Abstract
At one time, G protein-coupled receptors were envisioned to simply relay either inhibitory or stimulatory binary signals through engaging particular G proteins. These receptors are now viewed as complex, multidimensional triggers of a variety of potential signaling cascades. This review will showcase current attempts to elucidate biased signaling and functional selectivity in tissues and organs as well as in the whole animal. In addition, it will emphasize the challenges that are inherent in attributing bias in a living system as well as offer opinions as to the manner in which these problems may be approached.
Introduction
In the study of G protein-coupled receptors (GPCRs), the term functional selectivity has been used to describe the context-dependent actions of distinct ligands at a particular receptor [1•,2,3,4,5,6,7]. In this regard, a receptor can assume multiple configurations that are determined by the interplay of the chemical interface of the ligand binding and receptor interactions with the immediate cellular environment, including binding of scaffolds, components of membrane and potentially, interactions with other receptors. Most of the examples of functional selectivity in vivo have been obtained in mouse models wherein genetic deletion of key signaling elements has helped to delineate whether a receptor responds differently to an agonist in the absence of the signaling element. One of the most useful mouse lines for evaluating functional selectivity in vivo has been the βarrestin knockout mice. This may stand to reason as βarrestins associate directly with GPCRs upon agonist binding and therefore, represent a proximal point of potential ligand-directed signal divergence. Thanks to heroic efforts in the laboratory of Robert Lefkowitz, mouse embryonic fibroblasts lacking the individual as well as both βarrestin 1 and 2 have been developed [8]. These tools have proven very useful, in addition to the knockout mice and the tissues derived from them, in the validation and elucidation of βarrestin-dependent signaling mediated by GPCRs.
βArrestin1-KO mice
Mice lacking βarrestin1 (βarrestin1-KO mice) were very useful in identifying the potential for developing biased agonists at GPR109a receptors. Niacin (nicotinic acid), which activates GPR109a receptors, has been used for the treatment of cardiovascular disease for many years because it effectively aids in lowering triglyceride levels and raising high-density lipoprotein (HDL) levels in blood [9]. However, its therapeutic use is limited by side effect cutaneous flushing. The flushing response induced by niacin is alleviated in mice lacking βarrestin1 [10•]. Moreover, further studies into the mechanism reveal that niacin-activated GPR109a receptors signal through βarrestin1 to activate phospholipase A2 (PLA2) to increase arachidonic acid levels [11]. Although the cutaneous flushing is attenuated in the βarrestin1-KO mice, niacin remains efficacious in its ability to reduce serum free fatty acid levels. These in vivo studies suggest that biased ligands that activate GPR109a and yet do not recruit βarrestin1 could be useful in treating dyslipidemia while avoiding the adverse side effect of cutaneous flushing [12]. The question remains, however, whether niacin’s actions at GPR109a is the only means it has to lower serum fatty acid levels. A report by Lauring et al. [13] showed that mice lacking GPR109a were still responsive to niacin’s lipid lowering properties. This underscores the difficulty of knowing whether the effects of the agonist are bifurcated at particular signaling points or if there are additional targets that are not being accounted for when working in whole animal systems.
βArrestin2-KO mice
The βarrestin2-KO mice have proven to be very useful in identifying examples of functional selectivity in vivo, particularly pathways that utilize βarrestin2 to promote GPCR-mediated signaling. The first physiological phenotype observed reported for the βarrestin2-KO mice was their enhanced responsiveness to morphine-induced anti-nociception [14••,15], demonstrating a role for βarrestin2 in negatively modulating mu opioid receptor responsiveness in vivo. Further studies showed that fentanyl and methadone did not reveal enhanced response profiles in the βarrestin2-KO mice [16•], although fentanyl and methadone were also known to mediate their analgesic effects through the mu opioid receptor [17]. These early studies suggest that there is a ligand-directed bias at the MOR, such that the activity of some agonists, such as morphine, is more influenced by the presence of βarrestin2 than others. Further studies in cell-based assays support these observations, suggesting that agonists that promote more phosphorylation of the receptor can promote recruitment of both βarrestin1 and βarrestin2 while agonists such as morphine, which only modestly promote receptor phosphorylation, are more dependent on βarrestin2 for regulation as βarrestin1 is not recruited [18,19,20].
