Extranodal natural killer/T-cell lymphoma (ENKTL) is an aggressive subtype of non-Hodgkin lymphoma showing frequent resistance to anthracyclines.1 Although intensified, non-anthracycline-based chemotherapy regimens have improved clinical outcomes, the prognosis of ENKTL, especially advanced disease, is still poor. Gene expression analysis suggested that the interaction of platelet-derived growth factor (PDGF) with the Janus-family kinase (JAK) and signal transducer and activator of transcription 3 (STAT3) pathways could induce expression of downstream molecules such as survivin, vascular endothelial growth factor (VEGF) and interleukin-6 (IL-6) in ENKTL.2 Thus, survivin and VEGF might contribute to the aggressiveness of ENKTL in association with PDGF and IL-6 through PI3K/Akt and JAK/STAT3 pathways.3 However, there are few data about the prognostic relevance of these proteins, and most studies have focused on their tissue expression rather than their extracellular presence such as in the serum. We reasoned that the presence of these extracellular proteins might indirectly reflect the amount of tissue expression.
Thus, we analyzed whether serum levels of PDGF, survivin, VEGF, and IL-6 could predict tumor burden including stage and Epstein-Barr virus (EBV) DNA as well as prognosis in newly diagnosed ENKTL patients who received non-anthracycline-based chemotherapy. The study population was from two prospective cohort studies carried out between September 2008 and December 2012 (first study: 2008–2011, clinicaltrials.gov identifier:00822731; second study: 2012-present, clinicaltrials.gov identifier:01877109). In those cohorts, all patients’ serum samples prior to treatment were aliquotted and stored at −80 °C for further studies because cytokines could be maintained in cryopreserved samples (Figure 1A). At diagnosis, whole blood EBV DNA titer was also quantified with a Roche LightCycler-EBV Quantification Kit (Roche Diagnostics GmbH, Mannheim, Germany) or a Qiagen Artus EBV RG real-time PCR assay (Qiagen GmbH, Hilden, Germany) according to the manufacturers’ instructions.4,5 To analyze association between variables, we did Fisher exact test, and calculated coefficients of correlation (r) using the Spearman rank test. The final updates on the patients’ survival were completed in February 2014, and the log rank test was used to compare progression-free survival (PFS: the time from diagnosis to disease progression or death from any cause) and overall survival (OS: the time from diagnosis to death from any cause). For multivariate analysis of survival outcomes, Cox regression hazard analysis was used, and two-sided P<0.05 was considered significant.
Figure 1.
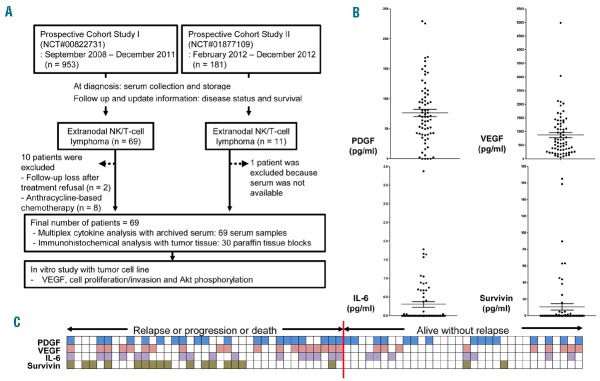
(A) Summary of study design and patient selection. (B) Serum levels of platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), IL-6 and surviving. (C) High VEGF (pink squares) showed a significant association with progression-free survival (PFS) events (68%, 19 of 28) compared to low VEGF (empty squares, 44%, 18 of 41; P=0.05). Positive survivin (green squares) showed a high rate of failure (89%, 16 of 18) than negative survivin (41%, 21 of 51; P<0.001). Positive IL-6 (purple squares) showed a marginal significance (70%, 16 of 23; P=0.06) and high platelet-derived growth factor (PDGF) (blue squares) was not related with the occurrence of a PFS event (57%, 20 of 35).
The median age of 69 patients at diagnosis was 48 years (range 17–75 years). Patients with stage IE or IIE (n=36) received primary treatments consisting of concurrent chemoradiotherapy (CCRT) and adjunct systemic chemotherapy, whereas stage III/IV patients (n=33) received systemic chemotherapy with SMILE (steroid, methotrexate, ifosfamide, L-asparaginase, and etoposide), as reported previously.6–8 Autologous stem cell transplantation (ASCT) was performed in 14 patients including upfront ASCT for stage III/IV patients as a consolidation (n=9) and salvage ASCT for relapsed or refractory patients (n=5). The International Prognostic Index (IPI) model designated 44 patients (64%) as having low or low-intermediate risk, and the NK/T-cell Prognostic Index (NKPI) model designated 43 patients (62%) as high risk (groups III and IV) (Table 1).9 We designated an EBV DNA level of 10 or more copies/mL as an elevated titer because the receiver-operating characteristic (ROC) curve analysis determined it as the optimal cutoff for OS. Thus, elevated EBV DNA at diagnosis showed a significant association with poor OS and PFS (P<0.001) (data not shown).
