Patients with myelodysplastic syndromes (MDS) harboring a clonal deletion of the long arm of chromosome 5 [del(5q)] display characteristic cytomorphological features.1,2 Flow cytometry (FCM) is currently considered complementary to the microscopic evaluation of bone marrow (BM) smears and karyotyping, and has been referred to as a supplementary method in otherwise non-informative cases.3 FCM-scoring-systems (FCSS and Ogata-score)4,5 have been developed from an analysis of progenitors and myelomonocytic cells. The European LeukemiaNet (ELN)-iMDS-FCM working group has been successful in its efforts to promote standardization in arguing for the inclusion of FCM in the diagnostic workup of patients with a suspicion of MDS.3,6 However, it is still not known whether cytogenetic subgroups are associated with a distinct FCM profile thus allowing classification and disease monitoring. The aim of this 2-center study was to compare the immunophenotype of different hematopoietic cell lineages in MDS patients with and without del(5q).
We investigated 156 untreated MDS patients within a training cohort; 38 patients showed a del(5q), including 26 patients with del(5q) as a single abnormality or with one additional aberration. An additional “validation cohort” consisted of 64 MDS [22 with del(5q), 12 showing del(5q) as single abnormality or with one additional aberration]. A standardized lyse-stain-wash procedure, using 8-color or 4-color staining (Dresden or Amsterdam laboratory, respectively) was performed. This allowed a comprehensive FCM analysis to be made according to FCSS and Ogata-score.4,5 We incorporated additional antigens, such as CD10, its decrease in granulopoiesis described as loss of synchronicity in relation to other maturation antigens,7 CD36/CD71 expression on granulopoiesis, reported to correlate with IPSS,8 and diminished (dim) expression CD71 on nucleated red cells (NRC), known as dyserythropoietic feature.9,10 For gating strategy and cutoff values see the Online Supplementary Appendix and Online Supplementary Tables S1 and S2.