Additional studies using morphine in the βarrestin2-KO mice suggest the contextual importance of receptor signaling. While morphine-induced antinociception is negatively regulated by βarrestins, morphine-induced constipation, respiratory suppression and physical dependence appear to involve βarrestin2 as these side effects are significantly diminished in the βarrestin2-KO mice [17,21••,22]. While these observations may imply a MOR-βarrestin2 dependent signaling pathway, such a mechanism has yet to be mechanistically demonstrated in vivo. The development of MOR agonists that are biased toward G protein signaling and against βarrestin2 recruitment may provide useful tools for ascertaining whether biased signaling underlies the separation of the antinociceptive properties and side effects induced by opiate narcotics. The first opioid agonist that was identified as a functionally selective agonist is herkinorin [23•] and while it has a limited bioavailability, it is antinociceptive when injected into the paw in the rat formalin test without producing tolerance with repeated dosing [24].
A recently developed biased agonist (TRV130) has shown promising efficacy in pre-clinical studies. This compound is potent in G protein-mediated signaling but has minimal efficacy for inducing βarrestin2 recruitment in cell-based assays. Importantly, in mouse and rat models, TRV130 has analgesic potencies that are better than that of morphine, yet produces less gastrointestinal transit delay and has less effect on respiratory parameters than morphine. TRV130 serves as a proof of concept demonstrating that the effects of an agonist that does not recruit βarrestin2 can recapitulate the behaviors seen in morphine treated mice that lack βarrestin2 and suggest that development of G protein signaling biased MOR agonists may be a means to promote opioid analgesia while limiting certain side effects [25••].
The βarrestin2-KO mice have also been useful for evaluating serotonin 2A receptors (5-HT2AR) function in vivo. Activation of 5-HT2ARs in mice produces a characteristic head twitch response. βArrestin2-KO mice, however, display the response to certain amphetamine agonists (DOI) and N-methyltryptamines, but not in response to serotonin. Using the mice and cortical neuron cultures from these animals, serotonin was shown to activate the receptor leading to assembly of a βarrestin2, Src and Akt complex and the signaling of this complex was necessary to invoke the head twitch response [26••]. In contrast, endogenous N-methyltryptamines, produced by tryptamines and serotonin methylation by N-methyl-transferases, lead to head twitch in the absence of βarrestin2 and these signaling events. Moreover, the N-methyltryptamines neither induce the recruitment of the βarrestin2-Src-Akt complex, nor require their activity to produce a head twitch in vivo. These findings suggest that 5-HT2ARs signal very differently in response to serotonin versus the metabolites of the tryptamines, and building on such differences in signaling may be important for directing 5-HT2AR signaling toward a serotonin-like path.
The dopamine D2 receptor (DAD2R) has been shown to signal via βarrestin2 interactions as well as through G protein-mediated mechanisms [27,28]. Using the anti-psychotic drug aripiprazole as a chemistry scaffold, a number of βarrestin-biased agonists were developed. When tested in mice using a phencyclidine (PCP)-stimulated locomotor activity model for schizophrenic behaviors, these compounds suppressed the PCP-induced hyperlocomotion in mice [29••]. The locomotor-attenuating actions of the βarrestin2-biased DAD2R agonists, however, were abolished in βarrestin2-KO mice suggesting that the βarrestin2 pathway may be therapeutically valuable for mediating the antipsychotic effects of targeting the DAD2R.
βArrestin2-KO mice have also been used to evaluate the effects of endogenous peptides and truncated versions of these agonists at the parathyroid hormone receptor (PTH1R) in promoting bone formation. Parathyroid hormones act on osteoblast PTH1Rs to modulate calcium homeostasis and bone remodeling [30]. The endogenous peptide for PTH1Rs, hPTH(1–34), behaves as a conventional agonist for both G protein coupling and βarrestin recruitment, whereas another peptide (D-Trp12,Tyr34)-PTH(7–34) was shown to function as an antagonist for G protein signaling and as an agonist for βarrestin-dependent signaling cascades [31]. In βarrestin2-KO mice, bone formation induced by PTH(1–34) is attenuated while it is not induced by treatment with the βarrestin-biased agonist PTH(7–34). While both PTH(1–34) and PTH (7–34) induce bone formation in wild-type mice, PTH (7–34) does so without producing hypercalcenemia or signs of bone resorption that are seen with PTH(1–34) administration [32••,33].
