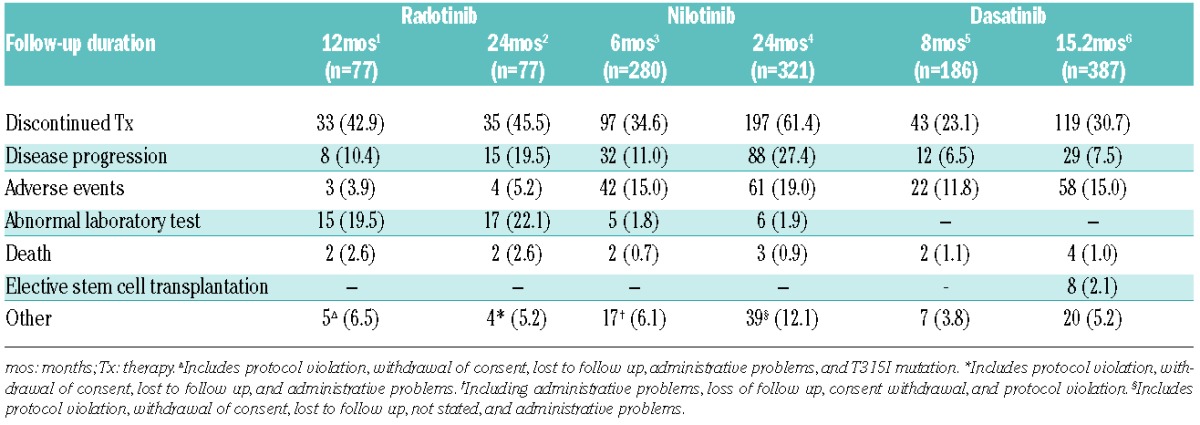
Radotinib is effective and well tolerated in chronic phase-chronic myeloid leukemia patients with resistance and/or intolerance to BCR-ABL1 tyrosine kinase inhibitors and may represent a promising alternative therapy.1,2 High discontinuation rates of second-line nilotinib or dasatinib have been reported at different time points3–6 and overall radotinib discontinuation rate was also comparable to other studies (Table 1). In detail, discontinuation rate at 12 months due to adverse events (AEs) and laboratory results was 23.4% in radotinib, but was respectively 16.8% at six months and 20.9% at 24 months with nilotinib. However, the radotinib discontinuation rate because of disease progression was lower than in other studies. The radotinib discontinuation rate because of abnormal laboratory results may be higher, but additional abnormalities have shown minimal increase after 12 months.2 Hyperbilirubinemia was studied and shows a different pattern to UGT1A1 gene promoter polymorphism of nilotinib.7 Overall, discontinuation rates for AEs and abnormal laboratory data should be interpreted with care due to different eligibility criteria, body size, and genetic background of patients. Previous studies suggested that potential higher rates of AEs in the Asian population may be associated with increased exposure to BCR-ABL1 tyrosine kinase inhibitors (TKIs).8,9 Therefore, a radotinib study including a majority of Asian patients might show higher AEs, including laboratory abnormality. As a starting dose, we consider that the current 400 mg twice daily dose may be appropriate for second-line therapy, but first-line studies with various reduced dosages are ongoing for further dose optimization. Regardless of the limitations of the radotinib study already mentioned in a prior manuscript,1 the data suggest the overall response rate with radotinib is comparable with other 2nd-generation tyrosine kinase inhibitors. In a radotinib phase II study, the primary end point was achievement of MCyR and patients who achieved MCyR were assessed as responders. Also the definition of treatment failure in our study was not that the base-line mutation remained, and, although the base-line mutation was maintained, if the patient achieved MCyR, this patient was assessed as a responder. Therefore, we assessed and explained in our article that if patients achieved MCyR and have no newly detectable mutation on treatment, these patients are defined as responders. Moreover, O’Hare T et al. are currently comparing radotinib resistance profiling to that of other TKIs using a panel of Ba/F3 cells (unpublished data, 2015) and the overall in vitro activity of radotinib was comparable to that of nilotinib against native BCR-ABL1 and single BCR-ABL1 mutants. As already stressed by a CML expert panel, globally, treatment penetration and compliance rates of TKIs are low due to high prices. However, Korean prices for nilotinib and dasatinib are approximately 20%–30% that of Western countries because of the approval of radotinib based on our study. Finally, cost-effectiveness of radotinib could improve drug accessibility and may be necessary in emerging regions.10
Table 1.
Reasons for discontinuation in second-line studies with radotinib, nilotinib and dasatinib.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kim SH, Menon H, Jootar S, et al. Efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 tyrosine kinase inhibitors. Haematologica. 2014;99(7):1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SH, Menon H, Jootar S, et al. Early response of radotinib therapy may predict long-term outcomes in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 TKIs: 24 month update of radotinib phase 2 trial. Haematologica (19th Congress of the European Hematology Association Abstracts). 2014: 284a [Google Scholar]
- 3.Kantarjian HM, Giles FJ, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistant and intolerance. Blood. 2007;110(10):3540–3546. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117(4):1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109(6):2303–2309. [DOI] [PubMed] [Google Scholar]
- 6.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22(6):1200–1206. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Choi SY, Lee SE, et al. Distinct Associations Between UGT1A1 Gene Promoter Polymorphism and Hyperbilirubinemia in Korean CML Patients Treated with Nilotinib and Radotinib. Blood. 2014;124(21):5511a [Google Scholar]
- 8.Kim DW, Goh YT, Hsiao HH, et al. Clinical profile of dasatinib in Asian and non-Asian patients with chronic myeloid leukemia. Int J Hematol. 2009;89(5):664–672. [DOI] [PubMed] [Google Scholar]
- 9.Chuah CT, Nakamae H, Shen ZX, Bradley-Garelik MB, Kim DW. Efficacy and safety of dasatinib versus imatinib in the East Asian sub-population of the DASISION trial of newly diagnosed chronic myeloid leukemia in chronic phase. Leuk Lymphoma. 2014;55(9):2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]


