Abstract
OBJECTIVES
Polysomy detected by fluorescence in situ hybridization (FISH) is associated with cholangiocarcinoma (CCA) in patients with primary sclerosing cholangitis (PSC). However, a subset of PSC patients with polysomy do not manifest CCA even after long-term follow-up. It is unknown if patients with chromosomal gains detected by FISH in multiple areas of the biliary tree (i.e., multifocal polysomy, MFP) are more likely to be diagnosed with CCA than patients with unifocal polysomy (UFP). Therefore, our aim is to determine whether patients with MFP are more likely to manifest CCA compared with patients with other chromosomal abnormalities including UFP and other FISH subtypes.
METHODS
We performed a retrospective review of PSC patients without a mass lesion who underwent FISH testing at our institution from 1 January 2005 to 1 July 2013.
RESULTS
Three-hundred and seventy-one PSC patients were included. Compared with patients with UFP, those with MFP were more likely to have weight loss (32 vs. 9%), suspicious cytology (45 vs. 13%) and develop serial polysomy (91 vs. 35%). MFP was associated with CCA (hazard ratio (HR), 82.42; 95% confidence interval (CI), 24.50–277.31) and was the strongest predictor of cancer diagnosis. Suspicious cytology (HR, 26.31; 95% CI, 8.63–80.24) and UFP (HR, 13.27; 95% CI, 3.32–53.08) were also predictive of CCA. MFP, UFP and suspicious cytology remained associated with CCA in the multivariable model.
CONCLUSIONS
Compared with other FISH subtypes, MFP is the strongest predictor of CCA. However, patients with UFP and suspicious cytology (regardless of FISH status) are also at an increased risk for CCA.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic liver disorder characterized by inflammation and fibrosis of the intra and/or extrahepatic bile ducts, which leads to cholestasis, liver injury, and fibrosis (1). Cholangiocarcinoma (CCA) may develop in 5–10% of patients with PSC and it may arise from a background of pre-malignant biliary tissue (field defect) including dysplasia (2,3). Although CCA is associated with a poor prognosis, a multicenter study reported a 72% 5-year survival free of CCA recurrence among PSC patients with early-stage CCA who undergo neoadjuvant chemoradiation followed by a liver transplant (4). Therefore, early detection of CCA is important as a subset of PSC patients will be eligible for curative therapy.
Distinguishing benign from malignant biliary strictures in PSC is challenging and the sensitivity of conventional biliary cytology is <45% (5). Although the presence of a malignant mass lesion with delayed venous phase enhancement on imaging is highly sensitive and specific for CCA, such lesions are uncommon in early-stage CCA (6,7). While an elevated carbohydrate antigen 19-9 (CA 19-9) is concerning for CCA, not all PSC patients with an elevated CA 19-9 will have biliary cancer (8–11). Advanced endoscopic techniques such as narrow-band imaging cholangioscopy have a highly variable appearance in PSC and do not appear to reliably increase the rate of biliary neoplasia detection (12). Therefore, complementary techniques such as fluorescence in situ hybridization (FISH) to assess for chromosomal aneuploidy (losses or gains of chromosomes) are used to assist in CCA detection (13). Polysomy has been associated with CCA (8,14,15). However, CCA may be absent in up to 45% of PSC patients with polysomy detected on a single exam (14). In contrast, CCA has been detected in up to 69% of PSC patients when polysomy is detected on subsequent examinations (serial polysomy) (15).
Because only a subset of PSC patients with polysomy will be diagnosed with CCA, it is important to identify other features that can predict the diagnosis of CCA once polysomy is detected to enhance our ability to risk stratify these patients. It is unknown if patients with multifocal polysomy (MFP) are at a higher risk of CCA compared with those with polysomy in a single location in the biliary tree (i.e., unifocal polysomy, UFP). Consequently, our primary aim is to determine if PSC patients with MFP detected on a single exam are more likely to be diagnosed with CCA compared with patients with UFP and other FISH types (serial polysomy, trisomy/tetrasomy, or a negative FISH study). Our secondary aim is to examine key clinical differences between those with UFP and MFP.
