Disease relapse still remains the most important cause of treatment failure in childhood acute myeloid leukemia (AML). Molecular monitoring of response to treatment by minimal residual disease (MRD) provides important information, widely used to tailor treatment in childhood acute lymphoblastic leukemia.1–3 On the contrary, prognostic relevance of MRD in pediatric AML has only been recently proposed and needs to be further investigated and confirmed.4–6 So far, the prognostic impact of the quality of response measured by flow cytometry after induction and consolidation therapy has been shown to provide independent prognostic information in pediatric AML,5 able to permit a refinement of risk stratification and to potentially improve AML patient outcome.
Intense efforts have been devoted to the development of methods able to measure residual AML burden in support of flow cytometry, and fusion transcript detection, which is undoubtedly useful in patient stratification at diagnosis,7 is currently under evaluation as a suitable MRD marker to predict relapse in AML.5 t(8;21)(q22;q22)RUNX1-RUNX1T1 and inv(16)(p13q22)CBFB-MYH11 are recurrent somatic lesions detected in approximately 20% of pediatric AML at diagnosis.8 In the AIEOP AML 2002/01 protocol, all patients carrying these abnormalities achieved morphological complete remission (CR) after the first induction course with idarubicin, cytarabine and etoposide (ICE) and were grouped as a unique subgroup called standard risk (SR). These children were given, after 2 courses of ICE induction therapy, three post-remissional courses of high-dose cytosine-arabinoside either in combination with etoposide (AVE cycle), or mitoxantrone (HAM cycle) or administered alone (high-dose Ara-c).7 A larger than expected proportion of patients carrying either t(8;21) or inv(16) relapsed, this leading to an 8-year probability of event-free survival of 63%. Although many of the relapsing patients were rescued by means of allogeneic hematopoietic stem cell transplantation (HSCT),8 there is a need to identify factors able to predict patients who might not respond to conventional chemotherapy to improve their outcome.9–11
In this retrospective study, we analyzed the role of MRD monitored by the absolute quantification of CBF fusion transcripts early during/after induction treatment, in order to assess its prognostic value in SR childhood AML. We enrolled 76 of the 99 children assigned to the SR group in the AIEOP AML 2002/01 Protocol:8 49 carried the t(8;21), 26 inv(16), and one carried the t(16;16) (see Table 1 for details). MRD measurement on bone marrow samples collected at time of diagnosis and after each of the two courses of ICE induction chemotherapy were analyzed. It was not possible to study the remaining 23 SR patients due to insufficient RNA extracted from cells collected after induction courses, but the outcome of patients who were or were not included in the study was comparable (data not shown). We used the real-time quantitative RQ-PCR (Ipsogene FusionQuant® kit for absolute quantification of fusions on the ABI 7900HD; Applied Biosystems) to detect RUNX1-RUNX1T1 and CBFB-MYH11 fusion expression following the manufacturer’s guideline for normalization (with ABL gene) and standard curve method for quantification (10−5 standard curve sensitivity by using plasmid serial dilutions already included in the kit).12,13 