality at five years were 38%, 46%, 36% and 26%, respectively. In multivariate analysis, tyrosine-kinase inhibitors given before allogeneic stem cell transplantation was associated with a better overall survival (HR=0.68; P=0.04) and was associated with lower relapse incidence (HR=0.5; P=0.01). In the post-transplant period, multivariate analysis identified prophylactic tyrosine-kinase inhibitor administration to be a significant factor for improved leukemia-free survival (HR=0.44; P=0.002) and overall survival (HR=0.42; P=0.004), and a lower relapse incidence (HR=0.40; P=0.01). Over the past decade, administration of tyrosine kinase inhibitors before allogeneic stem cell transplantation has significantly improved the long-term allogeneic stem cell transplantation outcome of adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Prospective studies will be of great interest to further confirm the potential benefit of the prophylactic use of tyrosine kinase inhibitors in the post-transplant setting.
Introduction
The Philadelphia (Ph) chromosome is one of the most frequent cytogenetic abnormalities in adult acute lymphoblastic leukemia (ALL). Its prevalence increases with age, accounting for 12%–30% in patients aged 18–35 years, 40% in patients aged 36–50 years, and over 50% in patients aged over 60 years.1,2 Philadelphia-positive (Ph+) ALL is associated with an at least 10% lower rate of achieving a first complete remission (CR1) with standard induction as compared to Ph-negative disease and has poor long-term prognosis, with a median survival of eight months.3,4 In recent years, BCR-ABL1-directed tyrosine kinase inhibitors (TKIs), such as imatinib mesylate (and more recently dasatinib or nilotinib), combined with chemotherapy have been found to be very effective for inducing disease remission in patients with Ph+ALL without additional toxicity, suggesting that better long-term outcomes may be possible.5–10 In the pre-TKI era, allo-SCT was considered to be the standard of care using either a matched sibling or unrelated donor in first CR (CR1) in adults.11–17 Although allo-SCT could offer a curative option in Ph+ ALL,17 relatively high rates of relapse and non-relapse mortality (NRM) limited the benefit of an allo-SCT.15,18–20 The international Bone Marrow Transplant Registry reported a leukemia-free survival (LFS) rate of 38% following human leukocyte antigen (HLA)-identical allo-SCT in CR1.13 Several groups have reported that TKI-based induction chemotherapy produced high CR rates, thus allowing a high proportion of patients to proceed to allo-SCT.18,21–25 However, there is little information on the efficacy of TKI administration after allo-SCT.26,27 Moreover, the impact of TKI-based therapy on long-term outcome after allo-SCT remains unclear. To address whether allo-SCT associated with TKI administration before and/or after allo-SCT is a valid therapeutic approach as compared to conventional chemotherapy, we conducted a retrospective comparative study assessing the outcome of 473 adult patients with Ph+ ALL who, between 2000 and 2010, underwent allo-SCT in CR1 with HLA-identical siblings or matched unrelated donors, with a special emphasis on the impact of TKIs.
Methods
Study design, data collection and selection criteria
Patients with Ph+ ALL reported to the registry of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) were included in this study. For the purpose of this specific analysis, participating centers were requested to enroll consecutive Ph+ALL cases diagnosed between January 2000 and December 2010. The study aimed to include cases of Ph+ B-ALL receiving first allo-SCT from an HLA-identical sibling donor (MSD) or HLA-matched unrelated donor (at least 6/6 HLA matching) (MUD) who: i) were aged 18 years or over at time of transplant; ii) were in CR1; iii) were transplanted between 2000 and 2010; iv) received allogeneic unmanipulated bone marrow (BM) or peripheral blood stem cells (PBSC) as stem cell source; v) received a standard myeloablative conditioning (MAC) regimen or a reduced-intensity conditioning (RIC) regimen according to Bacigalupo’s criteria;28 and vi) whose complete clinical data and outcomes were available. A total of 473 allo-SCT recipients from 77 participating centers met these eligibility criteria. Institutional review board approval was obtained from all participating institutions.
Minimal residual disease assessment
Investigators were asked to provide minimal residual disease (MRD) data at time of transplant. As the most commonly accepted level of sensitivity for a given sample to be considered PCR negative is 10−4 BCR/ABL copies, we distinguished two groups for the purpose of this analysis: a “low-risk” group with MRD ≤10−4, and a “high-risk” group with a ratio >10−4.29,30
Statistical analyses and definitions
The primary end points were leukemia-free survival (LFS), relapse incidence (RI), and non-relapse mortality (NRM). Secondary end points were overall survival (OS), acute graft-versus-host disease (aGvHD) and chronic graft-versus-host-disease (cGvHD).
Patient-related, disease-related, and transplant-related variables were compared between the 2 groups receiving or not TKI before transplantation using the χ2 statistics for categorical variables and the Mann-Whitney test for continuous variables. Factors that differed significantly between the two groups with P values less than 0.05, and all factors associated with a P value less than 0.10 by univariate analysis were included in the final models. Cumulative incidence functions (CIF) were used to estimate RI and NRM in a competing risk setting, because death and relapse compete with each other. To study cGvHD, we considered relapse and death to be competing events. Probabilities of LFS and OS were calculated using Kaplan-Meier estimates. Univariate analyses were performed using Gray’s test for CIF and the log rank test for LFS and OS. Associations of patients’ and graft characteristics with outcomes were evaluated in multivariate analysis, using Cox proportional hazards model. In order to assess the possible impact of acute GvHD, chronic GvHD and use of up-front prophylactic TKI after transplant on outcome, we used a Cox model with time-dependent variables.
