Abstract
Depression is a common comorbidity among HIV-infected individuals. We studied the relationship between depressive symptoms, risk behaviors (risky-sexual behavior, tobacco, alcohol and illicit drug use) and HIV outcomes. This cross-sectional study conducted in 2009 at Washington University HIV Clinic included screening for depression with patient health questionnaire (PHQ-9), survey of sexual behavior, illicit drug, alcohol and tobacco use within 30 days. Sociodemographics, plasma HIV RNA levels, CD4 cell counts and sexually transmitted disease (STD) test results were obtained from medical records. Multivariate logistic and linear regression models were used to assess the association between depressive symptoms severity and risk behaviors, HIV outcomes and combination antiretroviral therapy (cART) adherence. A total of 624 persons completed the assessment, of whom 432 (69%) were male and 426 (68%) African American. The median CD4 cell count was 410 cells/mm3 and 479 (77%) were on cART of whom 112 (23%) had HIV RNA level > 400 copies/mL. Overall, 96 (15%) had symptoms of major depressive disorder. Depressive symptom severity was associated with increased likelihood of high-risk drinking (Odds Ratio (OR), 2.4; 95% Confidence Interval (CI), 1.1–5.1), current tobacco use (OR, 1.8; 95%CI, 1.1–2.9), illicit drug use (OR, 1.7; 95%CI, 1.0–2.8) and risky-sexual behavior (OR, 1.5; 95%CI, 0.8–2.7). Sub-optimal cART adherence (visual analogue scale < 95%) was also associated with depressive symptoms severity (p< 0.05). After adjustment for age, sex, race, receipt of cART and cART adherence, depressive symptoms severity was independently associated with lower CD4 cell count (p< 0.05) but not with higher HIV RNA level (p= 0.39). Depression adversely affects HIV-infected individuals, requiring greater effort at utilizing multidisciplinary interventions.
Keywords: HIV, Depression, Risk Behaviors, Antiretroviral therapy, Adherence
Introduction
Depression is a major comorbidity among the HIV-infected population (Benton, 2008) with prevalence reaching as high as 48% depending on study (Rabkin, 2008). A national study of the prevalence of psychopathology in an HIV-infected population assessed 2864 individuals and estimated the prevalence of major depression to be 22% (Orlando et al., 2002).
Depressive symptoms have been associated with poor HIV-related outcomes, such as rapid progression of HIV disease, higher mortality rates, poor adherence to cART, and higher HIV viral loads (Evans et al., 2002; Horberg et al., 2008; Ickovics et al., 2001; E Shacham, Nurutdinova, Satyanarayana, Stamm, & Overton, 2009; Vinet-Oliphant et al., 2010). Depression has also been associated with high-risk behaviors: alcohol use in both HIV-infected and HIV-negative individuals (Sullivan, Goulet, Justice, & Fiellin, 2011) and illicit drug use (Berger-Greenstein et al., 2007; Bing et al., 2001). Data on tobacco use and depression in HIV-infected individuals are limited (Reisen et al., 2011). Depressive symptoms have been associated with diminished libido (K. Williams & Reynolds, 2006), but the few available studies examining the relationship between risky-sexual behaviors and depression in HIV-infected individuals have provided inconsistent results (Bradley, Remien, & Dolezal, 2008; Enbal Shacham, Basta, & Reece, 2009; C. T. Williams & Latkin, 2005). The majority of these studies assessed associations between high-risk behaviors and major depression, but none assessed whether the severity of depressive symptoms correlates with an increased probability of engaging in high-risk behaviors among individuals with HIV infection.
The primary objectives of this study were to characterize major depressive symptoms in a cohort of people living with HIV/AIDS who are engaged in care and to assess the relationship between depressive symptoms severity and the probability of engaging in high-risk behaviors. We hypothesized that increased severity of depressive symptoms was associated with increased probability of engaging in high-risk behaviors. The secondary objective was to assess the relationship between severity of depressive symptoms and HIV-related outcomes. Our hypothesis was that symptoms of depression were associated with lower CD4 cell counts and a higher HIV RNA level.
Measures and Methods
Study population
This cross-sectional study using behavioral assessments was conducted as part of standard patient care among individuals aged ≥ 18 years presenting for care at Washington University HIV outpatient clinic between June and September 2009. Data including CD4 cell count (current and nadir), plasma HIV RNA level and receipt of cART were collected from medical records. Sexually transmitted diseases (STD) test results (urinary nucleic acid amplification testing for Chlamydia trachomatis and Neisseria gonorrhoeae, and rapid plasma reagin for syphilis) within the previous 12 months from the most recent visit were collected. The study was approved by Washington University Human Research Protection Office.
Behavioral assessment
Behavioral assessments are conducted annually as part of standard patient care. Since most patients visit clinic every three months, we conducted our assessment in a three-month period in attempt to cover all the patients. Trained medical assistants performed interviews to each patient in the examining room prior to the physician encounter, and the collected information were stored directly to the medical records. Data regarding sociodemographics, depressive symptoms and behavioral risk factors (unprotected sex and use of alcohol, illicit drugs and tobacco) were collected from these behavioral assessments.
