Abstract
The acquisition of Philadelphia chromosome (Ph) as a secondary change during the course of hematopoietic malignancies is rare and is associated with poor prognosis. Few cases of secondary Ph have been reported after hematopoietic cell transplantation (HCT). A secondary Ph at relapse is of clinical importance because it provides a therapeutic target for tyrosine kinase inhibitors along with or in replacement of chemotherapy. We describe a case of relapsed acute myeloid leukemia after HCT that developed a BCR/ABL-1 translocation along with erythrophagocytosis by blasts as a secondary change at the time of relapse. The progression of this patient's myeloid neoplasm from myelodysplastic syndrome to acute myeloid leukemia and relapsed AML after HCT was accompanied by a stepwise cytogenetic evolution: a deletion 20q abnormality subsequently acquired deletion 7q and, finally, at relapse after HCT, a secondary Ph was gained. The relationship between the secondary Ph and the erythrophagocytosis by blasts is not clear. We review the possible pathogenesis and cytogenetic associations of erythrophagocytosis by blasts, a rare feature in acute leukemias.
Keywords: acute myeloid leukemia, hematopoietic cell transplantation, secondary Philadelphia chromosome, erythrophagocytosis
Introduction
The Philadelphia chromosome (Ph) is present in 90-95% of patients with chronic myelogenous leukemia (CML), 20% of adult lymphoblastic leukemia/lymphoma (ALL), 5% of pediatric ALL, and 1-2% of de-novo acute myeloid leukemia/myelodysplastic syndrome (AML/MDS). A secondary Philadelphia (Ph) chromosome appearing during the course of hematopoietic malignancies is rare and is associated with poor prognosis [1, 2] [3, 4]. Few cases of secondary Ph occurring after hematopoietic transplantation have been reported [5] [6]. We present a unique case of relapsed AML after allogeneic hematopoietic cell transplantation (HCT) that acquired a Ph along with erythrophagocytosis by blasts at the time of the relapse.
Materials and methods
A 66-year-old man with an 8 months history of a low-grade MDS with deletion 20q was evaluated for progressive cytopenias. Complete blood counts showed hemoglobin of 8.3 g/dL, leukocyte count of 1.4×109/L and platelets 0.24×109/L. A bone marrow biopsy revealed 40% blasts and multilineage dysplasia. The blasts expressed CD7, CD13, CD34, CD117, HLA-DR, and dim CD45 by flow cytometry. Routine cytogenetic analysis showed the previously documented deletion 20q (20q-) and demonstrated clonal evolution with acquisition of a 7q deletion (7q-). The diagnosis of AML with myelodysplasia-related changes was established.
The AML was refractory to a standard induction chemotherapy with daunorubicin and cytarabine. The patient subsequently failed two different salvage chemotherapy regimens; i.e., FMC (fludarabine, mitoxantrone, cyclophosphamide) and MEC (mitoxantrone, etoposide, cytarabine). At this point the patient received an experimental agent CPX-351 and finally reached a complete remission. He underwent matched allogeneic sibling hematopoietic cell transplantation (HCT). Unfortunately, 3 months after transplantation the patient relapsed with 68% circulating blasts. A bone marrow biopsy confirmed relapsed AML with blasts showing a new morphologic feature of extensive erythrophagocytosis, which was not present at the original diagnosis. The flow cytometric immunophenotype was unchanged compared to the immunophenotype at the first diagnosis of AML. Donor chimerism was not evaluated. The chromosome analysis at relapse showed deletion of 7q and 20q as documented previously, with a new finding of t(9;22)(q34;q11.2).
Treatment was initiated with an experimental agent SGI-110, however, the leukemia failed to respond after 3 cycles. Administration of dasatinib was intended but delayed due to severe mucositis, dysphasia, and several episodes of aspiration pneumonia. The patient expired due to hypoxemic respiratory failure 8 months after the original diagnosis of AML.
Cytogenetic analysis
G-banded karyotyping was performed on bone marrow samples according to conventional methods. When available, at least 20 metaphases were analyzed. Karyotypes of Giemsa-banded chromosomes were described according to the 2009 International System of Human Cytogenetic Nomenclature [7]. Abnormal clones were defined as 2 or more cells with the same structural abnormality, or the same extra chromosomes, or the presence of 3 or more cells with loss of the same chromosome.
Fluorescence in situ hybridization (FISH) analysis
FISH procedures were performed on cell suspensions prepared from fresh bone marrow aspirate pellets using a standard AML FISH panel and probes for detection of the BCR/ABL-1 fusion. FISH was performed by codenaturation on a HYBrite instrument (Vysis/Abbott) at a denaturation temperature of 72°C for 2 minutes for freshly dropped cells, followed by overnight hybridization at 37°C. At least 100 nuclei were examined for each probe whenever possible. Images were captured using CytoVision software on a Leica DM5000B microscope.