All tests were two-sided. The type-1 error rate was fixed at 0.05 for determination of factors associated with time-to-event outcomes. Statistical analyses were performed with SPSS 19 (SPSS Inc./IBM, Armonk, NY, USA) and R 3.0.1 (R Development Core Team, Vienna, Austria) software packages.
Results
Patients’ characteristics
Four hundred and seventy-three allo-SCT recipients were included in this study (Online Supplementary Table S1); 260 (55%) were males. Median age was 42 years (range 18–70). Sixty-nine (15%) patients presented with extramedullary disease at time of diagnosis and the median white blood cell (WBC) count was 20.9×109/L (range 0.3–640). Three hundred and seventy-five (79.3%) patients received a standard MAC regimen and 98 (20.7%) an RIC regimen. One hundred and fifty-five (33.3%) patients received antithymocyte globulin (ATG) as part of the conditioning regimen. The median follow up (F/U) of alive patients after allo-SCT was 45 months.
Tyrosine kinase treatment features
Three hundred and ninety (82.5%) patients received TKIs before transplant. Among them, 329 received TKIs at induction, 274 at consolidation, 100 at induction only, 61 at consolidation only, 213 both at induction and consolidation. For 16 patients who received TKIs at induction, information at consolidation was missing. As expected, imatinib was the most widely used TKI (89.1%) at a median dose of 600 mg/d, followed by dasatinib (9.3%) at a median dose of 140 mg/d, while 6 patients received nilotinib (3 unknown cases). The median time of TKI initiation was 11 days (range 0–363) after diagnosis and median duration of administration was 99 days (range 7–385). The groups “TKIs before allo-SCT” and “no TKIs before allo-SCT” were comparable in terms of age, WBC at diagnosis, interval from diagnosis to CR1 and from CR1 to HSCT, patient sex, donor sex, extramedullary disease at diagnosis, type of donor, and level of MRD (Online Supplementary Table S1). Median follow up of patients without TKIs before allo-SCT and with TKIs before transplant were 98 months (range 1–142) and 42 months (range 1.2–145), respectively (P<0.0001). The two groups were also different in terms of stem cell source: PBSC was given to 309 patients (79%) in the group with TKIs versus 55 patients (66%) in the group without TKIs (P=0.01). More patients received MAC regimen in the group without TKIs before allo-SCT (96% vs. 76%; P=0.001).
Finally, 157 patients received TKIs after transplant at a median of 83 days (range 0–1786) after transplant: 124 received imatinib, 26 dasatinib, 1 nilotinib, and 6 missing cases. In all, 60 patients received TKIs post transplant for primary prophylaxis of relapse.
Outcome
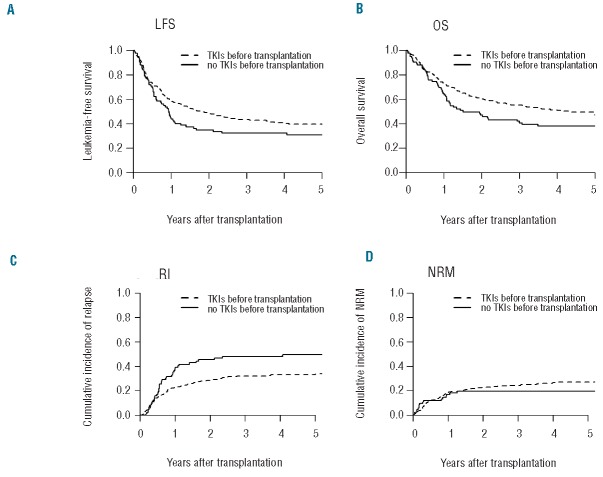
At five years, OS of the whole population was 46% (95%CI: 41–51). In univariate analysis, TKIs before transplant was the only factor associated with better OS [47% (95%CI: 41–53) vs. 38% (95CI: 27–48) in patients not receiving TKIs before allo-SCT, respectively; P=0.04] (Table 1 and Figure 1). As aGvHD, cGvHD and TKI post transplant were considered as a time-dependent variable, HR and 95%CI can only be estimated by using Cox model with time-dependent variable (Online Supplementary Table S2). In multivariate analysis for OS, 2 factors were favorable factors: TKI administration before allo-SCT (HR=0.68; 95%CI: 0.47–0.98; P=0.04), and TKIs given post transplant in prophylaxis of relapse (HR=0.42; 95%CI: 0.23–0.76; P=0.004) whereas older age of the patient, a longer interval from diagnosis to transplant, and aGvHD II or over were unfavorable factors (HR=1.02, 95%CI: 1.00–1.03, P=0.01; HR=1.002, 95%CI: 1.001–1.004, P=0.001; HR=1.43, 95%CI: 1.05–1.94, P=0.02, respectively).
Table 1.
Univariate analysis for pre-allo-SCT factors at five years.