For the t(8;21)-rearranged patients, we found a mean number of RUNX1-RUNX1T1 fusion transcript copies at diagnosis of 643,466 (range 56,752–3,387,522); it decreased to 190,741 mean copies after the I ICE course (range 11–3,062,045), and to 44,671 after the II ICE (range 0–1,671,638). To evaluate the impact of MRD levels measured as copies number during sequential monitoring, the Mantel-Byar test was used to calculate the cumulative incidence of relapse (CIR). We grouped patients in quartiles for copy number measured at diagnosis, after the first and second ICE course. The number of transcript copies at diagnosis, or after induction therapy did not correlate with the probability of both survival and the CIR. We then considered the logarithmic reduction of MRD after ICE I and II courses calculated with respect to values of copies number of transcript found at diagnosis for each patient (Online Supplementary Table S1). We subdivided patients in three Log-reduction groups: patients who reduced MRD less than 2 Log, between 2 and 3 Log, and those who reduced more than 3 Log in order to investigate whether this distribution into groups for MRD reduction was able to predict a different relapse risk. We interestingly found that 21 (43%) out of the 49 t(8;21) patients enrolled had a slow clearance of blasts after I ICE (<2 Log with respect to diagnosis), and, at the end of the II ICE course, 10 of them still had an MRD reduction lower than 2 Log. These slow-responding t(8;21) patients at the end of the two induction courses had a worse prognosis when compared to patients who reduced MRD more than 3 Log (patients who reduced less than 2 Log showed a 10-year OS of only 58.3% compared to 85.6% of patients who reduced more than 3 Log (P=0.2) (Figure 1). Next, we investigated if MRD might influence the risk of relapse. Nine out of the 49 patients with t(8;21) relapsed at a median time of 225 days (range 76–469) from diagnosis: 7 of the 9 relapsed t(8;21) patients reduced MRD less than 2 Log after I ICE, and 5 of these 9 relapsed patients still reduced MRD less than 2 Log at the end of the II induction course. The 10-year CIR of these patients after the two induction courses was significantly higher (50%) than that of patients with an MRD reduction greater than 2 Log (17% for 2–3 Log MRD reduction, and 9% for MRD Log reduction >3; I ICE P=0.02; 1B II ICE P=0.004) (Figure 2A). In univariate analysis both MRD Log reduction after induction therapy and white blood cell (WBC) count at diagnosis more than 100,000, as recently reported,8 were significant independent factors predicting leukemia relapse; however, they were not confirmed in multivariate analysis, probably due to the limited sample size analyzed. In view of these data, we show that monitoring of molecular MRD levels is instrumental to predict the risk of relapse for t(8;21)-rearranged patients, thus providing important prognostic information for the therapeutic management of these children.
Table 1.
Patients’ clinical characteristics.
Figure 1.

Overall survival (OS) in RUNX1-RUNX1T1 patients subdivided into three classes of MRD log reduction after two induction courses.
Figure 2.
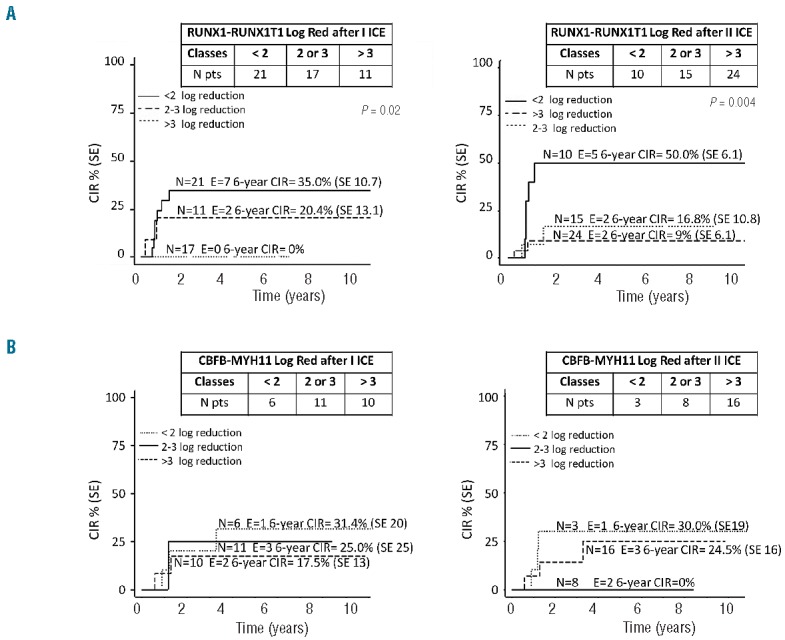
Cumulative incidence of relapse (CIR) in patients with RUNX1-RUNX1T1 (A) and CBFB-MYH11 (A) subdivided into different subgroups according to MRD log reduction after I ICE and II ICE.