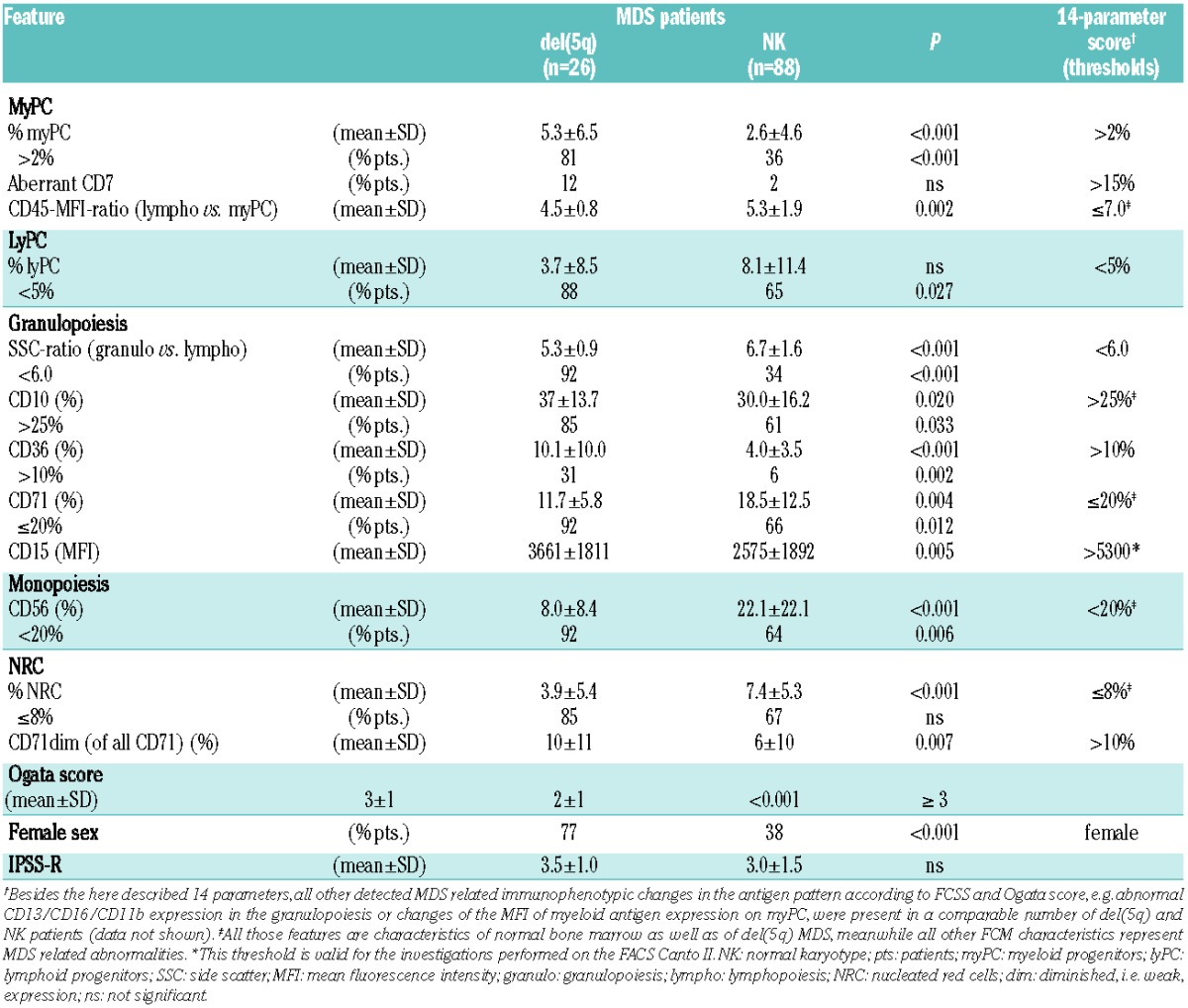
Initially, we compared the whole immunophenotype in MDS patients with isolated del(5q) or one additional cytogenetic abnormality (n=26) versus normal karyotype (n=88) as part of the training cohort. Among the distinct immunophenotype of del(5q) MDS (Table 1), the increased myeloid progenitor cells (myPC) in 81% versus only 36% of normal karyotype (NK) patients was one of the hallmarks even in the lower risk groups and was irrespective of cytomorphologically assessed blast count: less than 5% blasts [del(5q)=15 and NK=57 patients] with myPC of 3.2±2.8% versus 1.3±1.4% (P=0.003) and ≥5% [del(5q)=11 and NK=31 patients] with 8.2±8.8% versus 5.0±7.0% (P=0.024). The CD45-MFI-ratio, a feature associated with maturity within the progenitor cell compartment, was normal-to-low in each del(5q) patient.4 Granulopoiesis presented with an abnormally low sideward scatter (SSC)-ratio in almost all del(5q) patients reflecting hypogranularity. Interestingly, this is known to be a rather uncommon cytomorphological feature in del(5q) MDS.1,2 Notably, CD71 and CD10 expression were significantly more often normal compared to NK patients, pronouncing the maturity of granulopoiesis which is concordant with the low rate of dysgranulopoiesis evaluated cytomorphologically.11 The percentage of CD71dim NRC was distinctly higher in the del(5q) cohort. The entire immunophenotypic pattern of MDS with del(5q) as a single aberration or with only one additional abnormality provides further evidence for a clonal multilineage involvement which is supported by other studies.12
Table 1.
Immunophenotypic differences in MDS with isolated del(5q) or one additional abnormality versus MDS with normal karyotype (as a part of the training cohort).