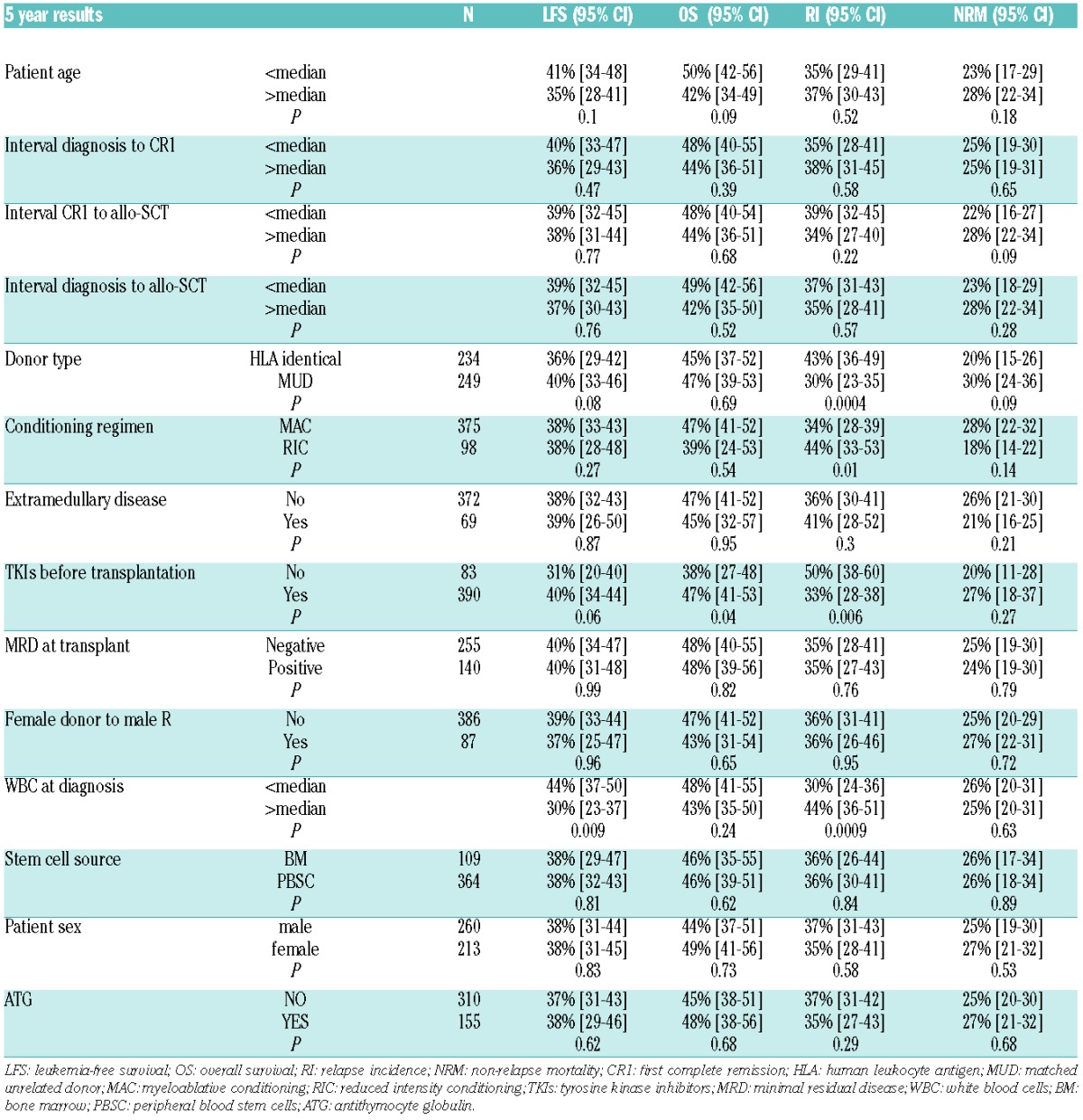
Figure 1.
Probability of (A) leukemia-free survival (LFS), (B) overall survival (OS), (C) relapse incidence (RI), and (D) non-relapse mortality (NRM) in allografted patients with Ph+ALL in first complete remission with TKIs before allogeneic stem cell transplantation versus without TKIs before allogeneic stem cell transplantation.
Overall LFS at five years was 38% (95%CI: 0.34–0.43). In univariate analysis, higher WBC at diagnosis was significantly associated with lower LFS [30% (95%CI: 23–37) vs. 44% (95%CI: 37–50); P=0.014]. Multivariate analysis for LFS identified 2 favorable factors: MUD (HR=0.65; 95%CI: 0.46–0.92; P=0.014) and, interestingly, TKIs given post transplant (HR=0.44; 95%CI: 0.26–0.74; P=0.002) (Table 2). Higher WBC at diagnosis and longer interval from diagnosis to transplant were associated with lower LFS (HR=1.002, 95%CI: 1.000–1.003, P=0.01, and HR=1.002, 95%CI: 1.001–1.003, P=0.003, respectively).
Table 2.
Multivariate analysis of leukemia-free survival, overall survival, reduced intensity and non-relapse mortality at 5 years.
