Prophylaxis for hemophilia is the scheduled infusion of the missing clotting factor with pre-specified dose, with the intention of preventing bleeds and subsequent hemophilic arthropathy. It is the treatment of choice for patients with severe hemophilia A in countries with available resources.1 Around the year 2000 it was reported that prophylaxis is most efficient when initiated early: before 3 years of age2 or directly after the first joint bleed.3,4 These strategies are reflected in the two most frequently used definitions of primary prophylaxis. The European Pediatric Network for Hemophilia Management (PedNet) first specified primary prophylaxis as starting before two years of age OR before the second joint bleed,5 and The World Federation of Hemophilia (WFH) as starting before the age of three years AND before the second joint bleed, in the documented absence of osteochondral joint disease.1
Early prophylaxis may require placing a central venous access device (CVAD) to facilitate frequent venous access, but these devices carry a risk of infections and thrombotic complications.6 In an attempt to reduce the need for CVADs while initiating early prophylaxis, Petrini and colleagues started prophylaxis with once-weekly infusions;2,7 many authors have subsequently published or recommended protocols starting with once-weekly infusions.8–10
The present study assesses how the increasing awareness of the importance of early prophylaxis affected bleeding before prophylaxis, CVAD use, initial prophylactic regimens, and achievement of primary prophylaxis.
Data on 919 patients with severe hemophilia A (FVIII<0.01 IU/mL), born 1990–2010, collected for the CANAL study11 (n=313) and the PedNet registry12 (n= 606) were used. Nine and 16 patients were excluded from CANAL and PedNet, respectively, because no treatment data were available.
Case report forms and inclusion and exclusion criteria were the same for both datasets.11,12 Anonymized data on patients’ demographics, bleeding, and details on all factor administrations were collected by the participating centers until 1st May 2013. For the present analysis, patients were followed from birth until the 50th treatment day with FVIII or the development of a clinically relevant inhibitor, defined as at least two positive inhibitor titers, combined with a decreased in vivo Factor VIII recovery. Start of prophylaxis was defined as the regular infusion of FVIII at least once-weekly and continued for at least two months. Data were analyzed in 5-year birth cohorts. Most parameters had a skewed distribution and were, therefore, presented as medians and interquartile range (IQR). Trends over time were analyzed using univariable linear or logistic regression. Kaplan-Meier survival analysis was used to assess the occurrence of the first joint bleed and cumulative incidences of start of prophylaxis and CVAD use, while accounting for differences in follow up due to inhibitor development. Differences in survival curves across birth cohorts were assessed using the log rank test.
Initiation of treatment and prophylaxis
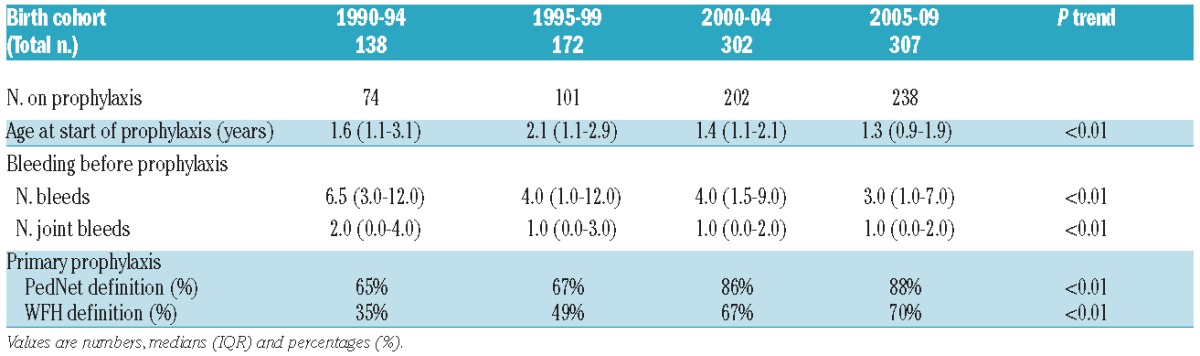
The median age at initiation of prophylaxis decreased from 1.6 years in the first birth cohort, to 1.3 years in the last birth cohort (Table 1) (P<0.01). Concomitantly, the proportion of patients starting prophylaxis before the age of three years increased from 45% to 84% (P<0.01).
Table 1.
Bleeding before prophylaxis and primary prophylaxis according to birth cohort.
