Abstract
The dephosphorylation of myosin by the MP causes smooth muscle relaxation. MP is also a key target of signals that regulate vascular tone and thus blood flow and pressure. Here, we review studies from the past two decades that support the hypothesis that the regulated expression of MP subunits is a critical determinant of smooth muscle responses to constrictor and dilator signals. In particular, the highly regulated splicing of the regulatory subunit Mypt1 Exon 24 is proposed to tune sensitivity to NO/cGMP-mediated relaxation. The regulated transcription of the MP inhibitory subunit CPI-17 is proposed to determine sensitivity to agonist-mediated constriction. The expression of these subunits is specific in the microcirculation and varies in developmental and disease contexts. To date, the relationship between MP subunit expression and vascular function in these different contexts is correlative; confirmation of the hypothesis will require the generation of genetically engineered mice to test the role of MP subunits and their isoforms in the specificity of vascular smooth muscle responses to constrictor and dilator signals.
Keywords: myosin phosphatase, cGMP, NO, PKG1, Mypt1, CPI-17, vascular smooth muscle
INTRODUCTION
It was just over 20 years ago that Alessi and coworkers purified from the chicken gizzard a protein phosphatase that dephosphorylates myosin [1]. They identified PP1β as the 37 kDa catalytic subunit, and 130 (Mypt1) and 20 kDa (M21) subunits that enhanced the activity of PP1β toward smooth muscle MLC and suppressed its activity toward other substrates, thereby providing regulation and specificity for its activity. (At the same time they identified the skeletal muscle MP and demonstrated that the smooth and skeletal muscle MP were not interchangeable due to differing regulatory (130 kDa) subunits, a subject beyond the scope of this review). Haystead and colleagues subsequently purified mammalian MP from pig bladder [59]. The Alessi study has been cited over 300 times, demonstrating the importance of the biochemical purification of MP as well as the tremendous progress that was made over the following decades. This progress can be dichotomized as (1) the identification of signaling pathways that regulate MP activity and thereby determine the balance between calcium-MLCK-mediated contraction and MP-mediated relaxation (depicted in Figure 1); and (2) the identification of the sequence and diversity in MP subunits and related signaling molecules. The number of identified serine–threonine phosphatases (~25) is quite small compared to the number of corresponding kinases (~400). This difference is due to different diversification strategies, with a large number of regulatory subunits responsible for the diversification of PP1 activities (reviewed in [3]). This review will focus on the diversity of the smooth muscle MP and related proteins and how their regulated expression might determine smooth muscle function and signaling responses specific to developmental, tissue specific, and disease contexts.
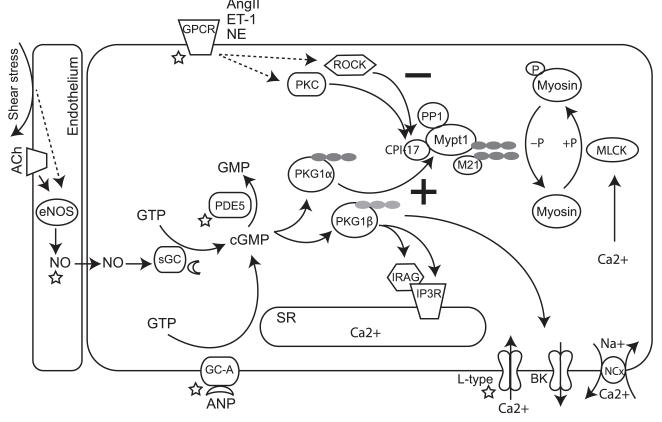
Figure 1.
Cartoon of signaling pathways that regulate smooth muscle force production. The regulatory (Mypt1) and inhibitory (CPI-17) subunits of MP are targets of multiple signaling pathways that modulate smooth muscle contractility. Pathways that decrease MP activity are denoted with a (−) and those that increase MP activity are denoted with a (+). Constrictor and dilator signals relax VSM through the combined effect on calcium flux and calcium sensitivity of the myofilaments. Several components of these signaling pathways are therapeutically targeted (star) or have targeting drugs in clinical trials (moon). LZ motifs are shown as filled circles. Abbreviations are defined in the abbreviations section in the text.
Smooth Muscle Contractile Diversity
Fundamental to a discussion of the role of MP isoforms in determining smooth muscle contractility is the distinction of smooth muscle phenotypes. Although there are many ways to classify smooth muscle, functionally they may be dichotomized as tonic vs phasic contractile properties [61], which is analogous to the slow vs fast contractile classifications of striated muscle. Large arteries and veins are characterized as tonic smooth muscle, which is continuously contracted. The smooth muscle of the portal vein, bladder, and gut is characterized as phasic and has rhythmic contractile activity and several-fold faster rates of contraction and relaxation as compared to tonic smooth muscle. Other tissues, such as small resistance arteries, have mixed properties (reviewed in [17,62]).
While phasic and tonic smooth muscles utilize the same basic mechanisms to contract and relax, i.e., MLCK- and MP-mediated phosphorylation and dephosphorylation of myosin, respectively, their force outputs are very different. In addition, the MP in tonic smooth muscle is subject to greater regulation by signaling pathways, both positive and negative, that set the sensitivity of the contractile apparatus to calcium-dependent force production, than is the phasic smooth muscle MP (discussed elsewhere in this article). These differences, like in striated muscle, are likely encoded within the genome, with regulated gene transcription and alternative splicing of genes producing great smooth muscle phenotypic diversity. In this review we will refer to gene products that are predominately expressed in phasic smooth muscle as “fast” isoforms and gene products that are predominately expressed in tonic smooth muscle as “slow” isoforms, analogous to descriptors used in striated muscle. Distinctions between the fast and slow gene programs include differential expression of splice variants of smooth muscle MHC (MYH11), MLC17 (MYL6), and MYPT1 (PPP1R12a). Other differences between phasic and tonic smooth muscle involve the level of gene expression, as, e.g., the 2- to 3-fold greater expression (and activity) of MP and MLCK in phasic vs tonic smooth muscle (reviewed in [17]). It is worth noting here that (1) the full extent of differences between these gene programs requires more complete investigation and (2) differential expression of a gene product in phasic vs tonic smooth muscle, although suggestive, are not sufficient to conclude causality in determining specific contractile properties of phasic vs tonic smooth muscle. Finally, phasic and tonic phenotypic specification should be distinguished from smooth muscle differentiation and dedifferentiation, a subject that has received much investigation due to the epidemic of atherosclerosis in the conduit vessels, but in and of itself is of little consequence to vascular function.