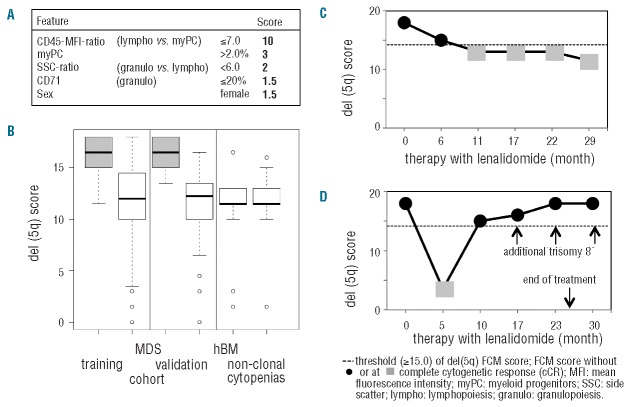
Second, we aimed at building a robust model by comparing all del(5q) samples of the training cohort [isolated del(5q) and del(5q) within complex aberrant karyotypes] with all non-del(5q) samples (normal; abnormal karyotypes). By incorporating 14 parameters, which appeared to best characterize the MDS with del(5q) and assigning one point each (Table 1), a score of 8 or over allowed us to distinguish del(5q) from non-del(5q) MDS with a remarkably high sensitivity and specificity (Table 2). Thus, both del(5q) subgroups, namely MDS with del(5q) as single aberration or with one additional abnormality as well as those patients with del(5q) as part of a complex aberrant karyotype could be equally well characterized with the above mentioned score. Next, a logistic regression analysis was performed in order to weight single parameters and to simplify the model (Online Supplementary Appendix). The final 5-parameter-del(5q)-score (Figure 1A) includes characteristics of myPC (percentage and CD45-MFI-ratio), granulocytes (SSC-ratio and CD71 expression), and was further refined by adding female sex, known to be associated with del(5q).11 The normal-to-low CD45-MFI-ratio is mandatory and received the highest ranking. Even the presence of all four further variables in parallel was not enough to characterize del(5q) MDS without an appropriate CD45-MFI-ratio. A 5-parameter-del(5q)-score of 15.0 or more was considered typical for the presence of del(5q) and could predict del(5q) in 95% of MDS harboring this abnormality. The robustness of the del(5q)-score was proven in a validation cohort (Table 2, Figure 1B and Online Supplementary Table S3A and B). In non-del(5q) MDS (normal and abnormal karyotype), considering the whole data set, the del(5q)-score was comparably low (mean: 11.5 vs. 11.0), with 25 patients comprising a score less than 10.0, never being present in del(5q) MDS. Some of the mentioned variables are part of the Ogata-score,5 accentuating the importance of these features in MDS. Remarkably, the addition of two parameters (CD71 and sex) and the differential weighting further refined the Ogata-score and allowed for a clear separation of del(5q) and non-del(5q) MDS, as well as nonclonal cytopenias and healthy BM (Table 2).
Table 2.
Sensitivity and specificity of immunophenotypic profile (training and validation cohort) analyzing del(5q) versus non-del(5q) patients as well as non-clonal cytopenias and healthy bone marrow.
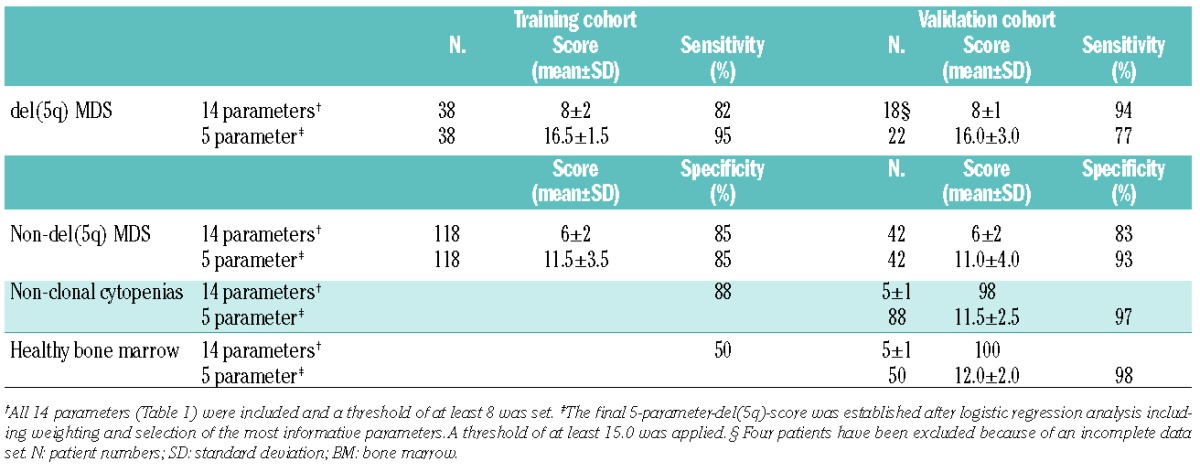
Figure 1.
