The overall short-term treatment efficacy of rituximab (R) in immune thrombocytopenia (ITP) is reported to be approximately 58%.1,2 With four once-weekly 375 mg/m2 doses, responses of 31% after two years,2 and 21% after five years2 can be expected with a median clinical improvement time of 10.51 months, and up to 49 months in other studies.3 Since lower doses have comparable efficacy,4 R remains an attractive second-line therapy especially in patients where splenectomy is contraindicated or should be delayed.1,3,5–8
However, there are no known response-modifying factors that can provide an optimal basis for choosing R and its dosing. Moreover, while numbers of ITP patients are limited, knowledge on these issues is mainly derived from (clustered) often small-sized and non-randomized studies.1–3,5,9 Therefore, in order to, firstly, delineate the efficacy and safety of two alternative dosing strategies, and secondly, to study the R-response-modifying factors (RMF), the Dutch Hemato-Oncology Cooperative Group (HOVON) randomized 156 immune thrombocytopenia (ITP) patients between four once-weekly standard 375 mg/m2 doses (arm A), a 2-weekly 375 mg/m2 in early responding patients (arm B), and a 2-weekly 750 mg/m2 regimen (arm C). In more detail, in arm B, when no early (at day 15) response was present or when response was lost within six weeks, another two once-weekly 375 mg/m2 rituximab infusions were given.
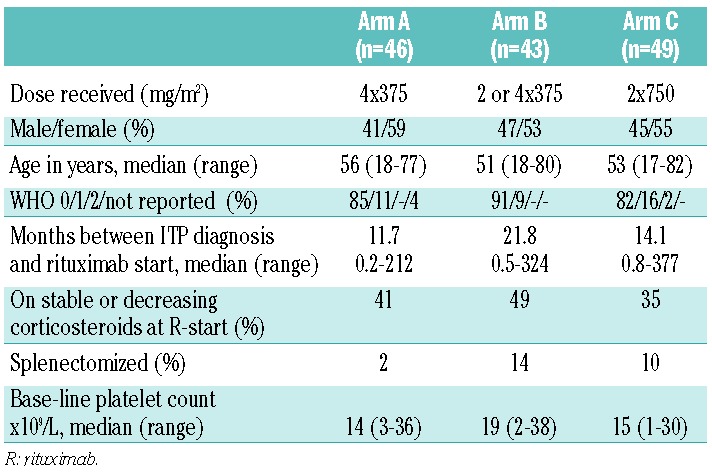
Eligible patients needed to be 18 years of age or older and with an ITP relapse or refractoriness (at least 2 platelet counts less than 30×109/L) and at least three weeks after high-dose corticosteroids (≥ 1mg/kg) before start of R (R start); further study details are available in the Online Supplementary Appendix. Complete (CR) good/partial (PR) and moderate (MR) response were defined as platelet counts of 150×109/L or more and 50×109/L or more on 2 consecutive occasions and a platelet count over 30×109/L with at least twice the base-line count, respectively. Retrospectively, responses were also analyzed according to the International Working Group (IWG) ITP trial guidelines.10 Relapse was defined as a further fall in platelet count to below 30×109/L or below the 2-fold increase of base-line platelet count. Relapse-free survival (RFS), defined as time from response until relapse, emergency treatment, or death, was analyzed using the actuarial Kaplan-Meier method. Patients still alive at the date of last contact were censored. The primary objective of this study was to evaluate the individual treatment arms as sufficiently promising (CR+ PR + MR > 50%) strategies11 but not to compare the results between the treatment arms. All analyses were performed according to the intention-to-treat principle, irrespective of patients’ compliance. Ineligible patients were excluded from all analyses. With 15 patients considered ineligible, and 3 patients who did not start with their assigned R treatment, 138 patients were evaluated for response as primary end point (Table 1).
Table 1.
Patients’ characteristics.
Twelve patients (9%) went off treatment for various reasons. In 4 of these, this was related to R-attributed adverse events and toxicity; one patient in arm B experienced a life-threatening infection. Four patients received emergency treatments (for details see Online Supplementary Table S1 and Figure S1). Weekly platelet counts were taken in all patients, up to ten weeks (day 71), after which responders were subsequently monitored monthly for at least 12 months. The median follow up of responding patients was 24 months (range 2-68; with 85% of responders monitored for >12 months).