Specific challenges in studying functional selectivity in vivo
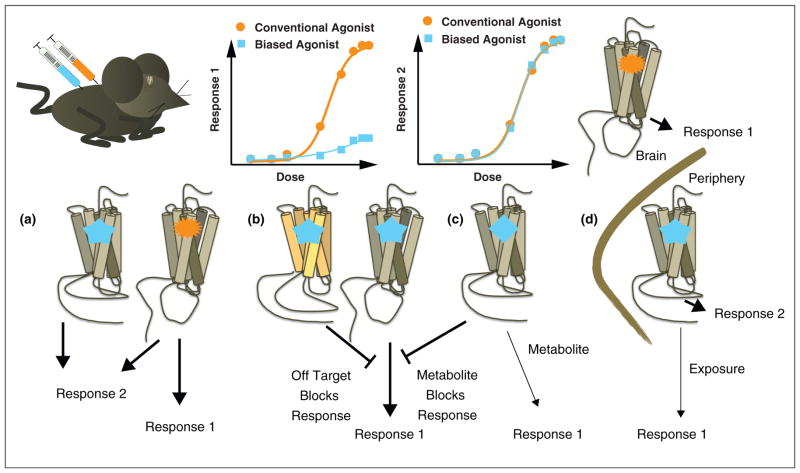
Functional selectivity (or ligand bias) has been repeatedly demonstrated at heterologously expressed receptors in cell-based assays [34]. The in vitro pharmacological profiles, however, may not be reliable predictors of the manner in which a ‘biased’ agonist behaves in vivo as multiple variables can impact on the physiological response resulting from administration of a drug to an animal. The greatest challenge in evaluating functional selectivity in vivo is in determining if physiological effects are due to a drug having ligand bias at a particular receptor or if the differences the drug is producing are due to effects at other receptors. A schematic outlining of these challenges is presented in Figure 1. In many cases, apparent functional selectivity may simply be due to differences in agonist ‘selectivity.’ In the whole animal, it is often difficult to determine if the response assessed is due to the agonist per se acting directly at the receptor to engage unique signaling cascades, or if it is due to the agonist acting at another receptor. If a compound acts at multiple targets which in turn leads to the release or accumulation of additional neurotransmitters, the response becomes even more difficult to trace back to the initial drug treatment. This could be the case for the amphetamines, which act at transporters to raise biogenic amine levels and also have high affinity for biogenic amine receptors [35,36,37]. If the transporter is affected, then the levels of neurotransmitters will be modulated and this combined effect reveals a ‘unique’ behavioral response that is not entirely due to an altered activation of the receptor that is in question. In an equally complex scenario, the test compound may be metabolized to derivatives with intrinsic pharmacological profiles of their own, acting in such a way to generate off-target effects. Or conversely, the alternate targets or metabolites of the reference compound may contribute to components of the physiological response that are lost when a surrogate agonist that is more selective or not metabolized in the same manner as the reference compound is used.
Figure 1.
The schematic depicts the predicted outcome of comparing a conventional drug to a biased drug in vivo where the conventional agonist promotes ‘Response 1’ while this response is absent when the animal is treated with the biased agonist. To distinguish a biased agonist from an inactive compound (or antagonist), both agonists should be similarly efficient at promoting a different response attributable to the receptor’s activation (Response 2). An example of biased agonism and alternative happenings are presented in the figure. (a) The depiction of biased agonism wherein the conventional agonist (orange starburst) binds to the receptor to induce Responses 1 and 2 and the biased agonist (blue star) only engages signaling at the receptor that leads to Response 2. (b) Alternatively, the ‘biased agonist’ could have activity at another target that opposes the actions it has at the primary target associated with Response 1. (c) This could also happen if the ‘biased agonist’ is metabolized in vivo to form a new compound that does not preserve the pharmacology of the parent compound (i.e. it could become an antagonist and competitively prevent Response 1. Moreover, the metabolite could simply be less potent and efficacious than the parent compound. (d) Additionally, if the ‘biased agonist’ has a different bioavailability profile than the conventional agonist in vivo, then the lack of its activity in Response 1 could be attributed to its inability to reach the target receptor that controls the response. In this example, the ‘biased agonist’ does not cross the blood–brain barrier efficiently and therefore, cannot reach the target receptor. In the examples of b–d, the ‘biased agonist’ would have non-selective effects rather than be considered functionally selective at the target GPCR and therefore, not a ‘biased agonist’.