Assessment of depression
Depressive symptoms were assessed by the Patient Health Questionnaire (PHQ-9), which focuses on nine diagnostic criteria for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) depressive disorders. The PHQ-9 has been validated for the diagnosis of major depressive disorder (MDD), other depressive disorder (ODD) and any depressive disorder (MDD or ODD) in diverse populations, including individuals living with HIV infection (Monahan et al., 2009). We defined MDD as the presence of ≥ 5 of 9 depressive symptoms at least “more than half the days” in the past 2 weeks, and 1 symptom of depressed mood or anhedonia (Kroenke, Spitzer, & Williams, 2001). The PHQ-9 scores were categorized as no depression (0), minimal depression (1 to 4), mild depression (5 to 9), moderate depression (10 to 14), moderately severe depression (15 to 19) and severe depression (20 to 27) (Kroenke et al., 2001).
Assessment of high-risk behaviors
Risky-sexual behavior was defined as unprotected sex (no condom use at last sexual encounter and reporting never using condoms in the last 3 months) or positive STD test within 12 months. Illicit drug use was defined as use of marijuana, cocaine, methamphetamines, heroin, any opioid, inhalants or any other illicit drugs within 30-days. High-risk drinking was defined as ≥ 14 drinks per week for men and ≥7 drinks for women as per the National Institute on Alcohol Abuse and Alcoholism (NIAAA) definition of “at-risk” or “heavy” drinking (Dawson, 2011). Tobacco use within 30 days defined current use.
Adherence to cART was assessed using the visual analogue scale (VAS), which requires participants to recall their adherence for the previous 30 days and estimate the percentage of medication doses (0% to 100%) that they had taken as prescribed (Giordano, Guzman, Clark, Charlebois, & Bangsberg, 2004; Kalichman et al., 2009). The VAS results were dichotomized to optimal adherence (≥ 95%) and sub-optimal adherence (< 95%) (Paterson et al., 2000; Reynolds et al., 2007).
Statistical analysis
Descriptive analysis and bivariate analysis were conducted to assess the difference in characteristics between those with and without MDD symptoms. Chi-square analyses were used for dichotomous variables and Mann-Whitney U test were used for non-parametric continuous variables. To assess the relationship between depressive symptoms severity and the probability of engaging in each risk behavior, multivariate logistic regression models were conducted. To assess the relationship between HIV-related outcomes (CD4 cell count and HIV RNA level) and severity of depressive symptoms, hierarchical linear regression models were used. All statistical analyses were performed using R 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria), and posterior simulation of beta from linear regression models were performed using the sim function in the package arm (Gelman & Hill, 2006, pp. 44–45) to demonstrate the uncertainty of the fitted regression in the figure. Statistical significance was defined as p< 0.05.
Results
Association between MDD symptoms and high-risk behaviors
Of 624 individuals included, 432 (69%) were male, 426 (68%) African American and 96 (15%) reported MDD symptoms. Table 1 details further characteristics. There were 479 (77%) individuals on cART, which 49% were on protease inhibitor (PI)-based and 43% on non-nucleoside reverse transcriptase (NNRTI)-based regimen. Individuals with MDD symptoms were more often African American (81% vs. 66%, p< 0.05), less likely to be receiving cART (66% vs. 79%, p< 0.01), more likely to report illicit drug use (35% vs. 26%, p< 0.05) and be current smokers (66% vs. 44%, p< 0.001). Among those receiving cART, sub-optimal adherence (VAS< 95%) was higher among individuals with MDD symptoms (38% vs. 24%, p< 0.01). Consequently, there was more virologic failure among individuals on cART with MDD symptoms than those with mild or no depressive symptoms (30% vs. 22%, p< 0.05). There were no significant differences in the proportion of individuals with MDD symptoms among those on either PI-based or NNRTI-based regimen. While risky-sexual behavior was common, neither risky-sexual behavior nor high-risk drinking was associated with MDD symptoms (p= 0.32 and 0.27, respectively). Among those with MDD symptoms, there were a higher proportion of individuals with low CD4 cell count of < 200 cells/mm3 (29% vs. 19%, p< 0.05) and HIV RNA level of > 400 copies/mL (55% vs. 40%, p< 0.05), although these include those off cART. The length of time from HIV diagnosis was not associated with MDD symptoms (p= 0.89).
Table 1.
Basic Characteristics of the Study Patients
| Characteristics | All patients (n =624) |
MDD (n =96) |
No MDD (n =528) |
p value |
|---|---|---|---|---|
| Age, years (IQR) | 43 (32–49) | 41 (30–48) | 43 (33–50) | 0.12 |
| Male Sex | 432 (69%) | 65 (68%) | 367 (70%) | 0.73 |
| Race White | 169 (27%) | 15 (16%) | 154 (29%) | < 0.05 |
| African American | 426 (68%) | 78 (81%) | 348 (66%) | |
| Other | 29 (5%) | 3 (3%) | 26 (5%) | |
| Years from HIV diagnosis (IQR) | 8.0 (3.1–13.0) | 8.0 (3.2–13.0) | 7.8 (2.8–14.0) | 0.89 |
| CD4 cell count * | 117 (19%) | 25 (29%) | 92 (19%) | < 0.05 |
| < 200 cells/mm3 | ||||
| HIV RNA Level * | 240 (38%) | 47 (55%) | 193 (40%) | < 0.05 |
| > 400 copies/mL | ||||
| Risky Sexual Behavior | 117 (19%) | 21 (22%) | 96 (18%) | 0.39 |
| Illicit Drug Use | 169 (27%) | 34 (35%) | 135 (26%) | < 0.05 |
| High-risk Drinking | 55 (9%) | 13 (22%) | 56 (16%) | 0.27 |
| Tobacco Use | 239 (47%) | 63 (66%) | 230 (44%) | < 0.001 |
| Currently on cART | 479 (77%) | 63 (66%) | 416 (79%) | < 0.01 |
| VAS < 95% | 123/479 (26%) | 24/63 (38%) | 99/416 (24%) | < 0.01 |
| PI-based cART | 237/479 (49%) | 36/63 (57%) | 201/416 (48%) | 0.19 |
| NNRTI-based cART | 208/479 (43%) | 25/63 (40%) | 183/416 (44%) | 0.52 |
| HIV RNA Level > 400 copies/mL on cART | 112/479 (23%) | 19/63 (30%) | 93/416 (22%) | < 0.05 |
MDD, major depressive disorder; IQR, interquartile range, cART, combination antiretroviral therapy; VAS, visual analogue scale; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor.