We then considered inv(16)-rearranged patients and performed similar analyses for the 27 patients enrolled. These patients showed a mean number of transcript copies at diagnosis of 143,015 (range 102–582,426), which decreased to 631 (range 0–3726) after the I ICE, and to 190 (range 0-686) after the II ICE course, respectively. We evaluated if the copy number of fusion transcripts distributed in quartiles found in BM of patients at diagnosis, and at the end of the two ICE courses may have a prognostic value, but no significant differences were identified either for CIR or for OS in inv(16) rearranged patients (Online Supplementary Figure S1). Furthermore, by using the Log MRD reduction distribution, we found that 21 of 27 patients (78%) after the I ICE, and 24 of 27 (89%) after the II ICE achieved an MRD reduction greater than 2 Log. At time of last follow up, 6 of 27 patients had relapsed; among them, we documented that MRD was reduced more than 2 Log since the end of I ICE. We conclude that the CBFB-MYH11 AML showed in most cases a rapid clearance of blasts after induction therapy. Thus, early MRD monitoring does not seem to be useful for predicting relapse occurrence (not significant at 10 years) (Figure 2B).
Through this analysis, we document that among pediatric Core Binding Factor-rearranged AML, two different patterns of molecular response after induction therapy with a different capacity of predicting relapse can be identified. In particular, we found that monitoring of MRD levels after induction provides a reliable prognostic parameter exclusively for the RUNX1-RUNX1T1-rearranged patients. These data confirm the clinical usefulness of monitoring MRD levels reported in a recent study on adults with RUNX1-RUNX1T1 AML,14 although this report differs from our analysis in both timing (after 2 courses of consolidation therapy) and levels of MRD (3 Log reduction) cutoff suggested to predict relapse.
In view of these results, we propose a novel MRD-directed risk stratification and treatment of t(8;21) slow-responders to induction therapy. These children can be identified early during treatment and, in the light of their high risk of relapse, can be considered candidates to more aggressive therapies, even including allogeneic HSCT. We therefore propose that RQ-PCR MRD monitoring complemented with the flow-cytometry MRD14 data might be incorporated directly into clinical practice of the RUNX1-RUNX1T1 risk class attribution, whereas further studies on extended MRD monitoring for CBFB-MYH11 rearranged patients are warranted to identify a possible role in predicting the risk of late relapse.
Acknowledgments
We thank Dr. Sabrina Gelain, Katia Polato and Dr. Anna Leslz for the molecular and cytogenetic analysis, and Maria Grazia Giacometti and Katia Polato for samples preparation.
Footnotes
Funding: this work was supported by grants from Cariparo, IRP-Istituto di Ricerca Pediatrica-Città della Speranza Padova and from AIRC (special grant 5×1.000) to FL.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Basso G, Veltroni M, Valsecchi MG, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27(31):5168–5174. [DOI] [PubMed] [Google Scholar]
- 2.Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077–2084. [DOI] [PubMed] [Google Scholar]
- 3.Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10(8):460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(16):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaba H, Coustan-Smith E, Cao X, et al. Comparative Analysis of Different Approaches to Measure Treatment Response in Acute Myeloid Leukemia. J Clin Oncol. 2012; 30(29):3625–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Cao Z, Ruan M, et al. Monitoring the AML1/ETO fusion transcript to predict outcome in childhood acute myeloid leukemia. Pediatr. Blood Cancer. 2014;61(10):1761–1766. [DOI] [PubMed] [Google Scholar]
- 7.Masetti R, Pigazzi M, Togni M, et al. CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood. 2013;121(17):3469–3472. [DOI] [PubMed] [Google Scholar]
- 8.Pession A, Masetti R, Rizzari C, et al. Results of the AIEOP AML 2002/01 multicenter, prospective trial for treatment of children with acute myeloid leukemia. Blood. 2013;122(2):170–178. [DOI] [PubMed] [Google Scholar]
- 9.Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22(21):4384–4393. [DOI] [PubMed] [Google Scholar]
- 10.Gibson BES, Webb DKH, Howman AJ, De Graaf SSN, Harrison CJ, Wheatley K. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155(3):366–376. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29(3):310–315. [DOI] [PubMed] [Google Scholar]
- 12.Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by RT-qPCR in core-binding factor AML allows risk-stratification and predicts relapse: results of the UK MRC AML-15 trial. Blood. 2012:120(14):2826–2835. [DOI] [PubMed] [Google Scholar]
- 13.Beillard E, Pallisgaard N, van der Velden VHJ, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using “real-time” quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17(12):2474–2486. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H-H, Zhang X-H, Qin Y-Z, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121(20):4056–4062. [DOI] [PubMed] [Google Scholar]