Bleeding before prophylaxis
The first joint bleed occurred at a median of 1.7 years (IQR 1.0–2.8). Over time, fewer bleeds were accepted before initiating prophylaxis (Table 1). While the median number of joint bleeds before initiation of prophylaxis decreased, the proportion of patients starting prophylaxis before any joint bleed increased from 29% to 43% (Figure 1) (P=0.06). Since 1990, especially the proportion of patients starting prophylaxis before the second joint bleed increased (from 43% to 72%) (P<0.01).
Figure 1.
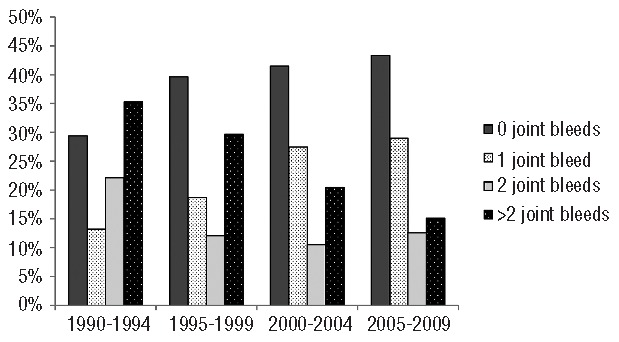
Joint bleeding before prophylaxis according to birth cohort.
The proportion of patients receiving primary prophylaxis according to the first PedNet definition (<2 years OR <2nd joint bleed)5 increased from 65% in the first, to 88% in the last birth cohort (P<0.01). According to the WFH definition (<3 years AND <2nd joint bleed), fewer patients started primary prophylaxis, yet the proportion still doubled from 35% to 70% (P<0.01). The WFH definition however, excludes the 21% of patients who had their first joint bleed after the age of three years and initiated prophylaxis afterwards.
Initiating prophylaxis
Use of a CVAD in patients on prophylaxis was relatively stable over time (P=0.85): by four years of age, approximately 40% of patients who started prophylaxis had used a CVAD. Prophylaxis was increasingly started using once-weekly regimens: from only 18% in the early 1990s to 59% in the last birth cohort (P<0.01). The increase was most prominent after the year 2000. Concomitantly, starting prophylaxis with 3 or more infusions a week decreased from 41% to 18% (P<0.01).
As this study focused on prophylaxis initiated within the first 50 days of treatment, results should not be extrapolated to a context in which prophylaxis is started later in life. Therefore, limiting the window of observation to the first 50 treatment days will have resulted in an underestimation of the overall age at start of prophylaxis and the number of bleeds incurred before prophylaxis, especially in the earlier cohorts. Differences between the first and last two cohorts could, therefore, be bigger than those presented in this study.
Comparison with other studies
National guidelines and recommendations issued in several European countries advised starting prophylaxis early,8,13 often combining criteria of both age and number of bleeds at initiation of prophylaxis. The results of this study confirm that there is an increasing tendency not only to start prophylaxis before the age of three years or before the second joint bleed, but also that more patients start on primary prophylaxis. The two circulating definitions of primary prophylaxis have different benefits and drawbacks. The 1999 PedNet definition uses a maximum age of two years OR a maximum of one joint bleed, and, therefore, potentially includes patients suffering many joint bleeds before the age of two years. The WFH definition, on the other hand, uses a maximum age of three years AND a maximum of one joint bleed.1 This makes it impossible to start ‘primary prophylaxis’ in the 21% of patients with a milder phenotype, characterized by the onset of joint bleeding after the age of three years.
The idea of initiating prophylaxis with once-weekly infusions originated in Sweden where it was applied with the aim of reducing the need for CVADs.2,7 This study shows that once-weekly infusions are now used in the majority of patients, even in countries without a formal protocol advising this strategy. CVAD use in this study was stable at around 40%. The two trends (starting prophylaxis earlier and starting with once-weekly infusions) likely balanced out and led to stable rates of use of CVADs. In addition, the frequency of venous access may be associated with the occurrence of CVAD complications. This question requires longer follow up and will be addressed in an ongoing study on CVAD management and complications.