Numbers and percentages include patients not on cART
Association between depression severity and high-risk behaviors
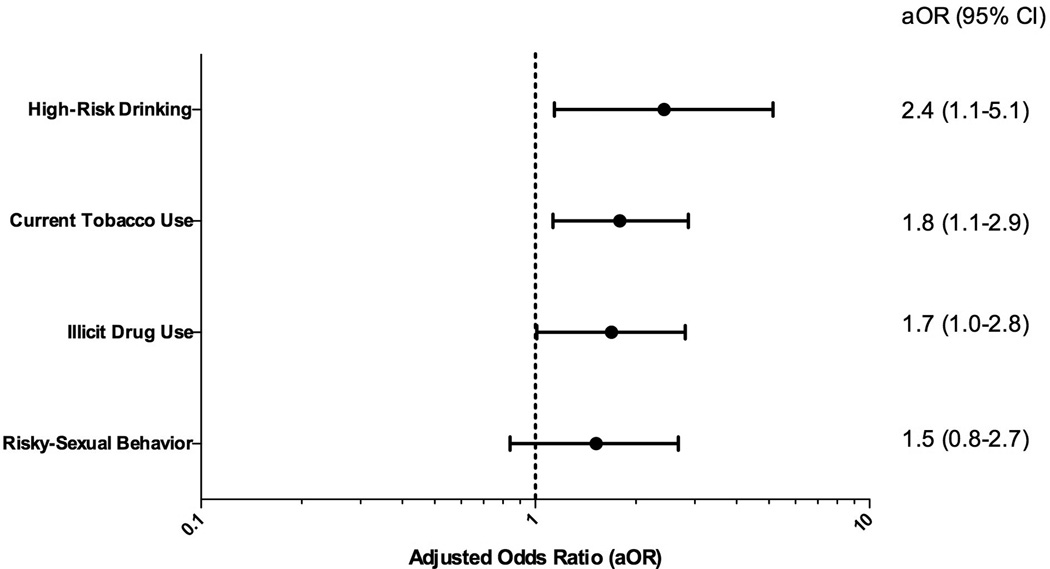
Figure 1 demonstrates the fitted logistic regression models estimating the probability of engagement to each risk behaviors by PHQ-9 score. These models were adjusted by age, gender, race, CD4 cell count, HIV RNA level, receipt of cART and cART adherence. As the severity of depressive symptoms increased (higher PHQ-9 score), the probability of engaging in each risk behaviors increased. Although risky-sexual behavior did not reach statistical significance (p= 0.33), there was a tendency (positive slope) for increased probability as severity of depression increased. Figure 2 illustrates the adjusted odds ratio (aOR) of individuals with PHQ-9 score ≥ 10 (moderate to severe depression) engaging in each risk behaviors. There was 2.4-fold (95% Confidence Interval (CI), 1.1–5.1) increased risk of high-risk drinking, 1.8-fold (95%CI, 1.1–2.9) increased risk of current tobacco use, 1.7-fold (95%CI, 1.0–2.8) increased risk of illicit drug use and 1.5-fold (95%CI, 0.8–2.7) increased risk of engaging in risky-sexual behavior in those with PHQ-9 scores ≥10 (all p< 0.05).
Figure 1.