Smooth muscle contractile phenotypes must be specified at some point during development. A priori, no assumptions can be made about when this will occur, a particularly important consideration given the variability in maturation of smooth muscle systems throughout the organism. Thorough investigation of multiple time points from early in the formation of vascular and visceral organ systems through maturity using markers of contractile phenotypes (e.g., MHC and MLC isoforms) allows for the identification of the developmental periods of phenotypic specification in individual tissues. These periods, which differ for individual vessels and organs, can then be more intensively studied. Smooth muscle phenotypic specification during development is discussed further in Section IIA. As a final point, the smooth muscle contractile phenotypic diversity within the vascular (and visceral) systems renders meaningless the approach of using a “representative” vessel for the study of smooth muscle responses in animal models of vascular disease. This is particularly important to emphasize here, as we shall see that the smooth muscle contractile phenotypes in the microcirculation are very different from the larger conduit arteries.
Smooth Muscle Relaxation
Identification of MP regulation by PKG1
Several decades passed between the groundbreaking discoveries of an EDRF (NO) signaling through the second messenger cGMP and the identification of the targets of the PKG1 (reviewed in [42]). Classic physiological studies by Kitazawa and others identified the MP as a target of cGMP signaling [39,73] (reviewed in [25,31]). In permeabilized blood vessels in which calcium clamp activates MLCK and force, cGMP reduces myosin phosphorylation and completely relaxes the smooth muscle, a process termed calcium desensitization. Surks and colleagues subsequently used the yeast two-hybrid method screening a library with the N-terminal LZ motif of PKG1α to identify a mechanism by which PKG1α is targeted to MP [64]. The N-terminal LZ motif of PKG1α mediates hetero-dimerization with the C-terminal LZ motif of the targeting/regulatory subunit of MP, Mypt1, and is required for PKG1 activation of MP. As would be expected, a number of phosphorylation sites in MP regulatory subunits have been identified (reviewed in [25,31]), but the precise mechanism by which cGMP/PKG1 activates MP is not defined with certainty.
Variability in Mypt1
In the 1990s, as MP was being identified and shown to be a target of cGMP/PKG1, the component parts were being cloned and sequenced from different species and tissues. Isoforms of the Mypt1 were identified, but had not been systematically studied [4,27,32,57]. The variability in Mypt1 results from alternative splicing of exons in two regions of the Mypt1: (1) the central portion of the transcript, near an inhibitory phosphorylation site (Thr696); and (2) at the 3′-end of the transcript, near the C-terminus LZ motif. The central exon Mypt1 variants are conserved in mammals, but not throughout evolution. Interestingly, although alternative splicing of specific exons is not conserved, all species from mammals to birds [8,57] and worm [71] have isoforms generated by alternative splicing in this region. However, the functional significance of alternative splicing in this region is not defined, nor even hypothesized, and will not be further discussed.
Our laboratory has focused for the past decade on the variants generated by an evolutionarily conserved 3′ alternative exon. Splicing (inclusion) of the 31 nt exon 24 (E24-included; Exon 23 [E23] in birds) at the 3′ end of Mypt1 transcript shifts the reading frame and introduces a premature termination codon, producing a completely different C-terminal amino acid sequence that lacks a LZ motif [34] (Figure 2). We have demonstrated that the splicing of this exon is tissue specific, developmentally regulated, modulated in disease, and appears to be functionally important.
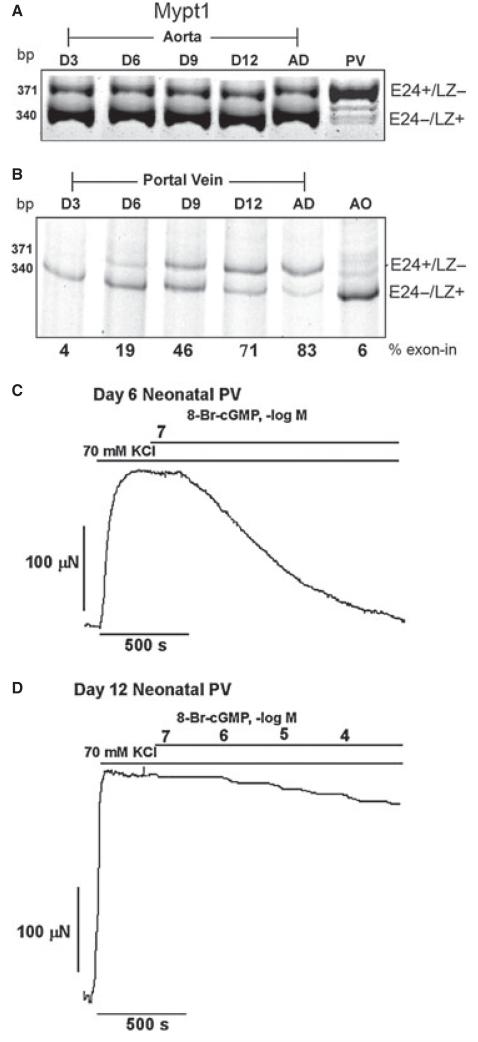
Figure 2.