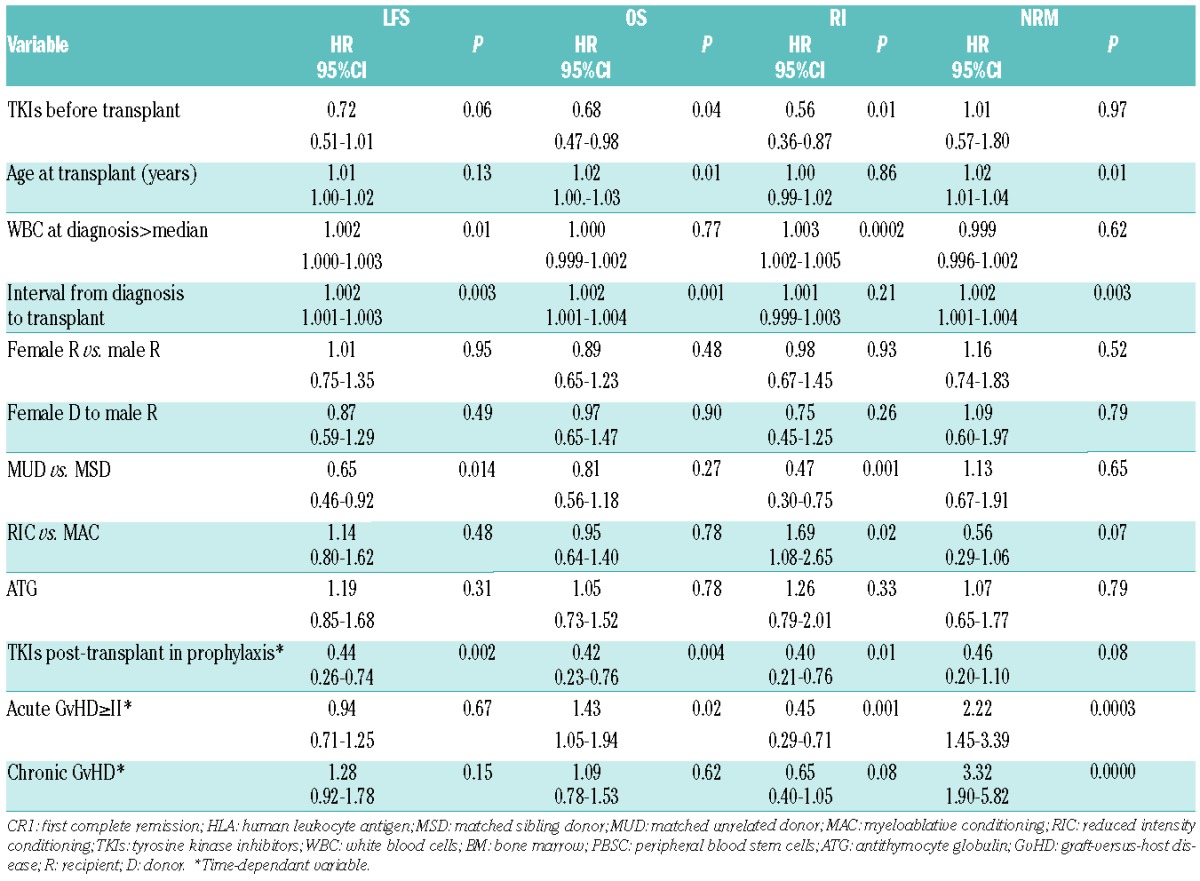
In this cohort, 94 (40%) patients died from disease relapse, with a cumulative incidence of relapse of 36% (95%CI: 0.32–0.41) at five years. In univariate analysis, the cumulative incidence of RI in the group of patients with TKIs before allo-SCT was 33% (95%CI: 28–38) versus 50% (95%CI: 38–60) in the group with no TKIs before transplant (P=0.006) (Figure 1). In multivariate analysis, use of TKIs before transplant (HR=0.56; 95%CI: 0.36–0.87; P=0.01) and the type of donor (MUD vs. MSD) (HR=0.47; 95%CI: 0.30–0.75; P=0.001) were the 2 significant protective factors against relapse. Two other “post transplant” factors were associated with lower RI in multivariate analysis: TKIs post transplant in prophylaxis (HR=0.40; 95%CI: 0.21–0.76; P=0.01) and aGvHD grade II or over (HR=0.45; 95%CI: 0.29–0.71; P=0.001). The use of an RIC regimen (HR=1.69; 95%CI: 1.08–2.65; P=0.02) and higher WBC at diagnosis (HR=1.003; 95%CI: 1.002–1.005; P=0.0002) remained a significant risk factor for higher RI.
Cumulative incidence of NRM was 26% (95%CI: 0.22–0.30) at five years. Sixty-six (28%) patients died from GvHD, 37 (15.7%) from infections and 8 (3.4%) from veno-occlusive disease. In uni- and multivariate analysis, the use of TKIs before allo-SCT did not influence NRM after transplant [27% (95%CI_18–37) in patients who received TKIs vs. 20% (95%CI: 11–28) in patients without TKIs; P=0.27]. Besides, in univariate analysis, no factor was significantly associated with NRM. In multivariate analysis, aGvHD and cGvHD were unfavorable factors for NRM (HR=2.22; 95%CI: 1.45–3.39; P=0.00003 and HR=3.32; 95%CI: 1.90–5.82; P<10−4, respectively) as well as age at transplant and time from diagnosis to transplant.