METHODS
Patients
This study was approved by the Institutional Review Board at Mayo Clinic, Rochester, Minnesota, USA. We performed a retrospective review of all patients with PSC who underwent cross-sectional imaging and biliary brushings for cytology and FISH who were seen at our institution between 1 January 2005 to 1 July 2013. The initial cohort was established by searching the electronic medical record system for patients with the term “primary sclerosing cholangitis” or “PSC” anywhere in their medical record and cross matched with those who had biliary brushings for FISH performed. The electronic medical records of this initial cohort were reviewed in detail.
Patients were included if the following were present: (i) retrograde, percutaneous or magnetic resonance cholangiography (MRC) demonstrating typical features of PSC including multifocal intrahepatic and/or extrahepatic biliary duct obstruction, beading or narrowing consistent with PSC; (ii) FISH was performed on biliary brushings obtained at the time of an endoscopic retrograde cholangiogram (ERC) or percutaneous transhepatic cholangiogram; and (iii) had cross-sectional abdominal imaging available for review. Patients were excluded from the primary analyses if one of the following were present: (i) history of a liver transplant, CCA, or gallbladder malignancy before the index FISH; (ii) gallbladder cancer or any intrahepatic malignancy (excluding CCA) at the time of index FISH; (iii) definitive radiographic features of CCA (mass lesion) seen at time of index FISH; or (iv) patients with polysomy who only had a single brushing performed and could not be classified as either UFP or MFP. The index FISH was defined as the most severe FISH result that occurred first. MFP was considered the most severe FISH subtype followed by UFP, trisomy, or tetrasomy and a negative FISH. If a patient only had negative FISH results, then the earliest FISH test was identified as the index FISH.
For CCA screening, it is our clinical practice to obtain liver tests every 3–6 months, an annual CA 19-9 and annual abdominal imaging (ultrasound or MRC) in all PSC patients. We will typically perform an ERC if patients develop clinical symptoms to suggest biliary obstruction, suspicious findings on imaging (including a dominant stricture), or worsening laboratory tests (including CA 19-9 elevations). Patients with suspicious findings will be followed closely with repeat imaging and/or ERC with brushings. Bile duct biopsies may also be obtained during ERC, if anatomically possible.
Data collection and key definitions
Data were obtained through the electronic medical record and abstracted on a standardized template. In addition to demographic features, the indication for undergoing a cholangiogram with FISH testing, cholangiography features, cytopathology data, the presence of symptoms, laboratory values (within 3 months of the index FISH), revised Mayo PSC risk, MELD and Child Pugh scores (16–18) and imaging features were abstracted at the time of the cholangiogram when the index FISH was identified. If a particular variable was not available for a patient, that individual was not included in the analysis for that missing covariate. A CA 19-9 ≥129 U/ml was selected as the cutoff on the basis of prior studies (8). Treating CA 19-9 as a continuous variable did not impact our results (data not shown). All patients underwent cross-sectional imaging (typically MRC) within 3 months of the index FISH.
The primary end point was CCA. CCA was defined as the presence of adenocarcinoma on cytology or biopsy specimens or a mass lesion with typical features of CCA seen on cross-sectional imaging (with or without metastases) (7). Imaging features considered indeterminate for CCA include bile duct wall thickening, irregularity or enhancement, marked biliary dilation, the presence of a dominant stricture, or focal biliary dilation with ipsilateral lobe atrophy. A dominant stricture is typically defined as a stricture with a diameter 1 mm or less in the hepatic duct or 1.5 mm or less in the common bile duct; determination of the presence of a dominant stricture was made by the endoscopist performing the ERC at the time of the index FISH (7). When dominant strictures are encountered, a wire-guided cytology brush is passed back and forth through the stricture repeatedly. Dominant strictures at distinct locations (right and left hepatic ducts, hilum, the common hepatic and common bile duct) are typically brushed separately and placed in different vials. If feasible, it is also our practice to brush irregular segments of the central bile ducts or areas that appeared thickened or showed enhancement on a MRC. The contents of each vial are split equally for routine cytology and FISH as previously described (15).
For conventional cytology, each patient specimen was evaluated by an experienced cytopathologist using standardized criteria and categorized as one of the following: inadequate cellularity, negative, atypical, suspicious for adenocarcinoma, or positive for adenocar-cinoma (19,20). FISH was performed with the UroVysion probe set (Abbott Molecular, Des Plaines, IL, USA) and specimens containing thousands of cells were assessed as previously described and categorized as either negative, trisomy, tetrasomy, or polysomy (Figure 1, (refs 14,15). One hundred consecutive epithelial cells from specimens categorized as polysomy were reviewed to generate an estimated proportion of cells positive for polysomy.