5-parameter-del(5q)-score. (A) The del(5q)-score is significantly higher (P<0.001) in del(5q) MDS (gray rectangles) versus non-del(5q) MDS, as well as healthy BM donors (hBM) and non-clonal cytopenias (white rectangles). (B) Composition of the final 5-parameter-del(5q)-score. (C and D) show monitoring of lenalidomide response in 2 representative MDS patients with isolated del(5q). In (C) the typical del(5q)-score before treatment and in partial CR is present; the loss of the del(5q) immunophenotype later on predicts cCR reliably at all appropriate investigation points. (D) Only data of a patient who achieved an intermittent cCR; already ten months after the start of the treatment, FCM could detect the reappearance of the disease with the presence of a typical del(5q)-score. At this time point, FCM analysis substantiated the cytogenetic result with metaphase FISH analysis detecting only 1 of 16 metaphases positive for del(5q) and interphase FISH was negative. In the following samples, the del(5q)-score increased further reaching the maximum of 18.0 seven months ahead of the full cytogenetic and hematologic relapse (month 30).
Finally, in 18 MDS patients (71 measurements) we tested whether the proposed 5-parameter-del(5q)-score could be used for monitoring response to lenalidomide and/or azacitidine.13,14 The del(5q)-specific-profile was lost (mean score=13.0) in all del(5q) patients achieving a complete cytogenetic response (cCR) after a lenalidomide-based therapy (n=13 measurements), including normal myPC and SSC-ratio (12 of 13 patients), whereas in most measurements in MDS without cCR (45 of 58 patients) a characteristic high del(5q)-score (mean=16.5) (Figure 1C and D) was present. A more complex aberrant karyotype as an expression of a higher grade of genetic instability might result in a different immunophenotype. However, a typical del(5q)-score reflected patients with a complex karyotype in a similar way as MDS with isolated del(5q). Namely, during all measurements in del(5q) MDS with complex karyotype, receiving azacitidine and not achieving a cCR, a typical high del(5q)-score was detectable. Additionally, only one patient without cCR but a non-typical del(5q)-score lower than the proposed threshold of 15.0 presented with a complex karyotype, both arguing for del(5q) as an early cytogenetic abnormality which drives the overall immunophenotype. In contrast, in del(5q) patients without a complete response to therapy but a non-typical low del(5q)-score this expression profile seems to be associated with the presence of a TP53 mutation. In 12 of 13 measurements with this non-typical low score (mean score=13.0; range=3.5-14.5) a TP53 mutation and/or a chromosome 17p deletion was present, whereas only one of 23 investigations in patients with wtTP53 had a non-typical low del(5q)-score (P=0.009). This observation sugests a possible link between TP53 mutations and concomitant alterations of the immunophenotypic profile. Thus, the del(5q)-score can be used as a reliable complementary tool for cytogenetic response in wtTP53 patients. TP53 mutations in MDS with isolated del(5q) have been reported in up to 20% of lower risk MDS.15 A mutational screening is recommended in patients with isolated del(5q) because of their potential resistance to lenalidomide and higher rate of AML evolution in TP53 mutated cases. From our data, we hypothesize that especially patients with isolated del(5q) but a non-typical low del(5q)-score raise suspicion of harboring a TP53 mutation and should subsequently be referred to mutational screening.