Impact of minimal residual disease on outcome
In this study, 395 patients were evaluated for MRD at time of transplant. MRD was determined by RT-PCR in 344 patients (93%), and by flow cytometry in 26 (7%) patients (25 missing cases). The median time for MRD evaluation prior to transplant was 16 (range 0–80) days. Two hundred and fifty-five (65%) had an MRD of 10−4 or under and 140 patients (35%) had an MRD at transplant over 10−4. Interestingly, there were no statistically significant differences in terms of OS, LFS, RI and NRM at five years between the “low-risk” and the “high-risk” MRD groups: LFS, 40% (95%CI: 34–47) versus 40% (95%CI: 31–48) (P=0.99); OS, 48% (95%CI: 40–55) versus 48% (95%CI: 39–56, %±4) (P=0.82), RI: 35% (95%CI: 28–41) versus 35% (95%CI: 27–43) (P=0.76); and NRM, 25% (95%CI: 19–41) versus 24% (95%CI: 19–30) (P=0.79), respectively. Also, no correlation was found between use of TKIs before transplant and MRD level at transplant (P=0.17).
Graft-versus-host disease
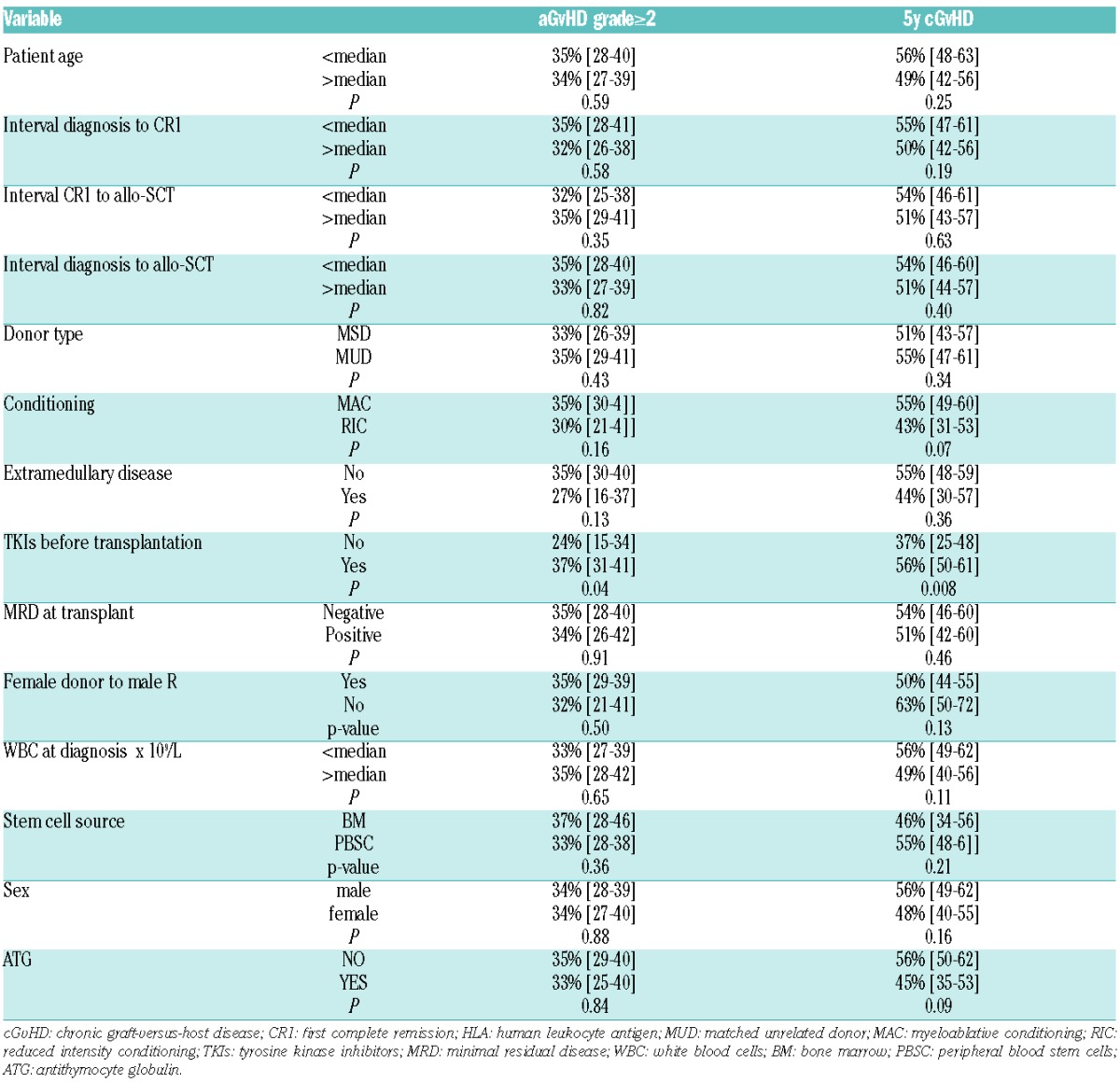
The cumulative incidence of grade 2–4 aGvHD at 100 days and cGvHD at five years were 40%±2 and 53%±2, respectively. In univariate analysis, the use of TKIs pre-transplant was significantly associated with a higher rate of aGvHD grade II+ [37% (95%CI: 31%-41%) vs. 24% (95%CI: 15%-34%); P=0.04] and of cGvHD [56% (95%CI: 50%-60%) vs. 37% (95%CI: 25%-48%) (P=0.008)] (Table 3). As TKIs post transplant was considered as a time-dependent variable, HR and 95%CI can only be estimated by using Cox model with time-dependant variable and are indicated in Online Supplementary Table S3. However, in multivariate analysis, the use of TKI before transplant after allo-SCT, studied as a time-dependant variable, was not associated with aGvHD or cGvHD, although TKIs post transplant was significantly associated with lower cumulative incidence of aGvHD (HR=0.21; 95%CI: 0.05–0.85; P=0.03). ATG was a protective factor of cGvHD (HR=0.57; 95%CI: 0.40–0.82; P=0.002).