Bone marrow evaluation
Bone marrow core biopsies were fixed in acetic acid-zinc-formalin fixative, decalcified in 10% formic acid-5% formaldehyde and embedded in paraffin. Sections, 1-μm-thick, were stained with haematoxylin and eosin and other histological stains. Peripheral blood and bone marrow aspirate smears were stained with Wright stain for morphologic evaluation.
Flow cytometric analysis
Four-color flow cytometric analysis was performed on bone marrow aspirates on a FACS Canto flow cytometer (Beckton Dickinson). Data analysis was performed using a FACS Diva software.
Results
Karyotype analysis at the diagnosis of acute myeloid leukemia with myelodysplasia-related changes showed the patient's previously documented deletion 20q abnormality and an additional deletion of chromosome 7 as follows: 46,XY,del(7)(q22q34),del(20)(q11.2;q13.1)[16]/46,XY[4]. (Figure 1A). The repeated karyotype at day 14 status post chemotherapy showed two metaphases with isolated 20q- and 5 metaphases with combined 20q- and 7q-. Interphase FISH performed at this stage documented 7q- and 20q- in 79% and 89.5% of the analyzed cells, respectively. The FISH panel included a probe for the BCR/ABL-1 fusion and it was negative for the translocation.
Figure 1.
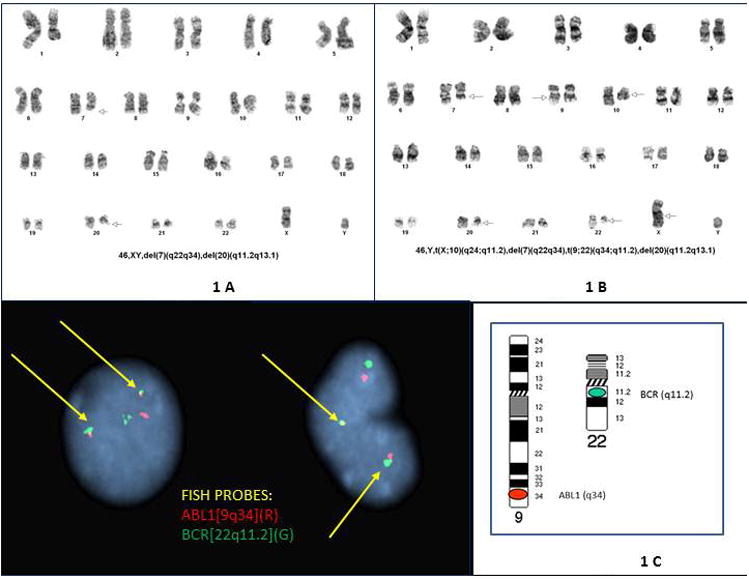
A: Cytogenetic findings at the diagnosis of acute myeloid leukemia with myelodysplasia-related changes. Chromosomes were characterized by a trypsin G-banding method and karyotypes described according to the standard ISCN nomenclature. The karyotype analysis shows the presence of deletion 20q, a previously documented chromosomal abnormality, and demonstrates clonal evolution as follows: 46,XY,del(7)(q22q34),del(20)(q11.2;q13.1)[16]/46,XY[4]. Figure 1B: Cytogenetic findings three months after HCT at relapse of the AML. At this time, the chromosome analysis shows deletion of 7q and 20q as documented previously, with a new finding of t(9;22)(q34;q11.2). Each of 20 metaphases had a 7q deletion, a 20q deletion and a t(9;22)(q34;q11.2) translocation. Two metaphases showed an apparently balanced X;10 translocation in addition to the three abnormalities above (featured karyotype). Figure 1C: Interphase FISH showing a double fusion pattern of ABL1 at 9q34 (red) with BCR at 22q11.2 (green). Image captured at room temperature using Leica DM5000B microscope at 1000× magnification (100×/1.40-0.70 oil immersion APO objective).
At the time of the relapse after HCT, the chromosome analysis showed deletion of 7q and 20q as documented previously, with a new finding of t(9;22)(q34;q11.2) as follows: 46,XY,del(7)(q22q34),t(9;22)(q34;q11.2),del(20)(q11.2q13.1)[18]/46,idem, t(X;10)(q24;q11.2)[2](Figure 1.B). There were no cells with either abnormality in isolation; each of 20 metaphases had a 7q deletion, a 20q deletion and a t(9;22)(q34;q11.2) translocation. Two metaphases represented a subclone which showed X;10 translocation in addition to the three abnormalities above. FISH of interphase nuclei with a standard AML panel confirmed the loss of 7q and 20q in 94.5% and 83% of nuclei respectively, revealed 3 copies of LAMP1 (at13q32) in 66% of nuclei, monosomy 20 in 11.5% of nuclei and the additional new finding of BCR/ABL-1 fusion in 91% of nuclei (Figure 1 C).