As far as R-efficacy is concerned (Table 2), 68 (49 %) patients responded within ten weeks (19% CR, 20% PR, 11% MR); in 16 of these patients, the response even improved after ten weeks. Response results were similar: 52% for arm A (39%–65%; 90% confidence intervals), 47% for arm B (33%–60%), and 49% for arm C (37%–62%). Of 43 patients in arm B, 7 (16%) responded early and received two 375 mg/m2 doses. Application of IWG response criteria defining CR as platelet counts above 100×109/L and ‘Response’ combining CR and PR (present in at least 2 measurements one week apart) led to even higher response rates: 63% (50%–75%), 59% (38%–64%), and 61% (48%–73%). Overall, responding patients showed a 72% RFS at one year and 58% at two years, and a median relapse-free survival of 29 months; comparisons between arms are, however, precluded by group patient numbers (Figure 1).
Table 2.
Treatment outcomes.
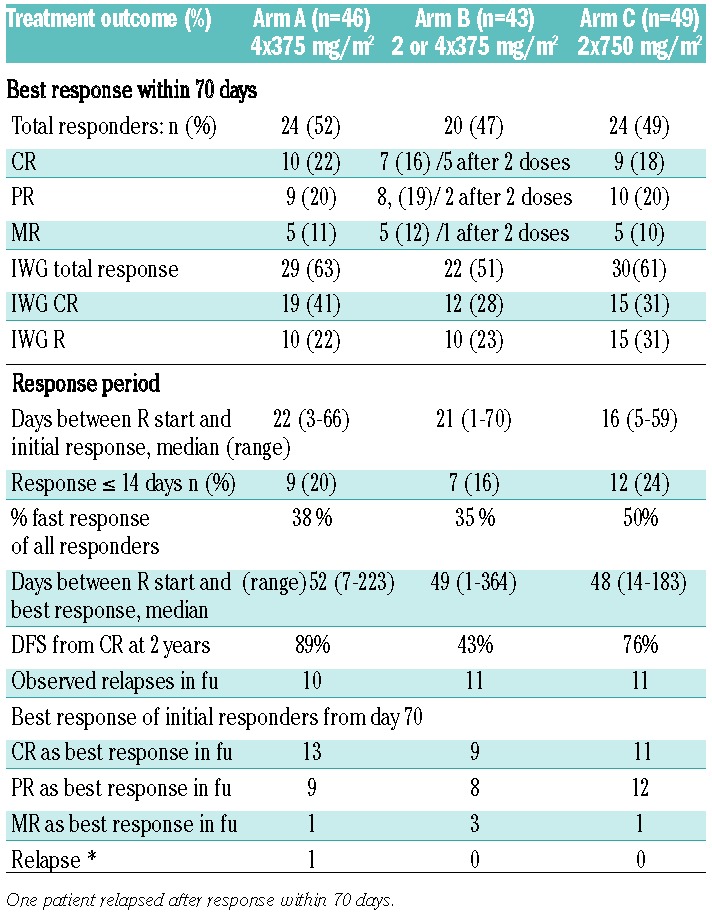
Figure 1.
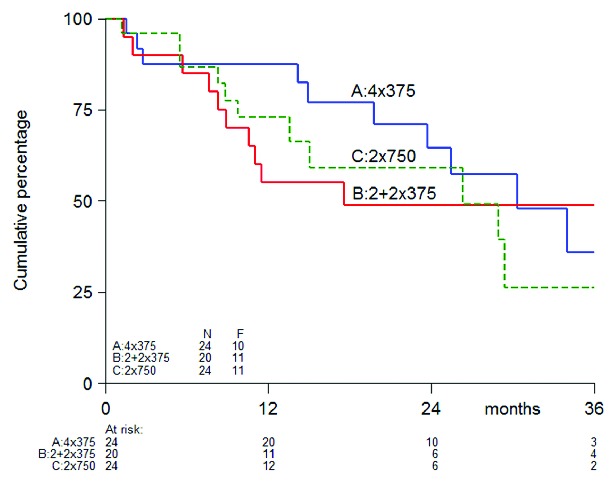
Relapse Free Survival (additional file).