Table 3.
Univariate analysis for acute graft-versus-host disease and chronic graft-versus-host disease at five years.
Discussion
This study confirms that TKIs are a critical treatment component for patients with adult Ph+ALL in whom an allo-SCT is indicated. We found a significantly superior 5-year post-transplant OS due to lower relapse rates in patients who received TKIs before allo-SCT. It is clear that inclusion of imatinib and newer TKIs into the various phases of the treatment of Ph+ ALL has re-shaped the therapeutic algorithm. Thomas et al.31 showed that the association of imatinib with induction chemotherapy resulted in a 2-year OS reaching 56%, including the group of patients who proceeded to standard MAC allo-SCT. Another single center study showed that incorporation of imatinib into induction chemotherapy with subsequent allo-SCT after MAC achieved a 3-year OS rate of 78%.22 In a smaller series of 25 patients with Ph+ ALL in CR1 who received post-transplant imatinib, the 3-year OS reached 62%.32 Although several earlier studies suggested a benefit for including TKIs in the treatment of Ph+ ALL, evidence of a long-term survival benefit has only become available more recently because many of the initial studies concerned early data with short-term follow up. The Japanese Adult Leukaemia Study Group recently reported a higher 3-year OS probability of 65% when combining imatinib-based induction and MAC allo-SCT.33 However, many of these studies included a significant proportion (up to 39%) of untransplanted patients. Our current study is the largest patient group yet reported to receive allo-SCT in Ph+ ALL in CR1 in the era of TKIs, with a long-term follow up at five years post allo-SCT. The use of pre-transplant TKIs had the beneficial effect in that all patients were in CR1 before allo-SCT. This is consistent with other observations that TKIs enhance the feasibility of allo-SCT for Ph+ALL by increasing remission rates and extending remission durations.17,18,23,25,31,34−38 In this study, pre-transplant TKI use was an independent variable in multivariate analysis for improving long-term OS in patients transplanted in CR1. This superior OS appeared to be largely related to a lower rate of relapse. Indeed, pre-transplant TKIs were associated with lower RI in both uni- and multivariate analyses. Consistent with other reports, we found that the use of TKIs before transplant was not associated with increased NRM.27
In this study, the MRD level at transplant was not predictive for outcome, and did not correlate with TKI treatment. Reports from the pre-imatinib era suggested a close correlation between BCR-ABL transcript levels and outcome.39,40 In the TKI era, BCR-ABL transcript levels have also been correlated with response in some studies.41 However, long-term outcome in relation to BCR-ABL response to any given therapeutic intervention is difficult to interpret. Unlike chronic myeloid leukemia, there is no clear agreement on what represents a therapeutically important BCR-ABL response at a given time point during Ph+ ALL therapy. Lee et al.20 showed that a 3-log reduction in BCR-ABL transcripts after one month of imatinib treatment predicted a reduced risk of relapse. In contrast, Yanada et al.42 observed no association between rapid achievement of BCR-ABL negativity and long-term outcome. In the present study, a 0.01% threshold was used to define MRD positivity, as widely used.43,44 However, using this threshold, we found no association between the MRD level before transplant and allo-SCT outcome. Several factors may account for this negative finding. First, despite some standardization in monitoring BCR-ABL fusion transcripts, this registry-based study included diverse methodologies for MRD determination. However, MRD was determined mainly by PCR for detection of chimeric mRNA arising from BCR-ABL1 genomic recombination in 344 patients (93%). This assay has been widely used to monitor response and guide therapeutic choices.20,40,42 Our results are quite consistent with two recently published studies. Schultz et al. reported 5-year outcomes of imatinib plus intensive chemotherapy in 91 children (age 1–21 years) with and without allogeneic BMT. MRD levels at the end of the second consolidation also did not predict outcome in those patients receiving either an unrelated or related donor BMT from all cohorts with 77±13% 5-year EFS for MRD 0.01% or under (n=14) compared with 40±22% for MRD over 0.01% (n=5; P=0.18).45 Bachanova et al. reported the outcome of 197 Ph+ALL patients who underwent allo-SCT. MRD before transplant, in the whole cohort, as determined by FISH and/or PCR, had no impact on relapse (P=0.13), disease-free survival (P=0.56) or overall survival (P=0.66). In sub-group analysis, patients receiving pre-HCT TKI in combination with MRD negativity pre-RIC HCT had superior OS (55%) compared with a similar MRD population after MAC (33%; P=0.0042).46 In our study, the number of patients in the RIC group who did not receive TKIs before allo-SCT (n=6) did not allow a subgroup analysis to be made. We can also hypothesize that, since TKIs induce profound molecular responses, a lower cutoff might have been more informative for correlating MRD with outcome. The lower relapse incidence seen in patients given pre-transplant TKI probably reflected a more profound molecular response in this patient group. It is also possible that post-transplant interventions with TKIs controlled allo-SCT patients with a higher MRD load until the development of the immune-mediated graft-versus-leukemia (GvL) effect. MRD monitoring of BCR-ABL fusion-transcript after allo-SCT for Ph+ ALL could shed some light on this possibility.47