Figure 1.
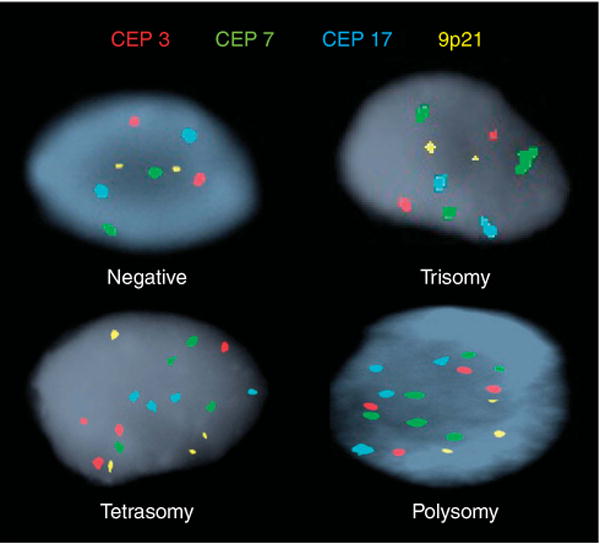
Subtypes of fluorescence in situ hybridization. Individual cells showing different fluorescence in situ hybridization results using CEP to chromosomes 3 (red), 7 (green), 17 (aqua) and a locus specific probe targeting 9p21 (gold). Negative (two copies of each probe); Trisomy 7 (at least 10 cells with ≥3 CEP 7 signals and ≤2 signals of the other probes); Tetrasomy (at least 10 cells with four signals for all four probes); Polysomy (at least five cells showing ≥3 signals for at least two of the four probes). CEP, centromere enumeration probe.
Patients with a negative FISH study, trisomy, or tetrasomy were included in the study regardless of the number of areas brushed. Patients were considered candidates for either UFP or MFP if brushings were performed in more than one location of the biliary tree during a single procedure and the specimens from different locations were placed in separate vials. If such patients had only one area positive for polysomy they were considered to have UFP, whereas MFP patients had polysomy detected in brushings from two or more areas. In contrast, if a patient had only a single area brushed which was positive for polysomy, they were defined as indeterminate for UFP or MFP and excluded from the primary analyses. Serial polysomy is defined as more than one FISH test positive for polysomy on more than one procedure (15).
Statistical analysis
All patients were analyzed on the basis of their index FISH results (i.e., negative, trisomy/tetrasomy, UFP, or MFP). Analysis was performed utilizing SAS 9.3 (SAS Institute; Cary, NC) and R 3.0.2 (R Foundation for Statistical Computing; Vienna, Austria). All tests were two-sided with a level of significance of P <0.05. Categorical data were compared using the Pearson chi-squared test and continuous variables were compared using the Kruskal–Wallis test. Continuous variables are expressed as median interquartile range (IQR). Follow-up was calculated from the time of the index FISH to the time of CCA diagnosis or censoring. The patients without CCA were censored at the time of liver transplantation and if this did not occur, then at their last cholangiogram or cross-sectional image (whichever occurred last). The Kaplan–Meier method was used to plot the survival free curves of the primary end point between FISH groups. The cumulative incidence of CCA was determined by the one minus Kaplan–Meier method. Cox proportional hazards regression analysis was utilized in both univariable and multivariable analyses to examine associations between covariates and the primary end point, and the results were expressed as hazard ratios (HRs) with respective 95% confidence intervals (CIs). Serial polysomy was treated as a time-dependent covariate, recognizing that this designation was often not determined until after the index FISH (baseline). Examination of the Schoenfeld residuals was used to check the proportional hazards assumptions. To avoid overfitting of our multivariable model, covariates associated with CCA (P value <0.05) in the univariable analysis were included in the multi-variable model as individual covariates plus the FISH subtype. As shown by Vittinghoff and McCulloch, the number of covariates included in our models is appropriate and would not be expected to cause model instability (21).