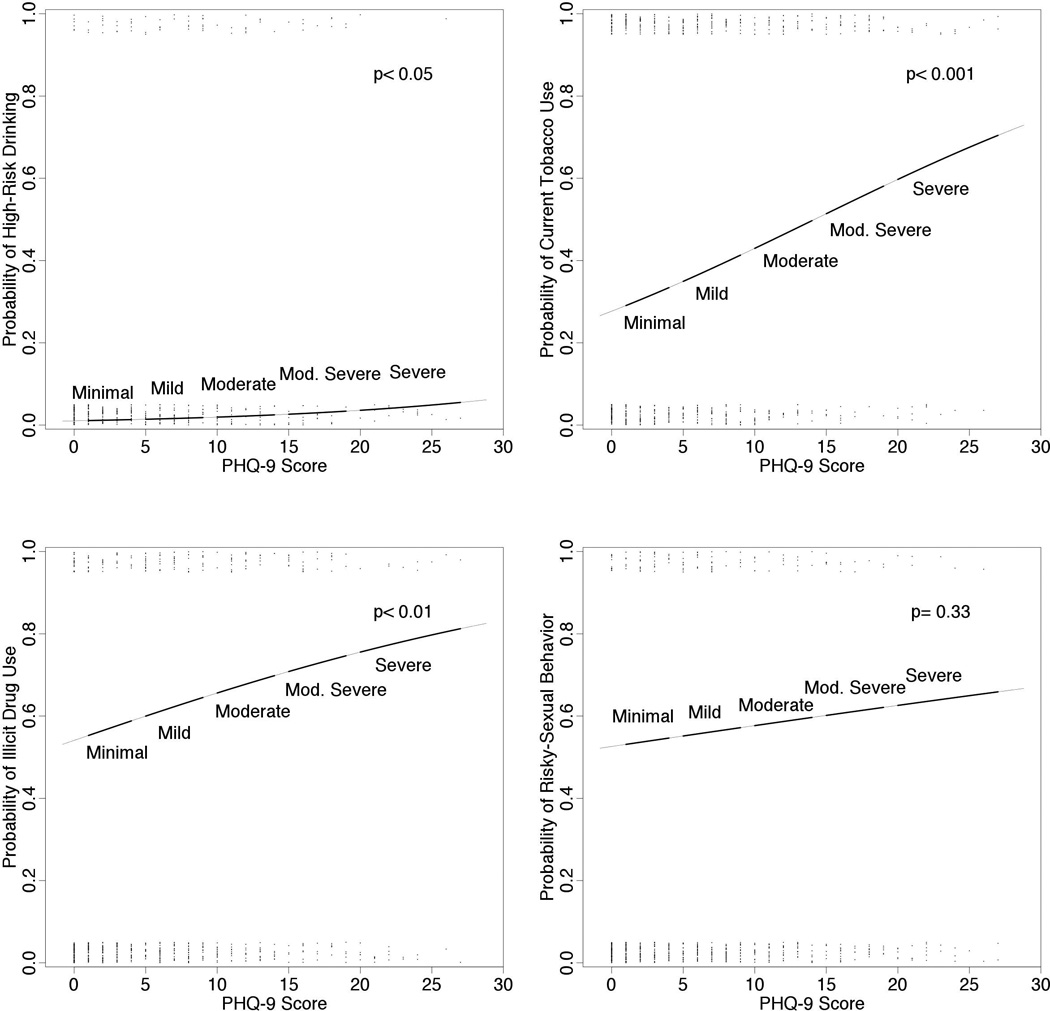
Probability of each risk behaviors estimated by PHQ-9 scores. Fitted logistic regression estimating the probability of engaging in each risk behaviors by PHQ-9 scores. Jittered dots represent the PHQ-9 score data. These multivariate models are adjusted by age, sex, race, current CD4 count, Log HIV viral load, receipt of cART and cART adherence.
Figure 2.
Adjusted odds ratios for depression (PHQ-9 ≥ 10) predicting each risk behavior. Adjusted by age, sex, race, HIV RNA level, current CD4 cell count, receipt of cART and cART adherence. 95% CI, reference for the odds ratios is no depression (PHQ-9 score of 0).
Association between cART adherence, depression and high-risk behaviors
In the univariate analyses, PI-based cART (Odds Ratio (OR), 1.9; 95%CI, 1.2–3.0), age ≤ 40 years (OR, 1.8; 95%CI, 1.2–2.8), presence of MDD (OR, 2.1; 95%CI, 1.2–3.6), African American race (OR, 3.3; 95%CI, 1.9–5.7), high-risk drinking (OR, 2.2; 95%CI, 1.1–4.2), current tobacco use (OR, 1.9; 95%CI, 1.3–2.8) and illicit drug use (OR, 1.7; 95%CI, 1.1–2.6) were associated with increased risk of sub-optimal adherence to cART (Figure S1). Multivariate logistic regression models were built to estimate sub-optimal adherence to cART by the PHQ-9 score (Figure 3). VAS (cART adherence) was significantly associated with depression severity (p< 0.05) when adjusted by age, sex and high-risk behaviors. Age ≤ 40 years (aOR, 1.7; 95%CI, 1.1–2.7), African American race (aOR, 2.6; 95%CI, 1.4–4.9), use of PI-based cART (aOR, 1.6; 95%CI, 1.0–2.6) and current tobacco use (aOR, 1.8; 95%CI, 1.1–2.8) were also significantly associated with depression severity.
Figure 3.