The issue of whether, and under what circumstances, TKIs should be administered after allo-SCT remains unresolved. We found a significant benefit of prophylactic TKIs on LFS, OS and RI. Study design prevented a full analysis of treatment and tolerability of TKI administration post allo-SCT. However, previous data suggest that a relatively short exposure to TKIs may control residual leukemia prior to the establishment of a GvL effect. Pfeifer et al.30 recently compared prophylactic with MRD-triggered imatinib after allo-SCT for Ph+ ALL. Prophylactic imatinib significantly reduced the incidence of molecular recurrence after allo-SCT compared with MRD-triggered imatinib (40% vs. 69%; P=0.046). Interestingly, 5-year survival in both interventional groups was high (80% and 74.5%), despite premature discontinuation of imatinib in the majority of patients because of poor tolerability. Ram et al.32 reported that imatinib, given after RIC allo-SCT, did not significantly affect relapse. Taken together the results suggest that prophylactic TKIs may improve outcome, but further confirmation is needed. However, patients we identified as high risk for relapse (higher WBC, an RIC regimen, and matched sibling donor SCT) might especially benefit from post-transplant TKI. Despite the potential of RIC regimen recipients to relapse, we found no difference in OS and LFS between patients who received an RIC regimen or a MAC regimen. This confirms that RIC is a valid option for patients ineligible for MAC, as shown by Bachanova et al.46
Interestingly, pre-transplant TKI was associated with a higher cumulative incidence of aGvHD [37% (95%CI: 31%-41%) vs. 24% (95%CI: 15%-34%); P=0.04] in univariate analysis. Although imatinib can be beneficial in steroid-refractory cGvHD,48,49 the main imatinib sideeffects are edema, skin rashes and diarrhea.50 The pathophysiology of these manifestations is still unknown. We can hypothesize that the association of TKIs and chemotherapy before HSCT can be deleterious and induce primary lesions. However, TKI pre-transplant was not associated with aGvHD in multivariate analysis (HR=1.52; 95%CI: 0.92–2.52; P=0.010). Whether TKIs are or are not deleterious when used before transplantation deserves further investigation. In multivariate analysis, TKIs post-HSCT were associated with a lower incidence of GvHD. This interesting issue of the potential beneficial impact of TKIs post transplant will need to be confirmed since the bias of patient selection by the physician for giving TKIs may have interfered with this result.
In conclusion, over the past decade, TKI administration before allo-SCT has significantly improved the long-term allo-SCT outcome of adult Ph+ALL. Prospective studies will be of great interest to further confirm the potential benefit of the prophylactic use of TKIs in the post-transplant setting.
Acknowledgments
We thank all European Group for Blood and Marrow Transplantation (EBMT) participating centers and national registries for contributing patients to the study and data managers for their superb work (see Online Supplementary Appendix). Supplementary information is available at the EBMT Web site. This work was presented during the Presidential Session of the European Group for Blood and Marrow Transplantation annual meeting, April 6–10, 2013, London, UK.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by an educational grant from Novartis, France.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Larson RA. Management of acute lymphoblastic leukemia in older patients. Semin Hematol. 2006;43(2):126–133. [DOI] [PubMed] [Google Scholar]
- 2.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Secker-Walker LM, Craig JM, Hawkins JM, Hoffbrand AV. Philadelphia positive acute lymphoblastic leukemia in adults: age distribution, BCR breakpoint and prognostic significance. Leukemia. 1991;5(3):196–199. [PubMed] [Google Scholar]
- 4.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109(8):3189–3197. [DOI] [PubMed] [Google Scholar]
- 5.Ravandi F, Kebriaei P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):1043–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruber F, Mustjoki S, Porkka K. Impact of tyrosine kinase inhibitors on patient outcomes in Philadelphia chromosome-positive acute lymphoblastic leukaemia. Br J Haematol. 2009;145(5):581–597. [DOI] [PubMed] [Google Scholar]
- 7.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110(7):2309–2315. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. [DOI] [PubMed] [Google Scholar]
- 9.Foa R, Vitale A, Vignetti M, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–6528. [DOI] [PubMed] [Google Scholar]
- 10.Rea D, Legros L, Raffoux E, et al. High-dose imatinib mesylate combined with vincristine and dexamethasone (DIV regimen) as induction therapy in patients with resistant Philadelphia-positive acute lymphoblastic leukemia and lymphoid blast crisis of chronic myeloid leukemia. Leukemia. 2006;20(3):400–403. [DOI] [PubMed] [Google Scholar]
- 11.Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia–results of the prospective multicenter LALA-94 trial. Blood. 2002;100(7):2357–2366. [DOI] [PubMed] [Google Scholar]
- 12.Chao NJ, Blume KG, Forman SJ, Snyder DS. Long-term follow-up of allogeneic bone marrow recipients for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1995;85(11):3353–3354. [PubMed] [Google Scholar]
- 13.Barrett AJ, Horowitz MM, Ash RC, et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992;79(11): 3067–3070. [PubMed] [Google Scholar]
- 14.Snyder DS, Nademanee AP, O’Donnell MR, et al. Long-term follow-up of 23 patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with allogeneic bone marrow transplant in first complete remission. Leukemia. 1999;13(12):2053–2058. [DOI] [PubMed] [Google Scholar]