RESULTS
Patients
During the 7.5-year study period, an estimated 3,100 unique patients with a diagnosis of PSC were seen at our institution. Among these individuals, 545 patients underwent FISH testing (Figure 2). The most common indication for exclusion from this study was definitive evidence of CCA seen on cross-sectional imaging at the time of the index FISH (n=107). Twenty-six patients with polysomy had only a single brushing performed and were therefore indeterminate for UFP or MFP. Ultimately, 371 patients (all with large duct PSC) were included in the primary analyses (negative FISH n=194, trisomy or tetrasomy n=114, UFP n=32, MFP n=31).
Figure 2.
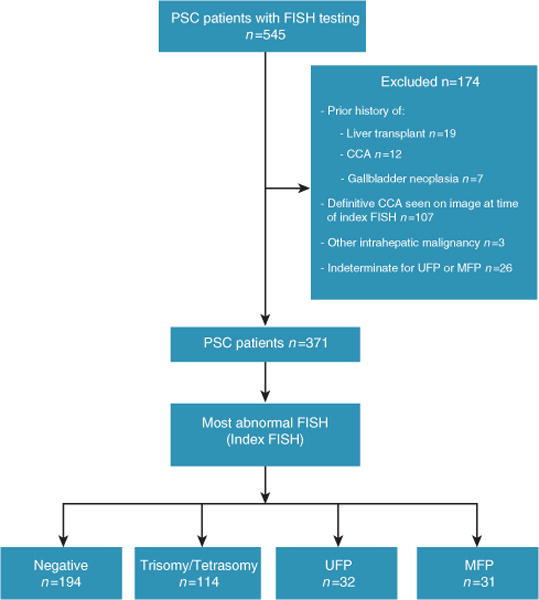
Patients included. CCA, cholangiocarcinoma; FISH, fluorescence in situ hybridization; MFP, multifocal polysomy; PSC, primary sclerosing cholangitis; UFP, unifocal polysomy.
The majority of patients underwent FISH testing due to a concern for CCA. This concern was raised by a varied clinical presentation, which typically suggested worsening biliary obstruction (Table 1). Weight loss was more common among those with MFP compared with UFP (32 vs. 9%, P=0.03). The most common suspicious imaging feature was a dominant stricture (61%). Although patients with polysomy were more likely to have a prior history of an abnormal FISH result (trisomy/tetrasomy or UFP among MFP patients) or cytology (suspicious or atypical), the proportion of UFP and MFP patients with a history of an abnormal FISH or cytology was similar (Table 1). Indeed, six patients initially had UFP but were subsequently found to have MFP after a median (IQR) of 170 (76–213) days and hence were included in the MFP subgroup.
Table 1.
Indications for cholangiogram and cholangiography features
| Negative (N=194) |
Trisomy/tetrasomy (N=114) |
UFP (N=32) |
MFP (N=31) |
Total (N=371) |
P value (total) |
P value (MFP vs. UFP) |
|
|---|---|---|---|---|---|---|---|
| Indication(s) for procedurea | |||||||
| Signs or symptomsb | |||||||
| Yes (any) | 97 (50%) | 52 (46%) | 16 (50%) | 11 (36%) | 176 (47%) | 0.47c | 0.24c |
| Fever | |||||||
| Yes | 20 (10%) | 11 (10%) | 2 (6%) | 4 (13%) | 37 (10%) | 0.84c | 0.37c |
| Weight loss | |||||||
| Yes | 28 (14%) | 20 (18%) | 3 (9%) | 10 (32%) | 61 (16%) | 0.06c | 0.03c |
| Abdominal pain | |||||||
| Yes | 41 (21%) | 25 (22%) | 10 (31%) | 9 (29%) | 85 (23%) | 0.51c | 0.85c |
| Pruritus | |||||||
| Yes | 39 (20%) | 21 (18%) | 5 (16%) | 9 (29%) | 74 (20%) | 0.54c | 0.20c |
| Jaundice | |||||||
| Yes | 50 (26%) | 29 (25%) | 8 (25%) | 12 (39%) | 99 (27%) | 0.47c | 0.24c |
| Suspicious imagingd | |||||||
| Yes | 86 (44%) | 48 (42%) | 7 (22%) | 14 (45%) | 155 (42%) | 0.12c | 0.05c |
| Prior abnormal FISHe or atypical/suspicious cytology | |||||||
| Yes | 22 (11%) | 20 (18%) | 14 (44%) | 15 (48%) | 71 (19%) | <0.001c | 0.71c |