In summary, we demonstrate a strong association of a common cytogenetic abnormality with an immunophenotypic profile in MDS, emphasizing the role of FCM as a reproducible and fast tool in the diagnostics and monitoring of response to therapeutic interventions. In addition, this contributes to a better understanding of the biology of del(5q) versus other MDS and emphasizes the uniqueness of this cytogenetically based MDS subgroup. Besides cytomorphology, FCM including the here proposed 5-parameter-del(5q)-score should be incorporated as part of the MDS diagnostics. It might also be used to speed up further cytogenetic and molecular diagnostics as well as confirming diagnosis, especially in cases where cytogenetic evaluation is inconclusive because of results around the cut-off percentage for this method or cannot be determined. Response monitoring in the context of immunomodulatory drugs might be possible using an easy-to-perform 4-color (CD45/CD34/CD19/CD71) immunophenotypic approach, which should be further substantiated in prospective studies.
Acknowledgments
We appreciated the excellent technical assistance of Claudia Klotsche, Cathleen Rüger, Catrin Theuser, and Susann Helas. Mutational analyses for mTP53 were performed by Dr. A. Kohlmann and colleagues at the MLL Munich Leukemia Laboratory (Munich; Germany).
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Brunning RD, Orazi A, Germing U, et al. Myelodysplastic syndromes/neoplasms, overview. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France, PA: IARC; 2008. p.88–93. [Google Scholar]
- 2.Giagounidis AA, Germing U, Haase S, et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia. 2004;18(1):113–119. [DOI] [PubMed] [Google Scholar]
- 3.Westers TM, Ireland R, Kern W, et al. Standardization of flow cytometry in myelodysplastic syndromes: a report from an international consortium and the European LeukemiaNet Working Group. Leukemia. 2012;26(7):1730–1741. [DOI] [PubMed] [Google Scholar]
- 4.Wells DA, Benesch M, Loken MR, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102(1):394–403. [DOI] [PubMed] [Google Scholar]
- 5.Ogata K, Della Porta MG, Malcovati L, et al. Diagnostic utility of flow cytometry in low-grade myelodysplastic syndromes: a prospective validation study. Haematologica. 2009;94(8):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Loosdrecht AA, Alhan C, Béné MC, et al. Standardization of flow cytometry in myelodysplastic syndromes: report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica. 2009;94(8):1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung JW, Park CJ, Cha CH, et al. A combination of CD15/CD10, CD64/CD33, CD16/CD13 or CD11b flow cytometric granulocyte panels is sensitive and specific for diagnosis of myelodysplastic syndrome. Ann Clin Lab Sci. 2012;42(3):271–280. [PubMed] [Google Scholar]
- 8.Maynadie M, Picard F, Husson B, et al. Immunophenotypic clustering of myelodysplastic syndromes. Blood. 2002;100(7):2349–2356. [DOI] [PubMed] [Google Scholar]
- 9.Kern W, Haferlach C, Schnittger S, Haferlach T. Clinical utility of multiparameter flow cytometry in the diagnosis of 1013 patients with suspected Myelodysplastic Syndrome. Cancer. 2010; 116(19):4549–4563. [DOI] [PubMed] [Google Scholar]
- 10.Xu F, Wu L, He Q, et al. Immunophenotypic analysis of erythroid dysplasia and its diagnostic application in myelodysplastic syndrome. Intern Med J. 2012;42(4):401–411. [DOI] [PubMed] [Google Scholar]
- 11.Hasserjian RP, Le Beau MM, List AF, Bennett JM, Thiele J. Myelodysplastic syndrome with isolated del(5q). In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France, PA: IARC; 2008 p. 102. [Google Scholar]
- 12.Tehranchi R, Woll PS, Anderson K, et al. Persistant malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med. 2010;363(11):1025–1037. [DOI] [PubMed] [Google Scholar]
- 13.Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118(14):3765–3776. [DOI] [PubMed] [Google Scholar]
- 14.Platzbecker U, Braulke F, Kündgen A, et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: a phase I study. Leukemia. 2013;27(6):1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebaa A, Ades L, Baran-Marzack F, et al. Incidence of 17p deletions and TP53 mutation in myelodysplastic syndrome and acute myeloid leukemia with 5q deletion. Genes Chromosomes Cancer. 2012;51(12):1086–1092. [DOI] [PubMed] [Google Scholar]