Table 1.
Associations between serological parameters and clinical characteristics
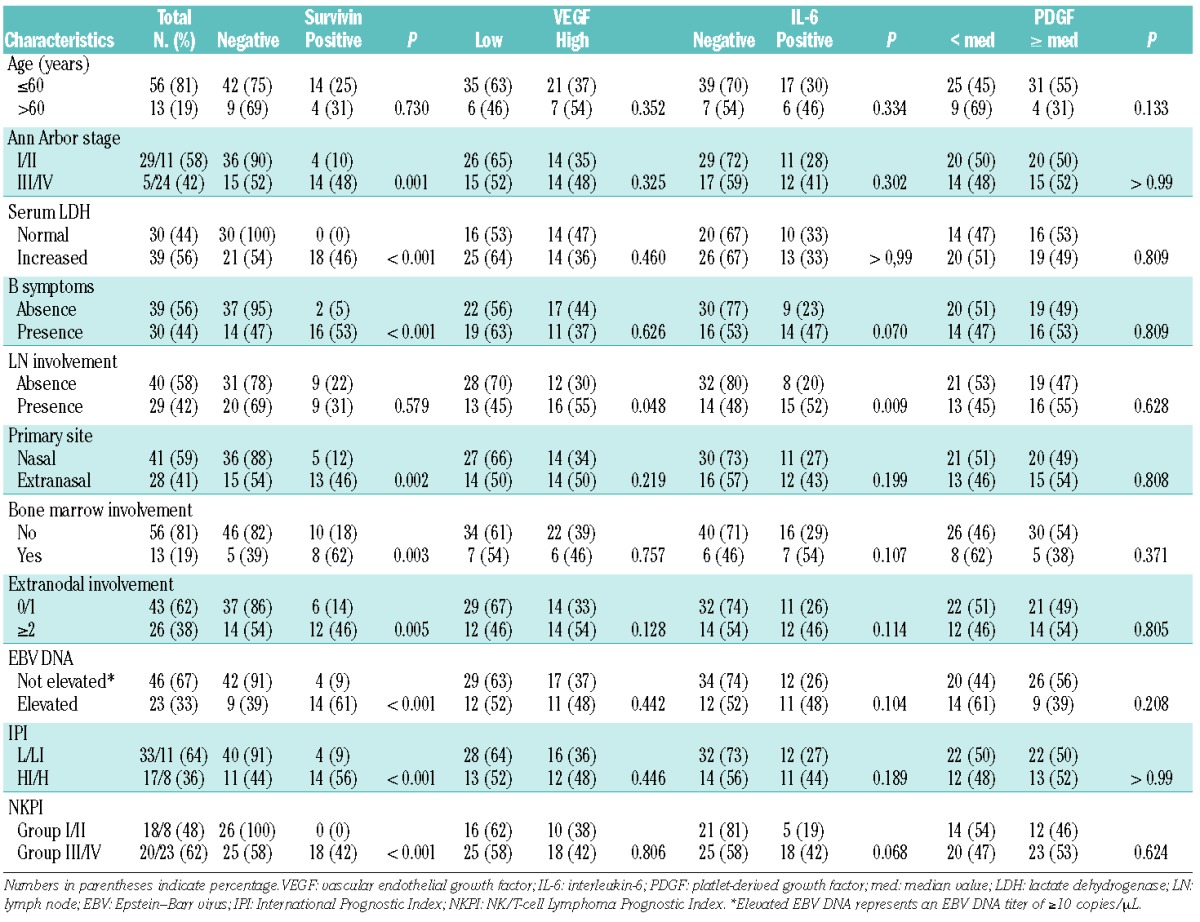
The concentrations of PDGF, survivin, IL-6, and VEGF (VEGF-A) in archived serum were measured using a multiplex cytokine assay with the Procarta cytokine profiling kit (Panomics, Fremont, CA, USA) in triplicate. The median concentration of serum PDGF was 70.0 pg/mL (range 0.0–229.4 pg/mL), and high PDGF group was determined by the median value because the optimal cutoff was not determined by ROC curve analysis. The mean (±SD) serum VEGF concentration was 875.8±802.1 pg/mL and patients were categorized into high and low VEGF groups according to the optimal cutoff for OS (843 pg/mL) determined by ROC curve. Survivin was detected in the serum of only 18 patients (range 0.8–165.0 pg/mL), and serum IL-6 was in only 23 patients (range 0.04–3.9 pg/mL) (Figure 1B). Thus, survivin and IL-6 were dichotomized into positive and negative groups. Although the number of cases was small, serum survivin concentration correlated significantly with IL-6 (r=0.263, P=0.029). Serum PDGF correlated significantly with VEGF concentration (r=0.334, P=0.004), but PDGF concentration did not correlate with survivin or IL-6 (data not shown) (P>0.05). At the time of analysis, 37 events occurred including disease progression during treatment (n=20), relapse during follow up (n=14), treatment-related mortality (n=2), and non-disease-related death (n=1). These events were enriched for the group with high VEGF or positive-survivin or IL-6 (Figure 1C). Accordingly, poor OS was significantly associated with high VEGF (>843 pg/mL) or serum positivity for survivin or IL-6 except PDGF (Figure 2A). The dose-intensity of primary treatments and the use of ASCT were not significantly different between two groups classified by these serum proteins (data not shown). As serum survivin correlated with EBV DNA titer (r=0.542, P<0.01), serum survivin-positive patients showed a significant association with elevation of EBV DNA and stage reflecting high tumor burden (P<0.05) whereas high serum VEGF was not