As response-modulating factors (RMF) studied throughout the 3 arms, early response (within 14 days vs. >14 days), showed more CRs within 70 days (18 of 28 vs. 8 of 40; P<0.001) and thereafter (19 of 28 vs. 14 of 40; P=0.01), but response duration and RFS were similar. Disease duration defined as time between initial diagnosis and start of R, was significantly shorter in patients reaching response (median 322 days; range 7–8964; P=0.008) and CR (237 days; range 29–7726; P=0.042) versus patients without response (median 783 days; range 23–11487). Vice versa, comparing 65 patients with ITP for less than one year, with 73 patients with ITP for more than a year, response (57% vs. 42%) and CR (27% vs. 22%) as well as 2-year RFS (66% vs. 46%; P=0.06) showed similar tendencies. No synergy was found between R and (still) having corticosteroids at study entry, and patient age was not associated with a higher probability to obtain a response (P=0.25) and RFS (P=0.57). The total response of 56% with 18 CR, 15 PR and 10 MR in female patients tended to be higher with 41% response (P=0.08), with 8 CR, 12 PR and 5 MR in males. Previous splenectomy was present in only 12 patients (with 1 CR, 1 PR and 3 MR) and could thus not be studied as RMF. In case of non-response or later relapse, patients often received other treatments. Of these, splenectomy was specifically studied with only 4 in 68 (6%) initially responding patients within one year after study initiation. In contrast, in the 70 non-responding patients, at least 15 patients (21%) underwent splenectomy within one year (P=0.01), while follow up data were not available for 17 of the non-responding patients.
Toxicities scored by the NCI Common Terminology Criteria for Adverse Events (CTCAE; v.3.0), as well as side effects, were clear and similar in all study arms (Table 3). In more detail, 22 patients (16%) experienced 23 CTCAE 3 or 4 events; of these, 10 patients (7%) with 10 CTC 3 and 4 events were recorded as SAEs. Another CTC 4 seizure should have been reported as SAE. As judged by more or less close occurrence of events to R-administration, and the absence or presence of other causal factors, 6 of a total 11 events were considered to be probably or possibly treatment-related. Two events were judged to be definitely related to R-administration (5.7%); all events reversed without morbidity (Online Supplementary Appendix).
Our study first of all confirms that lower4 but also more dense-dosed R are promising therapies for ITP patients. Interestingly, with 7 of 20 early responding patients receiving only two once-weekly 375 mg/m2 doses, arm B did not lead to inferior results in response quality or RFS. The value of such a dose sparing strategy should, however, be confirmed by a randomized study comparing no or additional treatment in early responding patients, A second important issue is that response delays other treatments. In this respect, 15 of 63 non-responding patients underwent splenectomy in the first year after study entry, while this was needed in only 4 of 63 previously non-splenectomized responders. However, it remains to be determined whether R can eventually help avoid splenectomy.1,2,5 Of the response-modulating factors studied in the pooled patients from all treatment arms, early response and female sex were associated with more frequent and better responses.12 The latter, although non-significant, is in contrast to a recent meta-analysis which found male ITP patients to have a better 1-year response. Age is a complicated response modulator1 because higher age is associated with longer disease duration and therapy resistance. In this light, we could indeed show that responding patients had shorter disease durations,3 while response, CR and longer CR duration tended to be superior in patients with an ITP diagnosis of less than one year. In other studies, up-front corticosteroids combined with R12–14 show synergy. We did not observe this effect, probably due to the fact that only corticosteroid-resistant patients were eligible for our study.