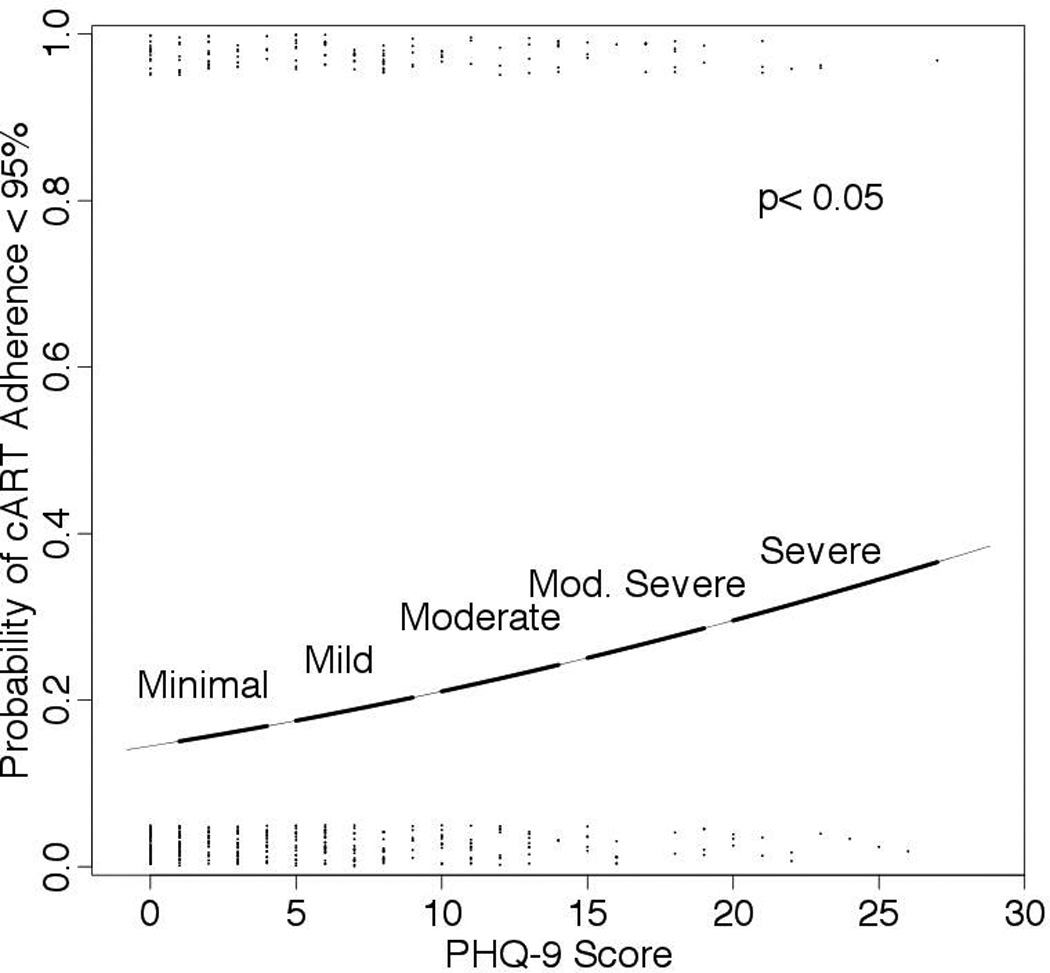
Probability of cART adherence < 95% in the multivariate model. Fitted logistic regression estimating the probability of cART adherence < 95%. Jittered dots represent PHQ-9 score data. This multivariate model includes age, race, tobacco use and receipt of PI-based vs. NNRTI-based cART.
Effect of severity depressive symptoms on HIV-related outcomes
Hierarchical linear regression models predicting CD4 cell count (both current and nadir) and HIV RNA level from the PHQ-9 score were built, adjusted by age, gender, race, receipt of cART and VAS. Table 2 summarizes both unstandardized and standardized coefficients of the two models; step 1 as an unadjusted and step 2 as an adjusted model. In the unadjusted models, both HIV RNA level and current CD4 cell count were associated with severity of depressive symptoms; a higher PHQ-9 score was associated with higher HIV RNA level and lower CD4 cell count (standardized beta = 0.18, −0.16, respectively, both p< 0.001). We did not detect an association between the nadir CD4 cell count and severity of depressive symptoms (p= 0.08) (Table S1 and Figure S2). In the adjusted models, we failed to identify an independent association between HIV RNA level and PHQ-9 score, but current CD4 cell count was independently associated with the PHQ-9 score (standardized beta = −0.11, p < 0.05). Significant factors associated with lower HIV RNA level were receipt of cART (standardized beta = −0.42) and adherence to cART (standardized beta = −0.18). African American race (standardized beta = 0.13) was associated with higher HIV RNA level. Significant factors predicting CD4 cell count were male sex (standardized beta = −0.14), African American race (standardized beta = −0.13), receipt of cART (standardized beta = 0.15) and adherence to cART (standardized beta = 0.12). Figure 4 shows the plot and regression line of these models. HIV RNA level was associated with PHQ-9 score with a positive slope in the unadjusted model in the left indicating that HIV RNA level increased with higher PHQ-9 score, but in the adjusted model on the right the slope decreased, indicating no independent association. The current CD4 cell count was associated with PHQ-9 score in both unadjusted (left) and adjusted (right) models with negative slopes, indicating the decline of CD4 cell count was independently associated with an increase in PHQ-9 score.
Table 2.
Hierarchical Linear Regression Model Predicting Slope of Log HIV RNA Level and CD4 Cell Count Change
| Log HIV RNA Level Changea | |||||
|---|---|---|---|---|---|
| Step | Clinical Features |
B | SE B | beta | P value |
| 1 | Intercept | 2.30 | 0.08 | ||
| PHQ-9 score | 0.04 | 0.01 | 0.18 | < 0.001 | |
| 2 | Intercept | 4.63 | 0.28 | ||
| PHQ-9 score | 0.005 | 0.006 | 0.04 | 0.39 | |
| Age | −0.004 | 0.003 | −0.05 | 0.22 | |
| Male sex | −0.13 | 0.09 | −0.06 | 0.14 | |
| African American | 0.25 | 0.08 | 0.13 | < 0.01 | |
| cART | −2.29 | 0.23 | −0.42 | < 0.001 | |
| VAS ≥ 95% | −0.35 | 0.09 | −0.18 | < 0.001 | |
| Current CD4 Cell Count Changeb | |||||
| 1 | Intercept | 493.46 | 17.34 | ||
| PHQ-9 score | −7.42 | 1.92 | −0.16 | < 0.001 | |
| 2 | Intercept | 273.28 | 105.18 | ||
| PHQ-9 score | −5.21 | 2.33 | −0.11 | < 0.05 | |
| Age | 0.40 | 1.28 | 0.01 | 0.75 | |
| Male sex | −89.12 | 31.17 | −0.14 | < 0.01 | |
| African American | −81.26 | 30.93 | −0.13 | < 0.01 | |
| cART | 272.19 | 85.36 | 0.15 | < 0.01 | |
| VAS ≥ 95% | 81.12 | 32.17 | 0.12 | < 0.05 | |
Log HIV RNA Level, log10 HIV RNA Level; B, unstandardized coefficient; SE, standard error; beta, standardized coefficient; VAS, visual analogue scale for cART adherence.