Approaches for evaluating functional selectivity in vivo
Since many of the limitations for evaluating downstream behavioral effects are that the responses may be due to agonists acting nonselectively, there is a need to minimalize the potential nonselective variables while maintaining the integrity of the endogenous system. One manner of approaching this challenge is to begin to break down the system and adopt isolated tissue preparations and primary cultures. While this approach does not eliminate selectivity questions entirely, it does greatly narrow the potential for nonspecific contributions by eliminating liver metabolism and the contribution of multiple sites of action that may regulate the same response (such as certain brain regions versus the spinal cord, or the autonomic nervous system versus the enteric nervous system, for example). The greatest challenge, however, is to determine the most appropriate biological system to study when investigating the actions of a biased agonist, as functional selectivity is context specific. Since a ligand may display bias in one cell type and not another, it is important to utilize a biological system wherein the receptor’s actions have been highly attributed to the physiological response observed in vivo. The next challenge come in elucidating receptor-generated signaling events that are due primarily to the activation of the receptor and not to downstream or off-target activations.
The most direct way to determine if two agonists differentially act at a receptor would be to determine structural changes that occur upon receptor binding. Tremendous advances were made during the crystallization of the β2 adrenergic receptor (β2AR) which were assisted by the development of nanobody-based approaches aided in stabilizing the receptor by mimicking an active state [38••,39]. Nanobodies are single-domain antibodies lacking light chain that naturally occur in camelid such as llama [40]. While these tools were useful in studying purified receptor preparations, more recently they have been applied to studying GPCRs in living cells. In this application, a nanobody (Nb80), was tagged with a green fluorescence protein (Nb80-GFP) and used as a conformation-specific biosensor of activated β2AR in live cells [41]. As a powerful tool for differentiating inactive vs. active state receptors, the labeled-nanobody approach may hold promise for developing new sensors for determining conformational states of GPCRs in native tissues if the sensor can be introduced in a manner that does not disrupt the system.
A proximal approach that is currently used to determine if the ligand has a differential effect on the receptor in question is to track the receptor’s movement. However, the primary limitation of this approach is that the receptor may not be specifically recognized by the antibody; further, antibodies that recognize epitopes in the regions that are involved in binding to signaling and trafficking proteins upon ligand treatment may fail to detect the relevant population of receptors that are indeed engaging in interactions with those trafficking proteins. A potential approach for improving receptor trafficking is the development of knock-in mice expressing fluorescent tags on the receptor behind the endogenous promoter. Such an approach has been successfully used to visualize delta opioid receptor internalization in response to agonists [42,43]. After confirming that the agonists to the DOR mediated the biological effects of interest by showing the effects were absent in the DOR-KO mice, the investigators showed that two DOR agonists (ADL5747 and ADL5859) that produce antinociception without inducing hyperlocomotor activity also failed to promote internalization of the DOR-GFP in neurons (spinal cord, dorsal root ganglion, striatum and hippocampus). Since this is in contrast to the actions of conventional agonist, which produces antinociception, hyperlocomotor activity, and internalizes DOR, it is attractive to conclude that ADL5747 and ADL5859 are biased agonists that activate pathways leading to antinociception while not activating pathways (involving internalization) leading to hyperlocomotor activity [43,44,45].
Another early indicator of GPCR activation is receptor phosphorylation. There is significant evidence that receptor phosphorylation at different sites will direct differential activation of distinct signaling cascades. In this manner, different agonists might be expected to activate different kinases that result in the phosphorylation of the receptor at different sites, producing a ligand-specific receptor phosphorylation ‘barcode’ [46,47]. These differences in phosphorylation profiles for the GPCRs may be a key point in delineating functional scaffolds as this post-translational modification may serve to facilitate or perturb interactions with neighboring scaffolds. Therefore, the phosphorylation state of a receptor may represent a reasonably proximal means for assessing differential actions at receptors in an endogenous setting that could be used to predict downstream changes in the receptor’s function.