Tissue-specific expression of isoforms of the Mypt1 generated by alternative splicing of Exon 24. E24 is skipped in tonic smooth muscle such as the aorta. This variant codes for a C-terminal LZ motif (LZ±) that is required for binding of the LZ motif of PKG1α and cGMP-mediated activation of MP. Activated MP dephosphorylates MLC, resulting in smooth muscle relaxation. Inclusion of the 31 nt E24 in phasic smooth muscle shifts the reading frame coding for a C-terminal that lacks the LZ motif (LZ−). E24 inclusion also generates a premature termination codon (*). As described in the text, a number of studies have shown developmental switching from Mypt1 E24− to E24+ as part of the implementation of the fast gene program (phasic phenotype) in smooth muscle tissues. In disease models these tissues revert to the Mypt1 E24−/LZ+ isoform as part of a modulation toward the tonic smooth muscle phenotype.
Tissue-specific and developmental regulation of Mypt1 isoforms
Extensive surveys of smooth muscle tissues have demonstrated specificity in the splicing of Mypt1 E24 [34,53]. The predominant isoform in tonic smooth muscle, such as the large arteries and veins, is the E24-skipped variant coding for the C-terminal LZ motif. In the phasic smooth muscle of the gut, bladder and portal vein, as well as the small resistance arteries, the predominant Mypt1 isoform is the E24-included variant coding for the LZ− isoform.
Developmental shifts in Mypt1 isoforms were identified as part of smooth muscle contractile phenotypic specification. As a generalization, smooth muscle fated to the tonic phenotype, as, e.g., in the large blood vessels, express the Mypt1 E24−/LZ+ isoform and slow gene program through-out development and into adulthood [52] (Figure 3A). Markers used in these studies included splice variants of the myosin heavy and light chains that also segregate with phasic vs tonic phenotype, although a more complete analysis of the gene programs remains to be done. Early in development, tissues destined to the phasic contractile phenotype, e.g., portal vein and intestines, predominately express the slow gene program [8,18], including the Mypt1 E24−/LZ+ isoform [34,52] (Figure 3B). These tissues switch to a fast gene program and become Mypt1 E24+/LZ− with variable timing, but usually around the time of birth through the first several postnatal weeks. This correlates with the developmental timing in the changes in their contractile properties, i.e., rates of contraction and relaxation [48]. Together, these observations suggest that the slow gene program is the developmental default, consistent with the observation that SMCs in culture do not express fast isoforms, i.e., are of the tonic phenotype. We have suggested that the splicing factor Tra2β may be responsible for the developmental implementation of the fast smooth muscle gene program [21,60], but for the most part the factors triggering this process remain unknown.
Figure 3.
Mypt1 isoform switching in vascular development correlates with relaxant response to 8-Br-cGMP. Aorta, a prototypical tonic smooth muscle tissue, expresses Mypt1 E24−/LZ+ slow isoform throughout development without isoform shifts (A). In contrast, the portal vein (PV), a prototypical phasic smooth muscle, between 3 and 12 days postnatally, shifts from the E24−/LZ+ to E24+/LZ− isoform. Shown are gel images in which E24+ and E24− variants are amplified in a single PCR, separated by size, and percent exon inclusion quantified (B). This shift in Mypt1 isoform expression correlates with vessel relaxation to 8-Br-cGMP: Day 6 neonatal PV completely relaxed to 10−7 M 8-Br-cGMP (C), whereas the Day 12 neonatal PV showed minimal relaxation to 10−4 8-Br-cGMP (D). These force recordings are from KCl depolarized unpermeabilized vessels. Similar results are obtained with permeabilized vessels activated by calcium. Figures reprinted from [52].
cGMP response
The tissue-specific expression of Mypt1 isoforms and complete isoform shifts during development provided a natural model to test the role of the Mypt1 LZ in cGMP activation of MP. Using the classic assay of cGMP activation of MP in intact muscle ex vivo, we demonstrated dichotomous responses depending on phenotype and Mypt1 isoform expression. In smooth muscle tissues expressing the Mypt1 LZ+ isoform, such as aorta at any developmental stage, or portal vein and intestine early in development, 8-Br-cGMP was able to activate MP, and completely, or nearly completely, relax the permeabilized muscle even under conditions where calcium maximally activated MLCK and force [34,52] (Figure 3C,D). In contrast, in smooth muscle tissues expressing the Mypt1 LZ− isoform, such as rat portal vein and intestine and chicken gizzard, 8-Br-cGMP had minimal effect under the same conditions. These experiments supported a role for Mypt1 LZ+ and LZ− isoforms in determining the response to NO/cGMP, the first such example identified. Of historical interest, both mammalian portal vein [16] and chicken gizzard [54] had been reported to be unresponsive to EDRF and cGMP, respectively, but no mechanism had been identified.
The studies reviewed above demonstrate a correlation between expression of Mypt1 LZ+ isoform and cGMP-mediated activation of MP/relaxation, but are not sufficient to conclude a causal relationship. The requirement for the LZ of PKG1α in mediating the relaxant effects of NO was demonstrated in a mouse model by replacement of the leucine residues with alanine [43]. These mice were hypertensive and their aortic and pial arteries showed impaired relaxation to ACh, the NO donor SNP, and 8-Br-cGMP. In vitro studies of LZ+ vs LZ− Mypt1 variants support the proposed model that PKG1α activation of MP requires interaction between the LZ motifs of PKG1α and Mypt1 [23,30,74], but these isoforms have yet to be tested in vivo through genetic modifications of mice.
Further considerations for specificity in smooth muscle responses to NO/cGMP
NO and other dilators exert their effect through inhibition of calcium flux and/or activation of MP (calcium desensitization). There is less evidence as to how regulated expression of other targets of PKG1 may determine this response. IRAG is another well-defined target of PKG1. PKG1 phosphorylation of IRAG inhibits opening of the IP3R and thereby reduces calcium flux (reviewed in [28]). A number of splice variants have been identified and carboxy-terminal truncations are proposed as deterministic of PKG1 inhibition of IP3R [69]. More broadly, there is suggestive evidence for a LZ code that provides specificity for targets of NO/PKG1 signaling. PKG1 itself has alternative N-terminal LZ motifs (α and β) generated from alternative first exons, sometimes mistakenly referred to as splice variants. Mypt1 was identified as a target of PKG1α, whereas IRAG was identified as a target of PKG1β and not vice versa.