For final model, F6,423 = 28.08, P < 0.001, R2 = 0.27
For final model, F6,427 = 7.53, P < 0.001, R2 = 0.083
Figure 4.
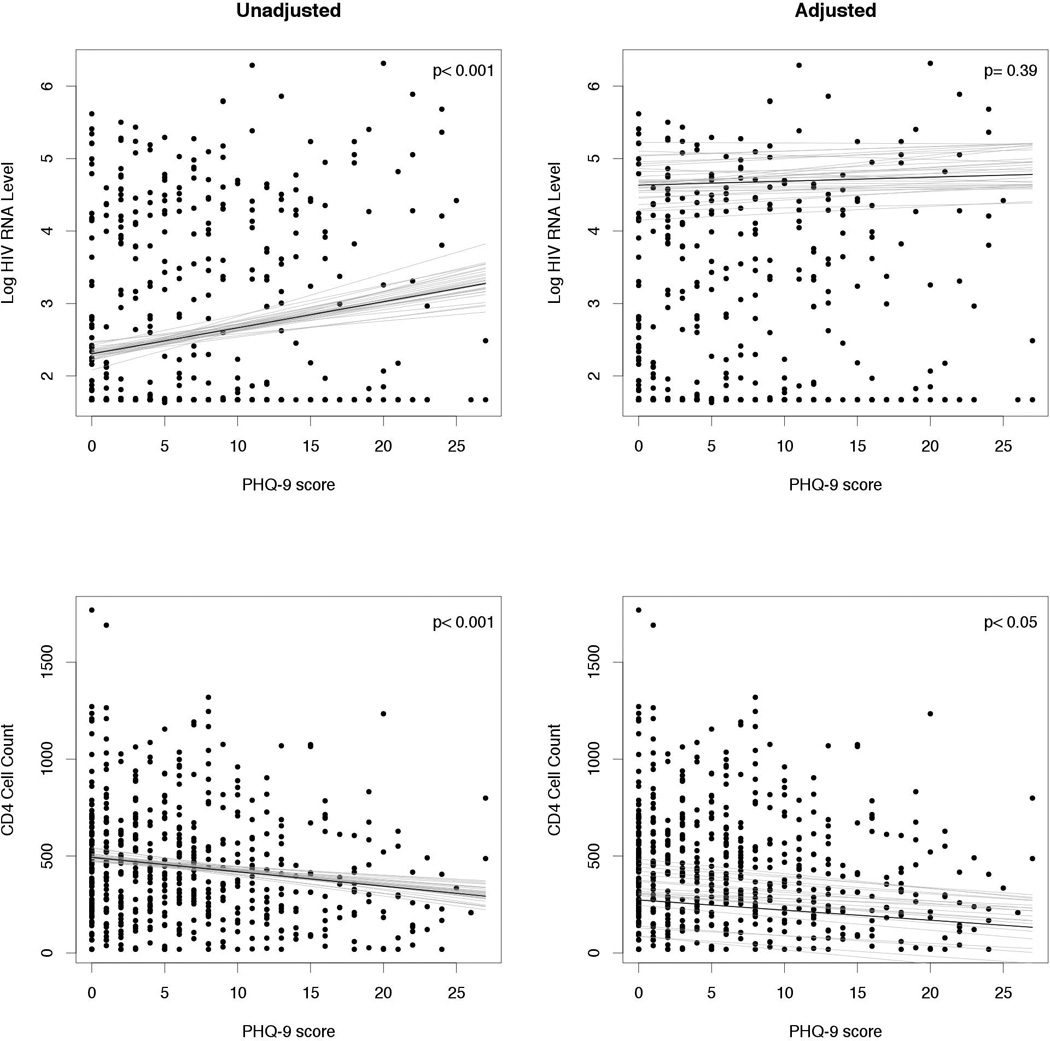
Linear regression models demonstrating the association between depression severity and HIV RNA level (top) or CD4 cell count (bottom) in unadjusted (left) or adjusted (right) analyses. These multivariate models are adjusted by age, sex, race, current CD4 count, Log HIV viral load, receipt of cART and cART adherence. Solid line shows the fitted regression model and light lines indicating uncertainty in the fitted regression with posterior simulations.