- 15.Laport GG, Alvarnas JC, Palmer JM, et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood. 2008;112(3):903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottmann OG, Pfeifer H. Management of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Hematology Am Soc Hematol Educ Program. 2009:371–381. [DOI] [PubMed] [Google Scholar]
- 17.Fielding AK, Rowe JM, Richards SM, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009; 113(19):4489–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644–3652. [DOI] [PubMed] [Google Scholar]
- 19.Mohty M, Labopin M, Volin L, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439–4443. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Kim DW, Cho B, et al. Risk factors for adults with Philadelphia-chromosome-positive acute lymphoblastic leukaemia in remission treated with allogeneic bone marrow transplantation: the potential of real-time quantitative reverse-transcription polymerase chain reaction. Br J Haematol. 2003;120(1):145–153. [DOI] [PubMed] [Google Scholar]
- 21.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–466. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Kim YJ, Min CK, et al. The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2005; 105(9):3449–3457. [DOI] [PubMed] [Google Scholar]
- 23.de Labarthe A, Rousselot P, Huguet-Rigal F, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–1413. [DOI] [PubMed] [Google Scholar]
- 24.Tanguy-Schmidt A, Rousselot P, Chalandon, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1):150–155. [DOI] [PubMed] [Google Scholar]
- 25.Ribera JM, Oriol A, Gonzalez M, et al. Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassmann B, Pfeifer H, Stadler M, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2005;106(2): 458–463. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter PA, Snyder DS, Flowers ME, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109(7):2791–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruggemann M, Schrauder A, Raff T, et al. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia. 2010; 24(3):521–535. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013; 27(6):1254–1262. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. [DOI] [PubMed] [Google Scholar]
- 32.Ram R, Storb R, Sandmaier BM, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96(8):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuta S, Matsuo K, Yagasaki F, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25(1):41–47. [DOI] [PubMed] [Google Scholar]
- 34.Fielding AK, Goldstone AH. Allogeneic haematopoietic stem cell transplant in Philadelphia-positive acute lymphoblastic leukaemia. Bone Marrow Transplant. 2008;41(5):447–453. [DOI] [PubMed] [Google Scholar]
- 35.Lee KH, Lee JH, Choi SJ, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2005;19(9):1509–1516. [DOI] [PubMed] [Google Scholar]
- 36.Wassmann B, Pfeifer H, Goekbuget N, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2006;108(5):1469–1477. [DOI] [PubMed] [Google Scholar]
- 37.Yanada M, Matsuo K, Suzuki T, Naoe T. Allogeneic hematopoietic stem cell transplantation as part of postremission therapy improves survival for adult patients with high-risk acute lymphoblastic leukemia: a metaanalysis. Cancer. 2006;106(12):2657–2663. [DOI] [PubMed] [Google Scholar]
- 38.Shimoni A, Kroger N, Zander AR, et al. Imatinib mesylate (STI571) in preparation for allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions in patients with Philadelphia-positive acute leukemias. Leukemia. 2003;17(2):290–297. [DOI] [PubMed] [Google Scholar]
- 39.Preudhomme C, Henic N, Cazin B, et al. Good correlation between RT-PCR analysis and relapse in Philadelphia (Ph1)-positive acute lymphoblastic leukemia (ALL). Leukemia. 1997;11(2):294–298. [DOI] [PubMed] [Google Scholar]
- 40.Pane F, Cimino G, Izzo B, et al. Significant reduction of the hybrid BCR/ABL transcripts after induction and consolidation therapy is a powerful predictor of treatment response in adult Philadelphia-positive acute lymphoblastic leukemia. Leukemia. 2005;19(4): 628–635. [DOI] [PubMed] [Google Scholar]
- 41.Stein AS, Palmer JM, O’Donnell MR, et al. Reduced-intensity conditioning followed by peripheral blood stem cell transplantation for adult patients with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2009;15(11):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanada M, Sugiura I, Takeuchi J, et al. Prospective monitoring of BCR-ABL1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol. 2008;143(4): 503–510. [DOI] [PubMed] [Google Scholar]
- 43.Raff T, Gokbuget N, Luschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910–915. [DOI] [PubMed] [Google Scholar]
- 44.Mortuza FY, Papaioannou M, Moreira IM, et al. Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20(4):1094–1104. [DOI] [PubMed] [Google Scholar]
- 45.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28(7):1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachanova V, Marks DI, Zhang MJ, et al. Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2014;28(3):658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radich J, Gehly G, Lee A, et al. Detection of bcr-abl transcripts in Philadelphia chromosome-positive acute lymphoblastic leukemia after marrow transplantation. Blood. 1997;89(7):2602–2609. [PubMed] [Google Scholar]
- 48.Olivieri A, Cimminiello M, Corradini P, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood. 2013; 122(25):4111–4118. [DOI] [PubMed] [Google Scholar]
- 49.Magro L, Mohty M, Catteau B, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009;114(3):719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deininger MW, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003; 21(8):1637–1647. [DOI] [PubMed] [Google Scholar]