Reports on the effects of early and/or low-dose prophylaxis on inhibitor development have been conflicting.9,14 As the etiology of inhibitor development is multifactorial, any analysis on the effects of timing and/or regimen of prophylaxis on inhibitor development should be adjusted for other risk factors of inhibitor development. The recent multivariable analysis by Gouw et al. on the RODIN data showed that early start of prophylaxis was associated with reduced inhibitor development in patients with low-risk mutations. However, the infusion frequency at start of prophylaxis was not associated with inhibitor development.15
In summary, publications in the late 1990s on the importance of early prophylaxis have led to a paradigm shift in clinical practice. Less bleeding is now accepted before the initiation of prophylaxis, and consequently, more patients start prophylaxis before three years of age and before the second joint bleed. In addition, initial prophylactic regimens increasingly use once-weekly infusions. To determine the consequences of the different regimens used to start prophylaxis, subsequent treatment and outcome, especially long-term joint status, need to be documented and analyzed.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47. [DOI] [PubMed] [Google Scholar]
- 2.Astermark J, Petrini P, Tengborn L, Schulman S, Ljung R, Berntorp E. Primary prophylaxis in severe haemophilia should be started at an early age but can be individualized. Br J Haematol. 1999;105(4):1109–1113. [DOI] [PubMed] [Google Scholar]
- 3.Fischer K, Van der Bom JG, Mauser-Bunschoten EP, et al. The effects of postponing prophylactic treatment on long-term outcome in patients with severe hemophilia. Blood. 2002;99(7):2337–2341. [DOI] [PubMed] [Google Scholar]
- 4.Kreuz W, Escuriola-Ettingshausen C, Funk M, Schmidt H, Kornhuber B. When should prophylactic treatment in patients with haemophilia A and B start? - The German experience. Haemophilia. 1998; 4(4):413–417. [DOI] [PubMed] [Google Scholar]
- 5.Ljung R. Second Workshop of the European Paediatric Network for Haemophilia Management, 17–19 September 1998 in Vitznau/Switzerland. Haemophilia. 1999;5(4):286–291. [DOI] [PubMed] [Google Scholar]
- 6.Ljung R. The risk associated with indwelling catheters in children with haemophilia. Br J Haematol. 2007;138(5):580–586. [DOI] [PubMed] [Google Scholar]
- 7.Petrini P. What factors should influence the dosage and interval of prophylactic treatment in patients with severe haemophilia A and B? Haemophilia. 2001;7(1):99–102. [DOI] [PubMed] [Google Scholar]
- 8.Meunier S, Trossaert M, Berger C, et al. French guidelines. Long-term prophylaxis for severe haemophilia A and B children to prevent haemophiliac arthropathy. Arch Pediatr. 2009;16(12):1571–1578. [DOI] [PubMed] [Google Scholar]
- 9.Kurnik K, Bidlingmaier C, Engl W, Chehadeh H, Reipert B, Auerswald G. New early prophylaxis regimen that avoids immunological danger signals can reduce FVIII inhibitor development. Haemophilia. 2010;16(2):256–262. [DOI] [PubMed] [Google Scholar]
- 10.Feldman BM, Pai M, Rivard GE, et al. Tailored prophylaxis in severe hemophilia A: interim results from the first 5 years of the Canadian Hemophilia Primary Prophylaxis Study. Journal of Thrombosis and Haemostasis. 2006;4(6):1228–1236. [DOI] [PubMed] [Google Scholar]
- 11.Gouw SC, Van der Bom JG, Van den Berg HM. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007; 109(11):4648–4654. [DOI] [PubMed] [Google Scholar]
- 12.Fischer K, Ljung R, Platokouki H, et al. Prospective observational cohort studies for studying rare diseases: the European PedNet Haemophilia Registry. Haemophilia. 2014;20(4):e280–e286. [DOI] [PubMed] [Google Scholar]
- 13.Richards M, Williams M, Chalmers E, et al. A United Kingdom Haemophilia Centre Doctors’ Organization guideline approved by the British Committee for Standards in Haematology: guideline on the use of prophylactic factor VIII concentrate in children and adults with severe haemophilia A. Br J Haematol. 2010;149(4):498–507. [DOI] [PubMed] [Google Scholar]
- 14.Auerswald G, Kurnik K, Blatny J, Reininger AJ. The EPIC Study: A clinical trial to assess whether early low dose prophylaxis in the absence of immunological danger signals reduces inhibitor incidence in previously untreated patients (PUPS) with hemophilia A. 55th ASH Annual Meeting and Exposition, New Orleans 2013;oral presentation-nr 576. [Google Scholar]
- 15.Gouw SC, Van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046–4055. [DOI] [PubMed] [Google Scholar]