Discussion
Our data demonstrate that more than 15% of our clinic population had symptoms consistent with major depression, which is comparable to other studies (Bing et al., 2001; Ciesla & Roberts, 2001; Morrison et al., 2002). This clinic-based study demonstrated that increases in severity of depressive symptoms were independently associated with a linear decline in current CD4 cell count, confirming that patients with advanced HIV disease are at greater risk of being affected by depressive symptoms. Compared to previous studies (Ickovics et al., 2001), our study included not only receipt of cART but also adherence to cART. We have showed that individuals with severe depressive symptoms had sub-optimal cART adherence. Another significant finding was that the increase in severity of depressive symptoms was associated with higher probability of engaging in high-risk drinking, tobacco, illicit drug use and risky-sexual behavior. Although association between severe depressive symptoms and risky-sexual behaviors did not reach statistical significance, it did show a clear tendency. This may be due to the small number of individual included to this study, therefore underpowered. The study also illustrates that depression screening as standard of care identifies at-risk patients and increases intervention opportunities for clinic providers, such as counseling and pharmacotherapy. Additionally, those with depression should be screened for engagement in high-risk behaviors, particularly given the independent association between these parameters. There was a concern that patients under care for longer periods had a higher probability of having treated for comorbid conditions, such as depression and other high-risk behaviors, and analyzing these patients with others who engaged in HIV care recently may bias the results. We hypothesized that the years from diagnosis served to control for better HIV management, but univariate analyses did not show any differences in the proportion of individuals with MDD symptoms and adjustment of multivariate model did not differ from the results shown. Therefore, length of time with HIV did not contribute to our model, highlighting the importance of periodic screening of depression and high-risk behaviors during routine patient care.
We have previously described the association between virologic suppression and depressive disorders in individuals with HIV infection receiving cART, and showed a 2.5-fold increased risk of detectable viral load (HIV RNA level of > 400 copies/mL) in depressed patients (E Shacham et al., 2009). In this current study, the association between depressive symptoms and uncontrolled virema was confirmed although it was mediated through inadequate adherence to cART. We then hypothesized that high levels of viremia may be contributing to worse depressive symptoms, but failed to identify independent linear association between PHQ-9 score and HIV RNA level. This finding is important since increase in HIV viral load (high level of viremia) itself did not have association with increase in depression severity, as shown in previous studies (Evans et al., 2002). These data suggest that depression may not be caused by the HIV infection itself, and that effective control of depressive symptoms can have potential benefit for management of HIV disease.
We also evaluated risk factors for sub-optimal adherence to cART. Given that the current adult treatment guidelines (Thompson MA, 2012) recommend cART for all HIV-infected individuals, delineating the risk factors for non-adherence will provide insight for the development of effective interventions. As predicted, we demonstrated that depressive symptoms indeed impact cART adherence. This relationship is particularly relevant given the evolution of HIV infection into a chronic disease state for which long-term adherence will prove critical to success.
There are limitations to this study. This was a single site study, and some of the statistical analyses were underpowered by the number of individuals included as previously mentioned. This was a cross-sectional study; therefore we are unsure of the directionality of the relationships identified. While we covered a large proportion of our clinic patients, there were patients who did not show to the clinic appointment and potentially missed three-month period that we conducted the behavioral assessment. This may cause bias, since the reason for not showing up to the clinic may be due to psychiatric illnesses, behavioral problems or other comorbidities. The measurement of adherence to cART using the VAS is limited in scope, as some studies have failed to demonstrate an association between the VAS and objective measures of adherence (Gill et al., 2010; Pearson, Simoni, Hoff, Kurth, & Martin, 2007). The definition of each high-risk behavior may lack adequate sensitivity or specificity. For example, the definition of high-risk drinking may underestimate the individuals at risk for alcohol dependence in the HIV-infected population (Enbal Shacham, Agbebi, Stamm, & Overton, 2011). The length of time with HIV does not necessarily represent the length of care. We are assuming that majority of the HIV diagnosed individuals enter to care right away, which is likely to be an inappropriate assumption. The behavioral assessment was conducted face-to-face, which may cause reporting bias, as people tend to under-report their engagement in risk behaviors when assessment is performed in such manner (Newman et al., 2002; Schackman et al., 2008). The next step will be to implement a computer-assisted self interview (CASI), which reduces reporting bias and allows prompt provider feedback of the behavioral assessment, and to obtain longitudinal data to study the causal directions (Johnson et al., 2001).
In conclusion, the results highlight associations between severity of depressive symptoms and engagement in high-risk behaviors and markers of advanced HIV disease. Our results support the implementation of routine screening for depression and high-risk behaviors in HIV clinics and multidisciplinary interventions to improve outcomes among depressed HIV-infected individuals.