related with tumor burden (Figure 2B). Likewise, the survivin-positive group showed a significant association with a high-risk group, as classified by the IPI and NKPI criteria (P<0.001) (Table 1). However, other proteins including PDGF, VEGF, and IL-6 were not associated with the IPI and NKPI (P>0.05). To evaluate the tissue expression of survivin, immunohistochemical analysis was performed in 30 patients who had available paraffin-embedded blocks. The percentage of survivin-positive tumor cells representing the presence of nuclear reactivity for survivin was determined by calculating the ratio of tumor cells with positive and negative nuclear staining (Figure 2C). The percentage of survivin-positive tumor cells was significantly associated with serum survivin concentration (r=0.443) (P=0.014).
Figure 2.
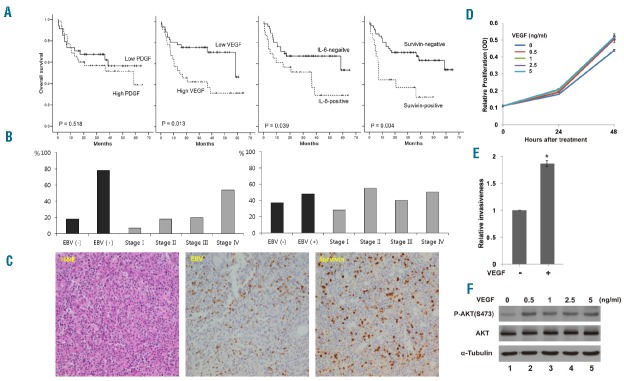
(A) Patients with high serum vascular endothelial growth factor (VEGF) concentration had better overall survival compared with patients with low VEGF. Serum IL-6-positive and serum survivin-positive patients had worse overall survival compared with patients who were negative for IL-6 and survivin. (B) Serum survivin-positive patients showed a higher proportion of Epstein-Barr virus (EBV) DNA elevation (78%, 14 of 18) and stage IV (54%, 13 of 24) whereas high VEGF did not show a significant association with EBV DNA elevation and stage. (C) Hematoxylin–eosin staining of a representative extranodal natural killer/T-cell lymphoma (ENKTL) sample; EBV-encoded small RNA in situ hybridization; strong nuclear survivin expression in lymphoma cells (X400). (D) NK-92MI cells treated with VEGF exhibited greater cell proliferation (P<0.01) compared with cells cultured without VEGF. Each condition with five replicates was repeated three times, and the data are expressed as mean±standard deviation. OD: optical density. (E) Treatment of VEGF-A (1 ng/mL) increased the invasiveness (*P<0.01). The values are from 3 independent experiments, and the data are expressed as mean±standard deviation. (F) Treatment of VEGF-A increased AKT phosphorylation (p-AKT).