The histologic evaluation of a bone marrow biopsy at the time of relapse after HCT showed a new morphologic feature of extensive erythrophagocytosis by blasts, which was not present at the original diagnosis (Figure 2).
Figure 2.
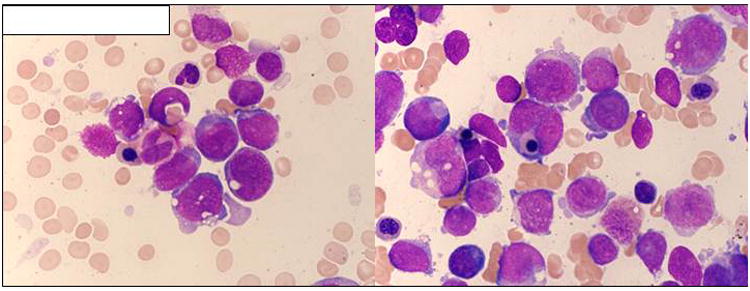
Bone marrow aspirate smear showing relapsed AML after HCT. (Wright Giemsa, 500×.) Several blasts seen with finely dispersed chromatin, basophilic cytoplasm with cytoplasmic vacuolization and erythrophagocytosis. Occasional nucleated red blood cell precursors and maturing neutrophil precursors are also present.
Discussion
The observation of Ph rearrangement as a secondary change suggests that Ph plays a role not only in leukemogenesis, but also in disease progression. Ph as a secondary change is usually late-appearing and may represent clonal evolution [5]. Clonal evolution is often apparent even before the occurrence of the Ph, and the original chromosomal abnormality commonly includes a monosomy 7, with or without additional abnormalities. Alternatively, late-onset Ph may represent a therapy-related myeloid neoplasm in the absence of the initial chromosomal abnormality [6]. In late-developing Ph chromosome, both p190bcr/abl mRNA and p210bcr/abl mRNA have been reported, though it appears that the p190 variant of BCR/ABL-1 rearrangement may occur more frequently [5]. Secondary Ph occurring after HCT has been reported rarely in the literature [5, 6]. The cytogenetic results in our case document a sequential evolution of the patient's clone that originally carried a 20q- in isolation, then acquired 7q- and finally, at relapse after HCT, acquired a Ph. Our data argue against the possibility that an independent clone different form the original AML gained advantage and acquired Ph after transplantation. Acquisition of a secondary Ph in the relapsed or terminal stage of disease may open up new therapeutic opportunities using tyrosine kinase inhibitors along with or in replacement of chemotherapy. In at least one report, a patient had developed a response to imatinib along with withdrawn immunosuppression and chemotherapy after a relapse post-transplant [6].
A distinctly unusual feature of our case is the development of erythrophagocytosis that parallels the acquisition of Ph chromosome. In acute leukemias, erythrophagocytosis is reported in less than 1% of cases and it is most frequently associated with monocytic or granulocytic types. The most commonly observed cytogenetic abnormalities are t(8;16)(p11;p13) and inv(8)(p11q13) in monocytic leukemias, and t(16;21)(p11;q22) and t(10;17)(p13;p12) in less differentiated AML. The deletion of the long arm of chromosome 20 has been described in both ALL and AML with erythrophagocytosis [8, 9]. Deletion of 20q is commonly seen in myeloid disorders such as MDS, myeloproliferative neoplasm and AML. Functional genes that contribute to cytophagocytosis might exist in this region. Though it has not been well defined, the mechanism by which the leukemic blasts phagocytize erythrocytes may involve aberrant premature expression of the complement receptors CR1, CR3 and the IgG receptors FcR and gp150 by the leukemic blasts, in addition to certain cytokines (i.e. TNF and IL2). Since our patient had a 20q- chromosomal abnormality, this may have contributed to the erythrophagocytosis. On the other hand, the 20q- abnormality was present at the time of the original diagnosis and erythrophagocytosis was not present until the acquisition of the Ph. Could the erythrophagocytosis be related to the secondary Ph chromosome?