Intriguing, and somewhat in conflict with one another, are isoform swap experiments. As noted above, mutation of the PKG1α LZ motif markedly impaired NO-mediated pial artery relaxation [43]. In contrast, either PKG1α or PKG1β can rescue PKG1 null mice [68]. However, the mice show different kinetics of NO-dependent relaxation of fundus smooth muscle dependent on rescue with either PKG1α or PKG1β [12], a result consistent with different targets. One limitation of this experiment is that transgenic (SM22α promoter driven) overexpression of each isoform was used for rescue and may not recapitulate the specificity encoded within the endogenous gene. Also consistent with specificity of PKG1 isoform signaling is the observation that cGMP relaxes jejunal smooth muscle predominately through calcium desensitization but ileal smooth muscle through inhibition of calcium flux [20], whereas the molecular basis of this specificity has not been determined. Other targets of PKG1 have been identified, and whether variation in the LZ motif is a more universal theme for generating diversity in the smooth muscle response to NO/cGMP or other signals requires further investigation. LZ motifs as part of coiled-coil domains are common mediators of protein interactions, yet there is no reliable method for computational prediction of these interactions.
Mypt1 variants in vascular function
What is the significance of Mypt1 isoforms and smooth muscle phenotypic diversity with respect to vascular function? This requires defining the patterns of gene expression and how they are modified in developmental or disease contexts. Vascular function is primarily determined at the level of the small arteries and arterioles, and it is well appreciated that these are not just smaller versions of the conduit arteries [2,5,10,36,65]. In the splanchnic circulation, the rat and mouse MA1 express predominately the fast Mypt1 E24+/LZ− isoform [53]. In general, where we and others have looked, the smaller resistance arteries, e.g., rat femoral artery and pig coronary arterioles, express the Mypt1 E24+/LZ−, as well as other fast isoforms, e.g., myosin heavy- and light-chain splice variants [9,33,40,53] (X Zhang, C Heaps, and SA Fisher, unpublished data; reviewed in [17]). As in prototypical phasic smooth muscle tissues there is a switch from slow to fast isoforms in the postnatal period ([52] and JJ Reho and SA Fisher, unpublished data).
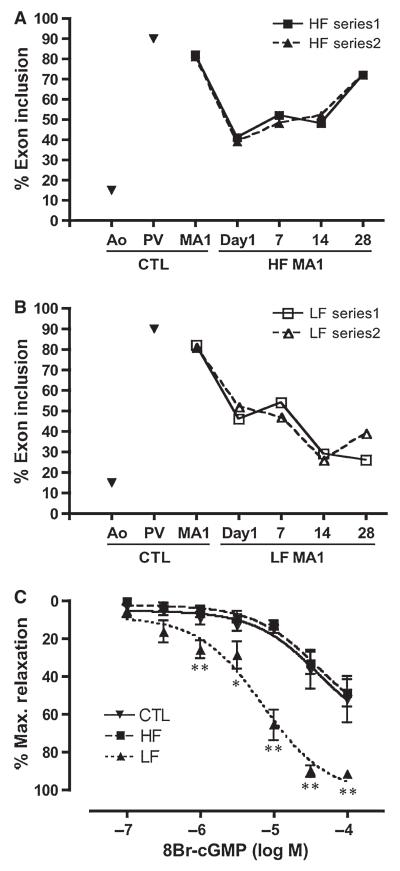
It has long been appreciated that resistance artery and conduit artery VSM exhibit distinct pathologic responses [19,46], but there has been limited study of VSM contractile phenotypic modulation in disease as the field has been focused on the study of the dedifferentiation of the conduit artery smooth muscle in atherosclerosis. We have studied this question in several disease models in which there was good evidence for altered VSM contractile function. The research groups of De Mey and Unthank separately pioneered what is termed the “HF/LF” model induced by ligation of the second-order mesenteric arteries [55,67]. De Mey’s group demonstrated altered gene expression in both the HF and LF upstream MA1s in this model, some of which fit into the paradigm of smooth muscle dedifferentiation (e.g., increased SMC proliferation and apoptosis and decreased expression of SMC markers) and some of which did not (e.g., lack of increase in expression of markers of dedifferentiation) ([70], reviewed in [7]). We implemented this model and observed dynamic changes in Mypt1 isoforms, with reversion to the Mypt1 E24−/LZ+ slow isoform in both the HF and LF MA1s [75]. The switch to the Mypt1 E24−/LZ+ isoform was transient in the HF MA1 (Figure 4A), likely due to outward remodeling of the vessel and abatement of the inciting stimulus. In contrast, the switch to the Mypt1 E24−/LZ+ isoform was sustained in the LF MA1 (Figure 4B), which has a sustained nearly no flow situation. This switch to the Mypt1 E24−/LZ+ isoform in the LF MA1s was accompanied by greater relaxation induced by 8-Br-cGMP (Figure 4C,D). Numerous other phenotypic changes were observed in this model [75,76], including reductions in Mypt1 mRNA and protein, which will not be further discussed due to space limitations.
Figure 4.
Mypt1 isoform switching in MA1s in the HF/LF model. Alternating sets of rat second-order mesenteric arteries were ligated, resulting in sustained LF in the upstream MA1s and HF in the adjacent MA1s supplying the occluded territory through the preexisting collateral arcade. Plotted is a time course of percent Mypt1 E24 inclusion in the HF and LF MA1s measured over a course of 4 weeks in two separate series of rats. Aorta and portal vein are shown as standards for the assay. HF and LF MA1s show a shift from the E24+/LZ− isoform to the E24−/LZ+ isoform within one day that is maintained for at least 14 days (A, B). The HF vessels recover by Day 28 to predominant expression of the E24+/LZ− fast isoform (A), while the LF vessels show further shifts to the E24−/LZ+ slow isoform similar to the ratio in the aorta (B). Phenylephrine activated Day 28 LF MA1s showed greater relaxation to 8-Br-cGMP than did the D28 HF or control MA1s (C) examined by wire myography ex vivo. Figures reprinted from [75].