| Worsening labs | |||||||
| Yes | 81 (42%) | 52 (46%) | 12 (38%) | 11 (36%) | 156 (42%) | 0.70c | 0.87c |
| Stent exchange only | |||||||
| Yes | 7 (4%) | 5 (4%) | 0 (0%) | 0 (0%) | 12 (3%) | 0.44c | NA |
| Diagnostic uncertaintyf | |||||||
| Yes | 7 (4%) | 5 (4%) | 0 (0%) | 0 (0%) | 12 (3%) | 0.44c | NA |
| Cholangiogram findings & sampling | |||||||
| Number of areas brushed | |||||||
| Median | 1.00 | 2.00 | 2.00 | 2.00 | 2.00 | <0.001g | 0.80f |
| IQR | 1.00–2.00 | 1.00–2.00 | 2.00–3.00 | 2.00–3.00 | 1.00–2.00 | ||
| Dominant stricture | |||||||
| Yes | 50 (26%) | 26 (23%) | 7 (22%) | 12 (39%) | 95 (26%) | 0.32c | 0.15c |
FISH, fluorescence in situ hybridization; IQR, interquartile range; MFP, multifocal polysomy; NA, not applicable; UFP, unifocal polysomy.
Multiple indications were present for 168 patients.
Multiple signs or symptoms may have been present in same patient.
Derived using chi-square test.
Suspicious imaging features indeterminate for cholangiocarcinoma (CCA) include bile duct wall thickening or enhancement, marked or worsening biliary dilation, a dominant stricture or focal biliary dilation with ipsilateral lobe atrophy.
Abnormal FISH includes trisomy/tetrasomy or UFP among patients who developed MFP.
Performed to confi rm PSC diagnosis.
Derived using Kruskall–Wallis test.
Baseline features and diagnosis of serial polysomy
Baseline features between the FISH groups are shown in Table 2. Inflammatory bowel disease (IBD) was present in 78% of patients (ulcerative colitis n=245, Crohn’s disease n=39, indeterminate colitis n=4). Although patients with MFP appeared to have a longer duration of inflammatory bowel disease compared with other FISH subtypes, this was not significant when compared with UFP patients. Twenty-eight patients with ulcerative colitis had medically refractory inflammatory bowel disease which required a colectomy. However, this was not associated with a diagnosis of CCA (HR, 1.98; 95% CI, 0.73–4.53). At baseline, patients with MFP were more likely than patients with UFP to have suspicious cytology (45 vs. 13%, P<0.001) and a higher proportion of cells with polysomy (13 vs. 3%, P<0.001). In contrast, a negative cytology was less common in MFP compared with UFP (7 vs. 47%, P<0.001). Laboratory testing, including CA 19-9 levels, were similar between those with UFP and MFP (Table 2). Among the MFP and UFP patients with suspicious cytology, the proportion with multifocal, compared with unifocal suspicious cytology was similar between MFP and UFP patients (75 vs. 79%, P=0.88), respectively. Among the 63 patients with polysomy at baseline, 55 had more than one examination and serial polysomy was subsequently detected in 91% (20/22) and 35% (8/23) of patients diagnosed with MFP and UFP (respectively) at baseline (P<0.001).
Table 2.