Supplementary Material
Acknowledgement
We would like to thank the patients and the staff at Washington University HIV clinic. TT was supported by the Bristol-Myers Squibb Virology Fellowship Program. ES was supported by Grant Number UL1 RR024992 from the NIH-National Center for Research Resources (NCRR), specifically KL2 RR024994. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH
References
- Benton TD. Depression and HIV/AIDS. Current Psychiatry Reports. 2008;10(3):280–285. doi: 10.1007/s11920-008-0045-y. [DOI] [PubMed] [Google Scholar]
- Berger-Greenstein JA, Cuevas CA, Brady SM, Trezza G, Richardson MA, Keane TM. Major depression in patients with HIV/AIDS and substance abuse. AIDS Patient Care and STDs. 2007;21(12):942–955. doi: 10.1089/apc.2006.0153. [DOI] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Bradley MV, Remien RH, Dolezal C. Depression symptoms and sexual HIV risk behavior among serodiscordant couples. Psychosomatic Medicine. 2008;70(2):186–191. doi: 10.1097/PSY.0b013e3181642a1c. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. The American Journal of Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Defining risk drinking. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2011;34(2):144–156. doi:Fea-AR&H-53. [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, Petitto JM. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. The American Journal of Psychiatry. 2002;159(10):1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. 1st ed. Cambridge University Press; 2006. [Google Scholar]
- Gill CJ, Sabin LL, Hamer DH, Keyi X, Jianbo Z, Li T, Desilva MB. Importance of dose timing to achieving undetectable viral loads. AIDS and Behavior. 2010;14(4):785–793. doi: 10.1007/s10461-009-9555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials. 2004;5(2):74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, Kovach DA. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes (1999) 2008;47(3):384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ for the HIV Epidemiology Research Study Group. Mortality, CD4 Cell Count Decline, and Depressive Symptoms Among HIV-Seropositive Women: Longitudinal Analysis From the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Copas AJ, Erens B, Mandalia S, Fenton K, Korovessis C, Field J. Effect of computer-assisted self-interviews on reporting of sexual HIV risk behaviours in a general population sample: a methodological experiment. AIDS (London, England) 2001;15(1):111–115. doi: 10.1097/00002030-200101050-00016. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, Swetzes C, Jones M, Macy R, Kalichman MO, Cherry C. A simple single-item rating scale to measure medication adherence: further evidence for convergent validity. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill.: 2002) 2009;8(6):367–374. doi: 10.1177/1545109709352884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine: Official Journal of the Society for Research and Education in Primary Care Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan PO, Shacham E, Reece M, Kroenke K, Ong’or WO, Omollo O, Ojwang C. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. Journal of General Internal Medicine. 2009;24(2):189–197. doi: 10.1007/s11606-008-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Ten Have T, Gettes DR, Chiappini MS, Weber AL, Evans DL. Depressive and anxiety disorders in women with HIV infection. The American Journal of Psychiatry. 2002;159(5):789–796. doi: 10.1176/appi.ajp.159.5.789. [DOI] [PubMed] [Google Scholar]
- Newman JC, Des Jarlais DC, Turner CF, Gribble J, Cooley P, Paone D. The differential effects of face-to-face and computer interview modes. American Journal of Public Health. 2002;92(2):294–297. doi: 10.2105/ajph.92.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando M, Burnam MA, Beckman R, Morton SC, London AS, Bing EG, Fleishman JA. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study. International Journal of Methods in Psychiatric Research. 2002;11(2):75–82. doi: 10.1002/mpr.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS and Behavior. 2007;11(2):161–173. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG. HIV and depression: 2008 review and update. Current HIV/AIDS Reports. 2008;5(4):163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- Reisen CA, Bianchi FT, Cohen-Blair H, Liappis AP, Poppen PJ, Zea MC, Labriola AM. Present and past influences on current smoking among HIV-positive male veterans. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2011;13(8):638–645. doi: 10.1093/ntr/ntr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: a cross-protocol analysis. Journal of Acquired Immune Deficiency Syndromes (1999) 2007;46(4):402–409. doi: 10.1097/qai.0b013e318158a44f. [DOI] [PubMed] [Google Scholar]
- Schackman BR, Dastur Z, Ni Q, Callahan MA, Berger J, Rubin DS. Sexually active HIV-positive patients frequently report never using condoms in audio computer-assisted self-interviews conducted at routine clinical visits. AIDS Patient Care and STDs. 2008;22(2):123–129. doi: 10.1089/apc.2007.0037. [DOI] [PubMed] [Google Scholar]
- Shacham E, Nurutdinova D, Satyanarayana V, Stamm K, Overton ET. Routine screening for depression: identifying a challenge for successful HIV care. AIDS Patient Care and STDs. 2009;23(11):949–955. doi: 10.1089/apc.2009.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacham Enbal, Agbebi A, Stamm K, Overton ET. Alcohol consumption is associated with poor health in HIV clinic patient population: a behavioral surveillance study. AIDS and Behavior. 2011;15(1):209–213. doi: 10.1007/s10461-009-9652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacham Enbal, Basta TB, Reece M. Relationship of psychological distress and unprotected sex among individuals with HIV seeking mental health care. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill.: 2002) 2009;8(2):93–99. doi: 10.1177/1545109709332468. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Goulet JL, Justice AC, Fiellin DA. Alcohol consumption and depressive symptoms over time: a longitudinal study of patients with and without HIV infection. Drug and Alcohol Dependence. 2011;117(2–3):158–163. doi: 10.1016/j.drugalcdep.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA AJ. Antiretroviral treatment of adult hiv infection: 2012 recommendations of the international antiviral society–usa panel. JAMA: The Journal of the American Medical Association. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- Vinet-Oliphant H, Alvarez X, Buza E, Borda JT, Mohan M, Aye PP, Lackner AA. Neurokinin-1 Receptor (NK1-R) Expression in the Brains of SIV-Infected Rhesus MacaquesImplications for Substance P in NK1-R Immune Cell Trafficking into the CNS. The American Journal of Pathology. 2010;177(3):1286–1297. doi: 10.2353/ajpath.2010.091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CT, Latkin CA. The role of depressive symptoms in predicting sex with multiple and high-risk partners. Journal of Acquired Immune Deficiency Syndromes (1999) 2005;38(1):69–73. doi: 10.1097/00126334-200501010-00013. [DOI] [PubMed] [Google Scholar]
- Williams K, Reynolds MF. Sexual dysfunction in major depression. CNS Spectrums. 2006;11(8) Suppl 9:19–23. doi: 10.1017/s1092852900026729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.