Similar changes in Mypt1 isoforms have been observed in other disease models (Table 1) including portal hypertension induced by stenosis of the portal vein [53] and hypertension of pregnancy [40], but not in animal models of systemic hypertension ([40], and JJ Reho and SA Fisher, unpublished data). In contrast, in a rat myocardial infarct model of congestive heart failure a decrease in the slow/LZ+ Mypt1 isoform was demonstrated in the aorta and iliac arteries [33]. More recently, this group demonstrated a rapid degradation of Mypt1 protein in peripheral arteries after induction of myocardial infarction in the rat [26]. Selective degradation of Mypt1 protein has also been observed in other models [41,75].
Table 1.
Change in Mypt1 E24 splice variant ratios in animal models of vascular disease. Abbreviation definitions can be found in the abbreviations section of the text
| Model | Description | Change in Myptl isoform ratio |
Vessel studied |
Reference |
|---|---|---|---|---|
| Portal hypertension | PV stenosis | ↑ E24-out/E24-in | Portal vein, MA1s | [53] |
| HF/LF | MA2 ligation | ↑ E24-out/E24-in | MA1s | [75] |
| Pregnancy | No | Uterine artery | [40] | |
| Hypertension pregnancy | l-NAME | ↑ E24-out/E24-in | Uterine artery | [40] |
| Hypertension | l-NAME or AngII | No | Uterine artery, MAs | [40] and (JR Reho and SA Fisher, unpublished data) |
| Chronic coronary occlusion | Occlusion of LCx | No | Coronary arteries and arterioles |
X Zheng and SA Fisher, unpublished data |
The significance of the vessel-specific expression of the contractile protein isoforms, and their switching in development and disease, in relation to vascular function and the regulation of blood flow, has not been clearly established, but is predicted to alter the sensitivity to NO/cGMP-mediated vasodilation. Supportive evidence for this hypothesis includes: (1) the inverse relation between vessel size and regulation by NO/cGMP in contrast to the increasing role for EDHF in smaller arteries [22,58]; and (2) a study demonstrating reduced control by NO during the postnatal maturation of the pig mesenteric circulation [47]. Definitive testing of this hypothesis awaits genetic engineering to interchange the Mypt1 LZ+ and LZ− isoforms.
Smooth Muscle Contraction: Inhibition of MP by Contractile Agonists
Ca2+ sensitization and identification of CPI-17
The discovery of the Ca2+ sensitization pathway follows a similar storyline to that of the relaxation pathway. Seminal physiological studies demonstrated that activation of ferret portal vein smooth muscle by the contractile agonist phenylephrine generated more force at any given level of intracellular calcium, as indicated by the Ca2+-binding photoprotein aequorin, than did potassium-induced depolarization [44]. The pathway for this calcium sensitizing effect was subsequently shown to involve Rho kinase and PKC signaling; ultimately the molecular mechanism was defined by the identification and characterization of the targets, Mypt1 [35], and an inhibitory subunit named CPI-17 [14,15].
CPI-17
CPI-17 (PPP1R14 gene family) is thought to be critical for agonist-mediated inhibition of MP activity (extensively reviewed in [13]). Phosphorylation of the inhibitory subunit increases its affinity for MP, thereby markedly potentiating its inhibitory activity resulting in greater force at any given concentration of activating calcium, i.e., calcium sensitization. As is the case with the regulatory subunit, there is correlative evidence for regulated expression of CPI-17 (PPP1R14A) being deterministic in smooth muscle response to contractile agonists. The ratio of CPI-17 to Mypt1 is higher in tonic smooth muscle than phasic smooth muscle correlating with the greater calcium sensitizing effect of the agonists [72]. In experiments using chemical inhibitors, PKC-mediated phosphorylation of CPI-17 and inhibition of MP was the predominant pathway for calcium sensitization in the resistance artery, whereas ROCK-mediated phosphorylation of Mypt1 was the predominant pathway for calcium sensitization in the larger artery (aorta) [36].
A natural experiment is chicken smooth muscle, as chickens lack the gene which codes for CPI-17. Chicken VSM shows reduced responses to agonists, but still contracts and vascular function is obviously normal [37]. There is in vitro evidence to suggest that other PPP1R family members, e.g., PHI-1 (PPP1R14B), may functionally substitute for CPI-17 or play nonredundant roles in VSM cells [11,51], but this has yet to be tested in vivo. CPI-17 transgenic overexpression experiments have been performed and demonstrate hypertension, increased contractile responses to agonists ex vivo, and increased pressor responses to NE in vivo, supporting a critical role of this subunit in determining vascular function [63]. There is also evidence for developmentally regulated [6,24] and disease-modulated [29,45,49,50,56] expression of CPI-17 correlating with sensitivity to contractile agonists. In summary, regulated expression of CPI-17 and other PPP1R14 family members may be deterministic of smooth muscle responses to contractile agonists in different contexts, a hypothesis that requires experimental validation in vivo.
CONCLUSIONS AND FUTURE DIRECTIONS
In this review, we have summarized studies over the past two decades that have examined the regulated expression of MP subunits and how this may determine vascular function in developmental, disease, and regional circulation-specific contexts. There is abundant evidence that the expression of the MP regulatory and inhibitory subunits that are signaling targets is highly specific and regulated and also correlates with responses to contractile agonists and antagonists. However, there is limited evidence and some controversy as to whether changes in VSM gene expression and contractility play a role in common vascular diseases such as heart failure and hypertension. This is in stark contrast to the situation with striated muscle, where research over the past two decades has clearly established the role of contractile phenotypic modulation in disease. How can we advance this field? We note four areas for further research:
It is clear that the VSM of the microcirculation is phenotypically distinct from the large arteries. It is difficult to study but must be completely characterized in normal, developmental, and disease models to understand important vascular changes in disease. Because of the difference between vessels, using a “representative” vessel is not informative. Luckily, the advent of deep RNA sequencing should facilitate complete characterization of microcirculations and catch up with the striated muscle field in this regard.