Recently, phosphosite-specific antibodies have been developed for detecting phosphorylated residues serine 363, 375 (Ser363, Ser375) and, threonine 370 (Thr370) on the C-terminal of MOR [48]. Using these antibodies, Doll et al. found a heirarchical role for agonist-induced phosphorylation of the MOR, wherein S375 serves as the initial priming phosphorylation site followed by phosphorylation at T376 and T379. The more phosphorylated the receptor becomes, the more likely it is to internalize [49]. The researchers found that morphine induces phosphorylation of S375 but not T370, whereas DAMGO causes the phosphorylations on both Ser375 and Thr370 with a faster rate at Ser375. Ser375 phosphorylation was sufficient and required for morphine-induced desensitization of MOR [20]. However, the differences in the phosphorylation ‘barcode’ can produce downstream consequences (such as internalization) that reflect the nature of the agonist. Some of these phosphorylation site-directed MOR antibodies have been shown to recognize MOR mouse tissues and will likely prove useful in determining agonist-directed signaling profiles in the endogenous setting [50]. The development of phosphorylation state antibodies again introduces the question of selectivity; however, these limitations may be overcome by the advances in developing highly sensitive phosphoproteomics methods that can distinguish receptor active-state specific phosphorylation barcodes. These highly sensitive technologies may serve as a means to determine differential receptor activity states induced by biased ligands in the endogenous setting.
The further one gets from the receptor, the more difficult it is to attribute the observed differences in signaling to the differential occupation of the receptor and not to off-target effects of the ligand. A proximal approach to detect receptor-mediated signaling downstream of differential ligand activation would be to trap the specific agonist-induced receptor-scaffold complex in the absence and presence of ligand and to assess the changes in complex composition using immunoprecipitation approaches [51]. However, this approach again relies upon antibody selectivity and recognition and also requires that the composition of the scaffold during sample preparation remains intact. Further, low numbers of receptors can make it difficult to isolate sufficient proteins for analysis. However, this is a recurring and intrinsic problem of studying receptor-mediated signaling events in vivo: it may only take a few receptors, at the right place and the right time, to induce the physiological effect; and this paucity of response may be extremely difficult to detect over the noise of the system.
Moving away from the receptor, one can examine changes in downstream signaling elements. While this approach introduces the confounding variable of non-selective stimulation contributing to the responses observed, the signal will, in many cases, be easier to detect. Moreover, upon identifying changes in proteins that are known to act as GPCR scaffolds, the process can serve as a means to evaluate receptor complexes with more traditional means such as immunoprecipitation of the receptor and validation of the players in the complex. While this may not necessarily be the most ideal approach, it remains one of the few ways that such interactions can be investigated in lieu of modifying the signaling components. Phosphoproteomics has been used to investigate drug-induced changes in signaling cascades in tissues and primary cultures [52,53]. In conjunction with elaborate bioinformatics signaling network analysis, mass spectroscopy (phospho)proteomics approaches may provide a means for comparing the downstream consequences of engaging diverse signaling pathways. Studies in HEK cells evaluating the angiotensin IIa receptor demonstrate the bifurcation of downstream signaling when a βarrestin2-biased agonist is used, demonstrating the potential utility of this approach [54]. The use of phosphoproteomics has tremendous potential for investigating bias in vivo as well as for determining physiologically relevant signaling pathways in vivo.
An approach that is perhaps the least proximal to the receptor involves monitoring changes in mRNA transcription in response to agonist-induced changes. In this case, the assumption is that the activation of a receptor by a ligand will promote a particular signaling cascade that will result in a particular pattern of gene responsiveness, or a transcription signature. This transcriptome approach has been used to dissect signaling mechanisms of functionally selective ligands of PTH receptor in vivo [55]. Both hPTH(1–34), the conventional agonist activating both G protein and βarrestin signaling cascades, and (D-Trp12,Tyr34)-PTH(7–34), an βarrestin-signaling biased agonist [32••], promote bone formation in vivo. By comparing the bone transcriptome profiles in wild-type or βarrestin2-null mice with long-term treatment with either vehicle, hPTH(1–34), or (D-Trp12,Tyr34)-PTH(7–34), the authors found signature patterns of gene clusters induced downstream of the βarrestin2-biased agonist [55,56].
The realization that GPCRs can signal via different pathways that are determined by the nature of the ligand binding to the receptor unveils a tremendous potential for new approaches in developing therapeutics at these receptors. On that front, the development of novel ligands that display functionally selective properties in cell-based assays is proving to be plausible and proficient. The future challenge, however, is in determining how the endogenous receptors, in the appropriate location, signal to control particular physiological responses. As we move forward with comparing agonists in vivo and attempting to attribute active signaling signatures to ligand-receptor interactions, the value of the classic tools of pharmacology and physiology will surely become apparent.