The present study demonstrated that downstream molecules of PDGF such as survivin, VEGF, and IL-6 significantly correlated with OS in patients with ENKTL. These findings suggest their greater contribution to the aggressiveness of ENKTL than PDGF. Elevated serum levels of VEGF and IL-6 in patients with ENKTL might be associated with the activation of the PI3K/Akt and JAK/STAT3 pathways in lymphoma cells, which leads to increased transcription of VEGF and IL-6 via phosphorylation of Akt and STAT3.10,11 Given that ENKTL is frequently accompanied by inflammation at diagnosis, the paracrine effect of the tumor microen-vironment is also possible through VEGF and IL-6 secretion from inflammatory myeloid cells and lymphocytes.12 In particular, VEGF was more abundant in serum compared with IL-6 because only 23 patients showed a detectable level of IL-6. Our in vitro study using the ENKTL cell line NK-92MI confirmed the pro-survival effect of VEGF. Exposing tumor cells to VEGF-A during in vitro culture increased the proliferative activity and invasiveness of tumor cells as well as phosphorylation of Akt compared with the control group (Figure 2D–F). These results imply that VEGF can contribute to the aggressive phenotype of ENKTL through the activation of the Akt pathway. Thus, we suggest the feasibility of serum VEGF as a prognostic marker and its potential therapeutic target in ENKTL patients. The worse OS in serum survivin-positive patients could be explained by anti-apoptotic activity of survivin. Impaired apoptotic activity can lead to impaired clearance of cells containing nuclear damage, and the persistence of damaged cells can result in genomic instability. Through this process, tumor formation can occur and tumor cells may become more aggressive.13 We found that the presence of survivin in the serum at diagnosis was significantly associated with an advanced stage and high EBV titer. Thus, serum survivin might originate from tumor cells with excessive survivin expression considering a significant correlation of survivin and EBV expression in tumor tissue. This finding is consistent with previous reports showing a high frequency of tissue expression of survivin in ENKTL patients.14 Thus, serum survivin positivity could be a surrogate marker for tumor burden, as is EBV DNA titer in ENKTL. However, serum survivin-positivity lost its independent prognostic power on multivariate analysis. Thus, only poor performance status (≥ECOG grade II) and high VEGF concentration were independent predictors of OS and PFS (Online Supplementary Table S1). The association of serum proteins with unfavorable clinical characteristics could explain this discrepancy between uni-and multivariate analyses. Thus, serum survivin lost its prognostic relevance whereas VEGF showed an independent prognostic value because VEGF was not related with clinical parameters (Table 1). Furthermore, a small sample size of this study and heterogeneity of treatments according to stage might influence the lack of prognostic power in the multivariate analysis.
In conclusion, serum VEGF and survivin concentrations may be suitable prognostic indicators of ENKTL outcomes in patients treated with non-anthracycline-based chemotherapy. Considering that drugs targeting VEGF (bevacizumab) and survivin (YM155) have been developed, further research should focus on evaluating their roles as a therapeutic target in ENKTL.
Footnotes
Trial registration: clinicaltrials.gov identifier: NCT#00822731, NCT#01877109
Funding: a grant from the Samsung Biomedical Research Institute (SMD1150931), and the National Research Foundation of Korea’s (NRF) Basic Science Research Program, which is funded by the Ministry of Education, Science and Technology (2014R1A2A1A11049853), supported this study.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.William BM, Armitage JO. International analysis of the frequency and outcomes of NK/T-cell lymphomas. Best Pract Res Clin Haematol. 2013;26(1):23–32. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, de Reynies A, de Leval L, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115(6):1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y, de Leval L, Gaulard P. Molecular underpinning of extranodal NK/T-cell lymphoma. Best Pract Res Clin Haematol. 2013;26(1):57–74. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS, Kim KH, Kim KH, et al. Whole blood Epstein-Barr virus DNA load as a diagnostic and prognostic surrogate: extranodal natural killer/T-cell lymphoma. Leuk Lymphoma. 2009;50(5):757–763. [DOI] [PubMed] [Google Scholar]
- 5.Germi R, Lupo J, Semenova T, et al. Comparison of commercial extraction systems and PCR assays for quantification of Epstein-Barr virus DNA load in whole blood. J Clin Microbiol. 2012;50(4):1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SJ, Kim K, Kim BS, et al. Phase II Trial of Concurrent Radiation and Weekly Cisplatin Followed by VIPD Chemotherapy in Newly Diagnosed, Stage IE to IIE, Nasal, Extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma Study. J Clin Oncol. 2009;27(35):6027–6032. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Eom HS, Kim JS, et al. Concurrent Chemo-Radiotherapy Followed by VIDL (Etoposide, Ifosfamide, Dexamethasone, L-asparaginase) Chemotherapy In Stage I/II Extranodal NK/T-Cell Lymphoma of Nasal Cavity/Nasopharynx. Blood. 2010;116(21):736. [Google Scholar]
- 8.Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–2980. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multi-center study. J Clin Oncol. 2006;24(4):612–618. [DOI] [PubMed] [Google Scholar]
- 10.Burger R. Impact of interleukin-6 in hematological malignancies. Transfus Med Hemother. 2013;40(5):336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krejsgaard T, Vetter-Kauczok CS, Woetmann A, et al. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia. 2006;20(10):1759–1766. [DOI] [PubMed] [Google Scholar]
- 12.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. [DOI] [PubMed] [Google Scholar]
- 13.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8(1):61–70. [DOI] [PubMed] [Google Scholar]
- 14.Ng SB, Selvarajan V, Huang G, et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J Pathol. 2011; 223(4):496–510. [DOI] [PubMed] [Google Scholar]