The paradigm of dedifferentiation and proliferation is well established in pathological changes in the tonic smooth muscle of the conduit vessels (reviewed in [38]). In contrast, it is less clear how much of a role this plays in the pathologies of the phenotypically distinct smooth muscle of various microcirculations [7,66,70]. It is clear that the gene program of the micro VSM is modulated in disease; its significance with respect to cell behavior, smooth muscle contractile function, and vascular function must be rigorously investigated and cannot be inferred from studies of conduit vessels or unrelated circulations.
The disease models that are used to study VSM phenotypic modulation in the microcirculation should be sufficiently robust to induce such a process. For example, it is unlikely that modest increases in blood pressure would produce a readily detectable change. Having appropriate gene targets, knowing the likely timing of the change, and having robust assays to measure changes in gene expression in these small samples are critical in measuring such changes. It would be useful to have an established set of VSM contractile gene products that are known to reliably change in models of vascular disease, analogous to what is routinely done in the study of the heart in animal and human models of heart failure.
Lastly, there is a critical need for genetically engineered mice to test the role of VSM isoform expression in the specificity of the response to constrictor and dilator signals in different contexts. Only these proof-of-concept reverse physiology experiments can convince vascular biologists that the VSM gene program does matter. And it should, given the many therapies that target VSM contractility (Figure 1).
It is our hope and expectation that a better understanding of this field, viz, diversity in VSM contractility within tissues, disease, and individuals, will promote a more rational and targeted approach to the treatment of vascular dysfunction.
PERSPECTIVE
Vascular dysfunction is common to hypertension, heart failure, and many other diseases. A great preponderance of research has revealed the role of altered signals in controlling smooth muscle force production in these diseases while relatively little is known about how an altered contractile state of the smooth muscle may influence the response. This is surprising given the many drugs used to treat these diseases that target the contractile state of the smooth muscle. Here we reviewed the data that show that the contractile gene program and function of the microcirculatory smooth muscle are quite distinct from the large vessels and modulate in disease and development, although much remains to be learned in this regard. This provides a rationale for developing specificity in pharmacological targeting of smooth muscle contractility depending on the organ bed, disease, and other factors.
ACKNOWLEDGMENTS
This study was supported by NIH grants HL66171 to SAF and T32 AR007592 to RPD.
Abbreviations used
- 8-Br-cGMP
8-bromoguanosine 3,5′-cyclic monophosphate
- ACh
acetylcholine
- AngII
angiotensin II
- BK
Ca2+-activated K+ channel
- Ca2+
calcium
- cGMP
cyclic GMP
- CPI-17
C-kinase potentiated protein phosphatase 1 inhibitor
- EDHF
endothelial-derived hyperpolarizing factor
- EDRF
endothelial-derived relaxing factor
- eNOS
endothelial NO synthase
- ET-1
endothelin-1
- GC-A
guanylate cyclase A
- GPCR
G-protein-coupled receptor
- HF
high flow
- IP3R
inositol triphosphate receptor
- IRAG
IP3 receptor-associated gating protein
- LAD
left anterior descending coronary artery
- LCx
left circumflex artery
- LF
low flow
- L-type
L-type Ca2+ channel
- LZ
leucine zipper
- MA1
first-order mesenteric artery
- MHC
myosin heavy chain
- MLCK
myosin light-chain kinase
- MLC
myosin light chain
- MP
myosin phosphatase
- Mypt1
myosin phosphatase target subunit 1
- NCx
sodium/calcium exchanger
- NE
norepinephrine
- NO
nitric oxide
- PDE
phospho-diesterase
- PHI-1
phosphatase holoenzyme inhibitor 1
- PKC
protein kinase C
- PKG1
cGMP-dependent protein kinase 1
- PP1β
protein phosphatase 1 beta
- ROCK
Rho-associated protein kinase
- sGC
soluble guanylate cyclase
- SM22α
smooth muscle protein 22 alpha
- SNP
sodium nitroprusside
- SR
sarcoplasmic reticulum
- VSM
vascular smooth muscle
REFERENCES
- 1.Alessi D, Macdougall LK, Sola MM, Ikebe M, Cohen P. The control of protein phosphatase-1 by targeting subunits. Eur J Biochem. 1992;210:1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- 2.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 3.Ceulemans HUGO, Bollen MATH. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 4.Chen YH, Chen MX, Alessi D, Campbell DG, Shanahan C, Cohen P, Cohen PTW. Molecular cloning of cDNA encoding the 110 kDa and 21 kDa regulatory subunits of smooth muscle protein phosphatase 1. FEBS Lett. 1994;356:51–55. doi: 10.1016/0014-5793(94)01231-8. [DOI] [PubMed] [Google Scholar]
- 5.Chilian WM. Coronary mircocirculation in health and disease. Circulation. 1997;95:522–528. doi: 10.1161/01.cir.95.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dakshinamurti S, Mellow L, Stephens NL. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Pediatr Pulmonol. 2005;40:398–407. doi: 10.1002/ppul.20290. [DOI] [PubMed] [Google Scholar]
- 7.De Mey JG, Schiffers PM, Hilgers RHP, Sanders MMW. Toward functional genomics of flow-induced outward remodeling of resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1022–H1027. doi: 10.1152/ajpheart.00800.2004. [DOI] [PubMed] [Google Scholar]
- 8.Dirksen WP, Vladic F, Fisher SA. A myosin phosphatase targeting subunit isoform transition defines a smooth muscle developmental phenotypic switch. Am J Physiol Cell Physiol. 2000;278:C589–C600. doi: 10.1152/ajpcell.2000.278.3.C589. [DOI] [PubMed] [Google Scholar]
- 9.DiSanto ME, Cox RH, Wang Z, Chacko S. NH2-terminal-inserted myosin II heavy chain is expressed in smooth muscle of small muscular arteries. Am J Physiol. 1997;272:C1532–C1542. doi: 10.1152/ajpcell.1997.272.5.C1532. [DOI] [PubMed] [Google Scholar]