Acknowledgments
Funding
LMB: R01DA033073, R01DA031927, R01DA025158.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1•.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. This is a highly collaborative review written to unify concepts on the pharmacological measures of functional selectivity as they were beginning to emerge. [DOI] [PubMed] [Google Scholar]
- 2.Kenakin T. Biased agonism. F1000 Biol Rep. 2009;1:87. doi: 10.3410/B1-87. http://dx.doi.org/10.3410/B3411-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohn LM. Selectivity for G protein or arrestin-mediated signaling. In: Neve KA, editor. Functional selectivity of G protein-coupled receptor ligands. 1. Humana Press; 2009. pp. 71–85. [Google Scholar]
- 6.Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63:1001–1010. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- 8.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. Beta-arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A. 2001;98:1601–1610. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258:94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 10•.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. Beta-arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. This study demonstrated that the flushing effects induced by niacin were due to niacin activation of GPR109a — βarrestin1 signaling. While βarrestin1-KO mice did not flush in response to niacin, the agonist was still able to lower serum free fatty acid levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamanna VS, Ganji SH, Kashyap ML. The mechanism and mitigation of niacin-induced flushing. Int J Clin Pract. 2009;63 :1369–1377. doi: 10.1111/j.1742-1241.2009.02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeWire SM, Violin JD. Biased ligands for better cardiovascular drugs: dissecting G protein-coupled receptor pharmacology. Circ Res. 2011;109:205–216. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- 13.Lauring B, Taggart AK, Tata JR, Dunbar R, Caro L, Cheng K, Chin J, Colletti SL, Cote J, Khalilieh S, et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci Transl Med. 2012;4:148ra115. doi: 10.1126/scitranslmed.3003877. [DOI] [PubMed] [Google Scholar]
- 14••.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. This first report on βarrestin-2 knockout mice provides in vivo evidence for the role of βarrestin-2 in regulating the function of mu-opioid receptor. [DOI] [PubMed] [Google Scholar]
- 15.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 16•.Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. This study compares opioid agonists in βarrestin2-KO mice and in cell-based assays, providing evidence that the agonists that robustly recruit βarrestins to MOR show no apparent phenotype in the absence of βarrestin2, while poor recruiters promote enhanced antinociceptive responses in the βarrestin2-KO mice. [DOI] [PubMed] [Google Scholar]
- 17.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohn LM, Gainetdinov RR, Caron MG. G protein-coupled receptor kinase/beta-arrestin systems and drugs of abuse: psychostimulant and opiate studies in knockout mice. Neuromol Med. 2004;5:41–50. doi: 10.1385/NMM:5:1:041. [DOI] [PubMed] [Google Scholar]
- 19.Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist-directed interactions with specific beta-arrestins determine mu-opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem. 2011;286:31731–31741. doi: 10.1074/jbc.M111.248310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23 :3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. This study showed that mice lacking βarrestin2 display less morphine-induced gastrointestinal delays and respiratory suppression. [DOI] [PubMed] [Google Scholar]
- 22.Bohn LM, Raehal KM. Opioid receptor signaling: relevance for gastrointestinal therapy. Curr Opin Pharmacol. 2006;6:559–563. doi: 10.1016/j.coph.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 23•.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce mu-opioid receptor — arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. Herkinorin was shown to have a bias for MOR for promoting G protein coupling over βarrestin2 recruitment in cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamb K, Tidgewell K, Simpson DS, Bohn LM, Prisinzano TE. Antinociceptive effects of herkinorin, a MOP receptor agonist derived from salvinorin A in the formalin test in rats: new concepts in mu opioid receptor pharmacology: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:181–188. doi: 10.1016/j.drugalcdep.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–717. doi: 10.1124/jpet.112.201616. This study presents work characterizing a biased MOR agonist that preferentially signals through G protein pathways over recruiting βarrestin2. It provides proof-of-concept evidence in mice that such compounds may produce potent antinociception with less gastrointestinal and respiratory suppression than morphine. [DOI] [PubMed] [Google Scholar]