Erythrophagocytosis is exceptional in the setting of Ph, as only two cases have been reported. A report of blast crisis of chronic myelogenous leukemia with erythrophagocytosis dates back to 1977 [10]. Unfortunately, no molecular data exists for this case, and therefore it is not certain that Ph was present in the phagocytic blasts. More recently, a de novo Ph-positive acute leukemia of ambiguous lineage with erythrophagocytosis was reported [11]. This patient was treated using the PETHEMA Ph+ 2003 protocol which includes imatinib. The patient reached a complete remission followed by a HCT. Nine months after the diagnosis, this patient was reportedly well.
In conclusion, this case illustrates the high degree of chromosomal instability and stepwise cytogenetic evolution that precedes the acquisition of a Ph as a secondary change. Though the secondary Ph may open therapeutic opportunities for tyrosine kinase inhibitors, data are insufficient at present to see the real impact of this approach. In our patient, the erythrophagocytosis by blasts likely contributed to the poor outcome. It is not clear whether certain genes linked to the 20q-deletion or to the secondary Ph may orchestrate erythrophagocytosis. Further reports may elucidate this association.
References
- 1.Sessarego M, Defferrari R, Dejana A, Salvidio E. Late-appearing Philadelphia chromosome in acute lymphoblastic leukemia. Cancer Genet Cytogenet. 1990;48:35–8. doi: 10.1016/0165-4608(90)90213-t. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchiya H, Migita M, Yamamori S, Kaneko Y, Adachi N, Nakamura T, Nobukuni Y, el-Sonbaty SS, Matsuda I. A late-appearing Philadelphia chromosome in acute lymphoblastic leukemia confirmed by expression of BCR-ABL mRNA. Leukemia. 1995;9:1689–93. [PubMed] [Google Scholar]
- 3.Matsue K, Miyamoto T, Ito M, Tsukuda K. Late appearance of the Philadelphia chromosome with monosomy 7 in a patient with de novo AML with trilineage myelodysplasia. Am J Hematol. 1995;49:341–6. doi: 10.1002/ajh.2830490413. [DOI] [PubMed] [Google Scholar]
- 4.Shimamoto T, Ohyashiki K, Ohyashiki JH, Fujimura T, Kodama A, Miyazawa K, Aizawa S, Toyama K. Late appearance of a Philadelphia translocation with minor-BCR/ABL transcript in a t(7;11)(p15;p15) acute myeloid leukemia. Leukemia. 1995;9:640–2. [PubMed] [Google Scholar]
- 5.Chen Z, Morgan R, Notohamiprodjo M, Meloni-Ehrig A, Schuster RT, Bennett JS, Cohen JD, Stone JF, Sandberg AA. The Philadelphia chromosome as a secondary change in leukemia: three case reports and an overview of the literature. Cancer Genet Cytogenet. 1998;101:148–51. doi: 10.1016/s0165-4608(97)00262-8. [DOI] [PubMed] [Google Scholar]
- 6.Prebet T, Michallet AS, Charrin C, Hayette S, Magaud JP, Thiebaut A, Michallet M, Nicolini FE. Secondary Philadelphia chromosome after non-myeloablative peripheral blood stem cell transplantation for a myelodysplastic syndrome in transformation. Bone Marrow Transplant. 2004;33:247–9. doi: 10.1038/sj.bmt.1704308. [DOI] [PubMed] [Google Scholar]
- 7.Shaffer LG, Slovak ML, Campbell LJ, editors. Recommendations of the International Standing Committee on Human Cytogenetic Nomenclature. Basel: Karger AG (Switzerland); 2009. An international system for human cytogenetic nomenclature. [Google Scholar]
- 8.Colon-Otero G, Li CY, Dewald WG, White WL. Erythrophagocytic acute lymphocytic leukemia with B-cell markers and with a 20q- chromosome abnormality. Mayo Clin Proc. 1984;59 doi: 10.1016/s0025-6196(12)62056-4. [DOI] [PubMed] [Google Scholar]
- 9.Mori H, Tawara M, Yoshida Y, Kuriyama K, Sugahara K, Kamihira S, Tomonaga M. Minimally differentiated acute myeloid leukemia (AML-M0) with extensive erythrophagocytosis and del(20)(q11) chromosome abnormality. Leukemia Research. 2000;24:87–90. doi: 10.1016/s0145-2126(99)00145-9. [DOI] [PubMed] [Google Scholar]
- 10.Shanley JD, Cline MJ. Phagocytosis of hematopoietic cells by blasts cells in blast crisis of chronic myelocytic leukemia. West J Med. 1977;126:139–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Ortuno FJ, Castilla C, Moreno MJ, del Mar Osma M, Gonzalez M, Vicente V. Erythrophagocytosis in de novo-philadelphia-positive acute leukemia of ambiguous lineage. Haematologica. 2006;91:ECR43. [PubMed] [Google Scholar]