Baseline features of patients included
| Negative (N=194) |
Trisomy/tetrasomy (N=114) |
UFP (N=32) |
MFP (N=31) |
Total (N=371) |
P value (total) |
P value (MFP vs. UFP) |
|
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| Median | 43.40 | 54.75 | 51.98 | 48.29 | 47.34 | <0.001a | 0.31a |
| IQR | 31.80–56.58 | 45.45–64.54 | 41.13–65.42 | 38.68–58.17 | 36.05–60.18 | ||
| Gender | |||||||
| Male | 127 (66%) | 73 (64%) | 19 (60%) | 22 (71%) | 241 (65%) | 0.80b | 0.34b |
| Smoking history | |||||||
| Yes | 14 (7%) | 15 (13%) | 3 (9%) | 4 (13%) | 36 (10%) | 0.35 b | 0.66 b |
| IBD | |||||||
| Yes | 152 (78%) | 85 (75%) | 24 (75%) | 27 (87%) | 288 (78%) | 0.49b | 0.22b |
| Duration of IBD (years)c | |||||||
| Median | 10.67 | 13.40 | 15.78 | 21.43 | 12.83 | 0.01a | 0.36a |
| IQR | 4.63–20.57 | 5.95–24.05 | 6.08–27.52 | 11.41–33.67 | 5.95–23.05 | ||
| Duration of PSC (years) | |||||||
| Median | 3.84 | 4.11 | 5.04 | 4.53 | 4.13 | 1.00a | 0.90a |
| IQR | 0.51–10.77 | 0.86–8.36 | 1.18–11.18 | 0.87–10.19 | 0.68–10.26 | ||
| UDCA | |||||||
| Yes | 99 (51%) | 53 (47%) | 17 (53%) | 13 (42%) | 182 (49%) | 0.70b | 0.37]b |
| CA 19-9 ≥129 U/mld | |||||||
| Yes | 22 (13%) | 24 (23%) | 6 (20%) | 11 (37%) | 63 (19%) | 0.01b | 0.15b |
| Alkaline phosphatase U/le | |||||||
| Median | 312.00 | 287.00 | 249.00 | 252.00 | 292.50 | 0.58a | 0.45a |
| IQR | 178.00–434.00 | 198.00–464.00 | 178.00–382.00 | 173.00–593.00 | 178.00–453.00 | ||
| Total bilirubin mg/dle | |||||||
| Median | 1.40 | 1.70 | 0.90 | 1.15 | 1.30 | 0.15a | 0.43a |
| IQR | 0.80–3.50 | 0.90–4.50 | 0.60–2.20 | 0.70–2.10 | 0.80–3.50 | ||
| MELD scoree | |||||||
| Median | 8.61 | 10.02 | 7.70 | 7.49 | 8.65 | 0.08a | 0.75a |
| IQR | 6.43–12.36 | 7.49–13.72 | 6.43–11.12 | 6.79–9.58 | 6.79–12.66 | ||
| Mayo risk scoref | |||||||
| Median | 0.54 | 1.06 | 0.44 | 0.55 | 0.75 | 0.01a | 1.00a |
| IQR | −0.32–1.75 | 0.31–2.02 | −0.27–1.48 | −0.17–1.35 | −0.16–1.81 | ||
| Child Pugh scoreg | |||||||
| Median | 5.00 | 6.00 | 5.00 | 5.00 | 5.00 | 0.03a | 0.78a |
| IQR | 5.00–7.00 | 5.00–8.00 | 5.00–7.00 | 5.00–6.00 | 5.00–7.00 | ||
| Negative cytology | |||||||
| Yes | 143 (74%) | 77 (68%) | 15 (47%) | 2 (7%) | 237 (64%) | <0.001b | <0.001b |
| Atypical cytology | |||||||
| Yes | 39 (20%) | 24 (21%) | 10 (31%) | 8 (26%) | 81 (22%) | 0.51b | 0.63b |
| Suspicious cytology | |||||||
| Yes | 12 (6%) | 11 (10%) | 4 (13%) | 14 (45%) | 41 (11%) | <0.001b | <0.002b |
| Adenocarcinoma | |||||||
| Yes | 0 (0%) | 2 (2%) | 3 (9%) | 7 (23%) | 12 (3%) | <0.001b | 0.15b |
CA 19-9, carbohydrate antigen 19-9; IBD, inflammatory bowel disease; IQR, interquartile range; MELD score (model for end-stage liver disease); MFP, multifocal polysomy; PSC, primary sclerosing cholangitis; UDCA, ursodeoxycholic acid; UFP, unifocal polysomy.
Derived using the Kruskal–Wallis test.
Derived using the chi-square test.
Available for 251 patients.
CA 19-9 values missing for 42 patients (negative FISH n=28, trisomy/tetrasomy n=11, UFP n=2, MFP n=1).
Available for 358 patients.
Available for 347 patients.
Available for 338 patients.
Importantly, the prevalence of dominant strictures was similar across all groups and the number of areas sampled was similar for UFP and MFP patients (Table 1). In addition, there were no differences between the number of dominant strictures present between UFP and MFP patients: 2 (1–2) vs. 1 (1–2), P=0.23, respectively. Among those with either UFP or MFP and a dominant stricture, polysomy was detected from the site of the dominant stricture in the majority of patients (71% UFP and 92% MFP, P=0.24). However, polysomy was also frequently collected from areas beyond a dominant stricture. For example, 29% of UFP patients and 58% of MFP patients (P=0.21) had polysomy detected from sites not associated with a dominant stricture.