- 26••.Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. Serotonin and tryptamine metabolites were shown to induce different kinase cascade activation profiles in mice. Serotonin leads to 5HT2AR activation of SRC and Akt in a βarrestin2-dependent manner, while the tryptamines do not. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 29••.Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B, et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. This study describes in vivo studies for βarrestin2-biased agonists at dopamine receptors. The authors propose that βarrestin2-mediated D2 signaling is the critical pathway for antipsychotic efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cupp ME, Nayak SK, Adem AS, Thomsen WJ. Parathyroid hormone (PTH) and PTH-related peptide domains contributing to activation of different PTH receptor-mediated signaling pathways. J Pharmacol Exp Ther. 2013;345:404–418. doi: 10.1124/jpet.112.199752. [DOI] [PubMed] [Google Scholar]
- 31.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 32••.Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. This article evaluates the ability of parathyroid hormone and a truncated PTH, which is biased for βarrestin2-mediated signaling, to induce bone formation. By only utilizing the βarrestin2 pathway, the authors show that bone formation could be increased while not increasing bone resorption, which usually occurs with PTH therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohinc BN, Gesty-Palmer D. beta-arrestin-biased agonism at the parathyroid hormone receptor uncouples bone formation from bone resorption. Endocr Metab Immune Disord Drug Targets. 2011;11:112–119. doi: 10.2174/187153011795564151. [DOI] [PubMed] [Google Scholar]
- 34.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tallman KR, Grandy DK. A decade of pharma discovery delivers new tools targeting trace amine-associated receptor 1. Neuropsychopharmacology. 2012;37:2553–2554. doi: 10.1038/npp.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotnikova TD, Caron MG, Gainetdinov RR. Trace amine-associated receptors as emerging therapeutic targets. Mol Pharmacol. 2009;76:229–235. doi: 10.1124/mol.109.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, Caron MG, Gainetdinov RR. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol. 2011;80:416–425. doi: 10.1124/mol.111.073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. The first crystallization of a GPCR utilizing the innovative nanobody approach to stabilize the active state of the receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steyaert J, Kobilka BK. Nanobody stabilization of G protein-coupled receptor conformational states. Curr Opin Struct Biol. 2011;21:567–572. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 41.Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS ONE. 2009;4:5425e. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Nozaki C, Le Bourdonnec B, Reiss D, Windh RT, Little PJ, Dolle RE, Kieffer BL, Gaveriaux-Ruff C. delta-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther. 2012;342:799–807. doi: 10.1124/jpet.111.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butcher AJ, Tobin AB, Kong KC. Examining site-specific GPCR phosphorylation. Methods Mol Biol. 2011;746:237–249. doi: 10.1007/978-1-61779-126-0_12. [DOI] [PubMed] [Google Scholar]
- 47.Tobin AB, Butcher AJ, Kong KC. Location, location, location…site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29:413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doll C, Konietzko J, Poll F, Koch T, Hollt V, Schulz S. Agonist-selective patterns of micro-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011;164:298–307. doi: 10.1111/j.1476-5381.2011.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Just S, Illing S, Trester-Zedlitz M, Lau EK, Kotowski SJ, Miess E, Mann A, Doll C, Trinidad JC, Burlingame AL, et al. Differentiation of opioid drug effects by hierarchical multi-site phosphorylation. Mol Pharmacol. 2013;83:633–639. doi: 10.1124/mol.112.082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grecksch G, Just S, Pierstorff C, Imhof AK, Gluck L, Doll C, Lupp A, Becker A, Koch T, Stumm R, et al. Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A mu-opioid receptor knock-in mice. J Neurosci. 2011;31:13890–13896. doi: 10.1523/JNEUROSCI.2304-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, Bohn LM. Functional selectivity of 6′-guanidinonaltrindole (6′-GNTI) at kappa-opioid receptors in striatal neurons. J Biol Chem. 2013;288:22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA. Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.014613. M111 014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stockton SD, Jr, Devi LA. An integrated quantitative proteomics and systems biology approach to explore synaptic protein profile changes during morphine exposure. Neuropsychopharmacology. 2013;38:1–16. doi: 10.1038/npp.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, 3rd, Lefkowitz RJ. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci U S A. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gesty-Palmer D, Yuan L, Martin B, Wood WH, 3rd, Lee MH, Janech MG, Tsoi LC, Zheng WJ, Luttrell LM, Maudsley S. Beta-arrestin-selective G protein-coupled receptor agonists engender unique biological efficacy in vivo. Mol Endocrinol. 2013;27:296–314. doi: 10.1210/me.2012-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bohinc BN, Gesty-Palmer D. Biased agonism at the parathyroid hormone receptor: a demonstration of functional selectivity in bone metabolism. Mini Rev Med Chem. 2012;12:856–865. doi: 10.2174/138955712800959125. [DOI] [PubMed] [Google Scholar]