- 10.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 11.El-Toukhy A, Given AM, Ogut O, Brozovich FV. PHI-1 interacts with the catalytic subunit of myosin light chain phosphatase to produce a Ca2+ independent increase in MLC20 phosphorylation and force in avian smooth muscle. FEBS Lett. 2006;580:5779–5784. doi: 10.1016/j.febslet.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ertl C, Lukowski R, Sigl K, Schlossmann J, Hofmann F, Wegener JW. Kinetics of relaxation by cGMP/cGKI signaling in fundus smooth muscle. Eur J Pharmacol. 2011;670:266–271. doi: 10.1016/j.ejphar.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009;284:35273–35277. doi: 10.1074/jbc.R109.059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase c. isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- 15.Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997;410:356–360. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- 16.Feletou M, Hoeffner U, Vanhoutte PM. Endothelium-dependent relaxing factors do not affect the smooth muscle of portal vein. Blood Vessels. 1989;26:21–32. [PubMed] [Google Scholar]
- 17.Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics. 2010;42A:169–187. doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher SA, Ikebe M, Brozovich FV. Endo-thelin-1 alters the contractile phenotype of cultured embryonic smooth muscle cells. Circ Res. 1997;80:885–893. doi: 10.1161/01.res.80.6.885. [DOI] [PubMed] [Google Scholar]
- 19.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 20.Frei E, Huster M, Smital P, Schlossmann J, Hofmann F, Wegener JÃW. Calcium-dependent and calcium-independent inhibition of contraction by cGMP/cGKI in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2009;297:G834–G839. doi: 10.1152/ajpgi.00095.2009. [DOI] [PubMed] [Google Scholar]
- 21.Fu K, Mende Y, Bhetwal BP, Baker S, Perrino BA, Wirth B, Fisher SA. Tra2beta is required for tissue-specific splicing of a smooth muscle myosin phosphatase targeting subunit alternative exon. J Biol Chem. 2012;287:16575–16585. doi: 10.1074/jbc.M111.325761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garland JG, McPherson GA. Evidence that nitric oxide does not mediate the hyperpo-larization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Given AM, Ogut O, Brozovich FV. MYPT1 mutants demonstrate the importance of aa 888-928 for the interaction with PKGIalpha. Am J Physiol Cell Physiol. 2007;292:C432–C439. doi: 10.1152/ajpcell.00175.2006. [DOI] [PubMed] [Google Scholar]
- 24.Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol. 2009;297:11. doi: 10.1152/ajpheart.00681.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassie ME, Moffat LD, Walsh MP, Mac-Donald JA. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys. 2011;510:147–159. doi: 10.1016/j.abb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Han YS, Brozovich FV. Altered reactivity of tertiary mesenteric arteries following acute myocardial ischemia. J Vasc Res. 2013;50:100–108. doi: 10.1159/000343015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haystead CMM, Gailly P, Somlyo AP, Somlyo AV, Haystead TAJ. Molecular cloning and functional expression of a recombinant 72.5 kDa fragment of the 110 kDa regulatory subunit of smooth muscle protein phosphatase 1M. FEBS Lett. 1995;377:123–127. doi: 10.1016/0014-5793(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 29.Hu W, Mahavadi S, Li F, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1beta. Am J Physiol Cell Physiol. 2007;293:24. doi: 10.1152/ajpcell.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang QQ, Fisher SA, Brozovich FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem. 2004;279:597–603. doi: 10.1074/jbc.M308496200. [DOI] [PubMed] [Google Scholar]
- 31.Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 32.Johnson D, Cohen P, Chen YH, Chen MX, Cohen PTW. Identification of the regions on the M110 subunit of protein phosphatase 1M that interacts with the M21 subunit and with myosin. Eur J Biochem. 1997;244:931–939. doi: 10.1111/j.1432-1033.1997.00931.x. [DOI] [PubMed] [Google Scholar]
- 33.Karim SM, Rhee AY, Given AM, Faulx MD, Hoit BD, Brozovich FV. Vascular reactivity in heart failure: role of myosin light chain phosphatase. Circ Res. 2004;95:612–618. doi: 10.1161/01.RES.0000142736.39359.58. [DOI] [PubMed] [Google Scholar]
- 34.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem. 2001;276:37250–37257. doi: 10.1074/jbc.M105275200. [DOI] [PubMed] [Google Scholar]
- 35.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 36.Kitazawa T, Kitazawa K. Size-dependent heterogeneity of contractile Ca2+-sensitization in rat arterial smooth muscle. J Physiol. 2012;590:5401–5423. doi: 10.1113/jphysiol.2012.241315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitazawa T, Polzin AN, Eto M. CPI-17-deficient smooth muscle of chicken. J Physiol. 2004;557:515–528. doi: 10.1113/jphysiol.2004.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 39.Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Zhang H, Gokina N, Mandala M, Sato O, Ikebe M, Osol G, Fisher SA. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat L-NAME model of hypertension of pregnancy. Am J Physiol Cell Physiol. 2008;294:C564–C571. doi: 10.1152/ajpcell.00285.2007. [DOI] [PubMed] [Google Scholar]
- 41.Ma H, He Q, Dou D, Zheng X, Ying L, Wu Y, Raj JU, Gao Y. Increased degradation of MYPT1 contributes to the development of tolerance to nitric oxide in porcine pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2010;299:L117–L123. doi: 10.1152/ajplung.00340.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh N, Marsh A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharacol Physiol. 2000;27:313–319. doi: 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- 43.Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci U S A. 2008;105:6702–6707. doi: 10.1073/pnas.0802128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan JP, Morgan KG. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueed I, Zhang L, MacLeod KM. Role of the PKC/CPI-17 pathway in enhanced contractile responses of mesenteric arteries from diabetic rats to alpha-adrenoceptor stimulation. Br J Pharmacol. 2005;146:972–982. doi: 10.1038/sj.bjp.0706398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normo-tensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 47.Nankervis CA, Nowicki PT. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol. 1995;268:G949–G958. doi: 10.1152/ajpgi.1995.268.6.G949. [DOI] [PubMed] [Google Scholar]