In conclusion, our detailed data can serve as a basis for future studies; for example, in which R is compared with other splenectomy-delaying approaches, such as TPO receptor agonists and splenectomy itself, and which include cost effectiveness and quality of life outcomes. Such studies might additionally generate the (immunological) predictors to help select the optimal ITP therapy and identify which patients would benefit most.8,16
Acknowledgements
The authors thank the local data managers, and central data managers of the HOVON Data Center for collecting patient data. Rituximab as study medication was free of charge and further conditions supplied to participating centres by Roche via HOVON. The following institutes and investigators of the Dutch-Belgian Cooperative Trial group for Hemato-Oncology HOVON participated in the study: Leiden: Leiden University Medical Center, dr. Brand and Zwaginga; Rotterdam: Erasmus MC - Daniel den Hoed Cancer Center dr. te Boekhorst; Amsterdam: Academic Medical Center Amsterdam, dr. Koene and Biemond; Dordrecht: Alb. Schweitzer Hospital, dr Levin; Veldhoven: Máxima Medical Center, dr. Vreugdenhil; Amsterdam: VU-Medical Center, dr. Zweegman and dr. Huijgens; Utrecht: Diakonessenhuis, dr. van der Griend; Den Bosch: Jeroen Bosch Hospital, dr Pruijt; Nieuwegein: St. Antonius Hospital, dr. de Weerdt and Koene; Maastricht: Maastricht University Medical Center, dr van Pampus; Nijmegen: Radboud University Medical Centre, dr. Novotny and van Pampus; Enschede: Medisch Spectrum Twente, dr. de Groot; Hoorn: Westfriesgasthuis, dr. van Maanen-Lamme; Amersfoort: Meander Hospital, dr. Wittebol; The Hague: HagaZiekenhuis, dr. Schipperus; Blaricum: Tergooiziekenhuizen, dr. Silbermann; Hoofddorp: Spaarnehospital dr. Schrama; Bergen op Zoom: Lievensberg hospital, dr. Valster; Ede: Gelderse Vallei hospital, dr. Velders; Amsterdam: Slotervaart hospital, dr. Soesan; Amsterdam: OLVG hospital, dr. Leeksma; Gouda: Groene Hart hospital, dr. Tanis; Winterswijk: Beatrix hospital, dr. Smeets; Amsterdam: Sint Lucas Andreas hospital, dr Vasmel.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Auger S, Duny Y, Rossi JF, et al. Rituximab before splenectomy in adults with primary idiopathic thrombocytopenic purpura: a meta-analysis. Br J Haematol. 2012;158(3):386–398. [DOI] [PubMed] [Google Scholar]
- 2.Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeot M, Zaja F, Vianelli N, et al. Rituximab therapy in adult patients with relapsed or refractory immune thrombocytopenic purpura: long- term follow-up results. Eur J Haematol. 2008;81(3): 165–169. [DOI] [PubMed] [Google Scholar]
- 4.Zaja F, Vianelli N, Volpetti S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85(4):329–334. [DOI] [PubMed] [Google Scholar]
- 5.Godeau B, Porcher R, Fain O, et al. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura–results of a prospective multicenter phase 2 study. Blood. 2008;112(4):999–1004. [DOI] [PubMed] [Google Scholar]
- 6.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia Blood. 2011;117(16):4190–4207. [DOI] [PubMed] [Google Scholar]
- 7.Ghanima W, Godeau B, Cines DB, et al. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood. 2012;120(5):960–969. [DOI] [PubMed] [Google Scholar]
- 8.Godeau B, Stasi R. Is B-cell depletion still a good strategy for treating immune thrombocytopenia? Presse Med. 2014;43(4 Pt 2):e79–85. [DOI] [PubMed] [Google Scholar]
- 9.Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146(1):25–33. [DOI] [PubMed] [Google Scholar]
- 10.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. [DOI] [PubMed] [Google Scholar]
- 11.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. [DOI] [PubMed] [Google Scholar]
- 12.Bussel JB, Lee CS, Seery C, et al. Rituximab and 3 dexamethason cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia less than 2 years duration. Haematologica. 2014;99(7):1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Mou W, Lu G, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 2011;93(1):91–98. [DOI] [PubMed] [Google Scholar]
- 14.Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone mono-therapy in adults with primary immune thrombocytopenia. Blood. 2010;115:2755–2562. [DOI] [PubMed] [Google Scholar]
- 15.Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232–239. [DOI] [PubMed] [Google Scholar]
- 16.Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007; 110(8):2924–2930. [DOI] [PubMed] [Google Scholar]