- 48.Ogut O, Brozovich FV. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am J Physiol Cell Physiol. 2000;279:C1722–C1732. doi: 10.1152/ajpcell.2000.279.6.C1722. [DOI] [PubMed] [Google Scholar]
- 49.Ohama T, Hori M, Momotani E, Iwakura Y, Guo F, Kishi H, Kobayashi S, Ozaki H. Intestinal inflammation downregulates smooth muscle CPI-17 through induction of TNF-alpha and causes motility disorders. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1429–G1438. doi: 10.1152/ajpgi.00315.2006. [DOI] [PubMed] [Google Scholar]
- 50.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- 51.Pang H, Guo Z, Xie Z, Su W, Gong MC. Divergent kinase signaling mediates agonist-induced phosphorylation of phosphatase inhibitory proteins PHI-1 and CPI-17 in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C892–C899. doi: 10.1152/ajpcell.00378.2005. [DOI] [PubMed] [Google Scholar]
- 52.Payne MC, Zhang HY, Prosdocimo T, Joyce KM, Koga Y, Ikebe M, Fisher SA. Myosin phosphatase isoform switching in vascular smooth muscle development. J Mol Cell Cardiol. 2006;40:274–282. doi: 10.1016/j.yjmcc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Payne MC, Zhang HY, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phospha-tase in a model of portal hypertension. Am J Physiol Heart Circ Physiol. 2004;286:H1801–H1810. doi: 10.1152/ajpheart.00696.2003. [DOI] [PubMed] [Google Scholar]
- 54.Pfitzer G, Merkel L, Ruegg JC, Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflugers Arch. 1986;407:87–91. doi: 10.1007/BF00580726. [DOI] [PubMed] [Google Scholar]
- 55.Pourageaud F, De Mey JG. Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. Am J Physiol Heart Circ Physiol. 1997;273:H1699–H1706. doi: 10.1152/ajpheart.1997.273.4.H1699. [DOI] [PubMed] [Google Scholar]
- 56.Sato K, Ohkura S, Kitahara Y, Ohama T, Hori M, Sato M, Kobayashi S, Sasaki Y, Hayashi T, Nasu T, Ozaki H. Involvement of CPI-17 downregulation in the dysmotility of the colon from dextran sodium sulphate-induced experimental colitis in a mouse model. J Neurogastroenterol Motil. 2007;19:504–514. doi: 10.1111/j.1365-2982.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J Biol Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- 58.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 59.Shirazi A, Iizuka K, Fadden P, Mosse C, Somlyo AP, Somlyo AV, Haystead TA. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J Biol Chem. 1994;269:31598–31606. [PubMed] [Google Scholar]
- 60.Shukla S, Fisher SA. Tra2beta as a novel mediator of vascular smooth muscle diversification. Circ Res. 2008;103:485–492. doi: 10.1161/CIRCRESAHA.108.178384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968;159:129–145. [PubMed] [Google Scholar]
- 62.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 63.Su W, Xie Z, Shu L, Calderon LE, Guo Z, Gong MC. Smooth muscle-selective CPI-17 expression increases vascular smooth muscle contraction and blood pressure. Am J Physiol Heart Circ Physiol. 2013;305:H104–H113. doi: 10.1152/ajpheart.00597.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 65.Tanko LB, Matrougui K. Can we apply results from large to small arteries? Circ Res. 2002;90:e68. doi: 10.1161/01.res.0000013737.02527.15. [DOI] [PubMed] [Google Scholar]
- 66.Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281:H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 67.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am J Physiol. 1996;271:H914–H923. doi: 10.1152/ajpheart.1996.271.3.H914. [DOI] [PubMed] [Google Scholar]
- 68.Weber S, Bernhard D, Lukowski R, Wein-meister P, Worner R, Wegener JW, Valtcheva N, Feil S, Schlossmann J, Hofmann F, Feil R. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res. 2007;101:1096–1103. doi: 10.1161/CIRCRESAHA.107.154351. [DOI] [PubMed] [Google Scholar]
- 69.Werder A, Mayr M, Schneider G, Oesterle D, Fritsch RM, Seidler B, Schlossmann J, Hofmann F, Schemann M, Allescher HD, Schmid RM, Saur D. Truncated IRAG variants modulate cGMP-mediated inhibition of human colonic smooth muscle cell contraction. Am J Physiol Cell Physiol. 2011;301:C1445–C1457. doi: 10.1152/ajpcell.00304.2010. [DOI] [PubMed] [Google Scholar]
- 70.Wesselman JP, Kuijs R, Hermans JJ, Janssen GM, Fazzi GE, van Essen H, Evelo CT, Struijker-Boudier HA, De Mey JG. Role of the Rhoa/Rho kinase system in flow-related remodeling of rat mesenteric small arteries in vivo. J Vasc Res. 2004;41:277–290. doi: 10.1159/000078826. [DOI] [PubMed] [Google Scholar]
- 71.Wissmann A, Ingles J, Mains PE. The Caenorhabditis elegans mel-11 myosin phosphatase regulatory subunit affects tissue contraction in the somatic gonad and the embryonic epidermis and genetically interacts with the Rac signaling pathway. Dev Biol. 1999;209:111–127. doi: 10.1006/dbio.1999.9242. [DOI] [PubMed] [Google Scholar]
- 72.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca (2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu X, Somlyo AV, Somlyo AP. c-GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochem Biophys Res Commun. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]
- 74.Yuen S, Ogut O, Brozovich FV. MYPT1 protein isoforms are differentially phosphorylated by protein kinase G. J Biol Chem. 2011;286:37274–37279. doi: 10.1074/jbc.M111.282905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res. 2007;100:730–737. doi: 10.1161/01.RES.0000260189.38975.35. [DOI] [PubMed] [Google Scholar]
- 76.Zhang H, Pakeerappa P, Lee HJ, Fisher SA. Induction of PDE5 and de-sensitization to endogenous NO signaling in a systemic resistance artery under altered blood flow. J Mol Cell Cardiol. 2009;47:57–65. doi: 10.1016/j.yjmcc.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]