Diagnosis of cholangiocarcinoma
CCA was detected in 37 patients (negative FISH n=3, trisomy/tetrasomy n=5, UFP n=6, MFP n=23). The 1- and 3-year cumulative incidence (95% CI) of CCA of the entire cohort was 8% (6–12%) and 11% (8–15%), respectively. Ultimately, metastatic CCA and death secondary to CCA occurred in 16 (negative FISH n=2, trisomy/tetrasomy n=1, UFP n=4, MFP n=9) and 14 (negative FISH n=2, trisomy/tetrasomy n=1, UFP n=4, MFP n=7) patients, respectively. The diagnosis of CCA was established on the basis of the following: cytology or biopsy alone n=21, definitive radiographic features alone n=4, both cytology or biopsy and definitive radiographic features n=12.
Brushings positive for adenocarcinoma were detected at the time of the index FISH in 12 patients (Table 2). Among these 12 patients, 5 (42%) had a dominant stricture identified on the ERC at the time of the index FISH. Polysomy was frequently detected from additional locations other than those with cytology-proven CCA. For example, among UFP and MFP patients with a brushing positive for adenocarcinoma (at any time point), 71% had polysomy detected at another region where adenocarcinoma was not detected on routine cytology.
Patients with MFP were more likely to be diagnosed with CCA in contrast to those with negative FISH, trisomy or tetrasomy, and UFP (Figure 3). The 1- and 3-year cumulative incidence (95% CI) of CCA among MFP patients was 65% (46–81%) and 83% (62–93%), respectively. After including those who were indeterminate for either MFP or UFP, MFP continued to be the strongest FISH predictor of CCA (Supplementary Figure 1 online). In addition, MFP remained predictive of a CCA diagnosis after excluding those patients with a biopsy or cytology positive for adenocarcinoma at the time of the index FISH (Supplementary Figure 2). The median (IQR) time to CCA diagnosis among those without adenocarcinoma on the index FISH and MFP was 146 (57–357) days.
Figure 3.
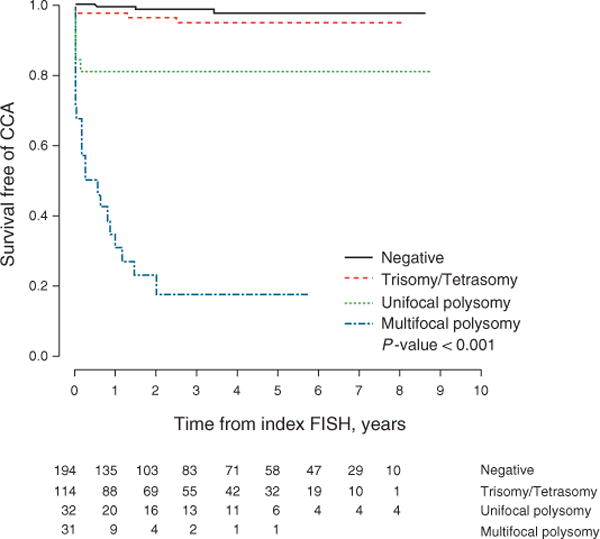
Survival free of cholangiocarcinoma among FISH subtypes. CCA, cholangiocarcinoma; FISH, fluorescence in situ hybridization.
In the univariable analysis, a prior abnormal FISH (trisomy/tetrasomy or UFP among those who developed MFP) or prior abnormal cytology (atypical or suspicious), CA 19-9 ≥129 U/ml, the number of areas sampled at the time of the index FISH, presence of a dominant stricture, atypical cytology, suspicious cytology, UFP, MFP, serial polysomy, and the proportion of cells positive for polysomy were associated with CCA (Table 3). As shown in Figure 4, MFP and UFP (compared with negative FISH) remained strong predictors of CCA after adjustment for the individual covariates that were significant in the univariable analysis: MFP (unadjusted HR, 82.42; adjusted HR ranged from 27.57 to 91.77) and UFP (unadjusted HR, 13.27; adjusted HR ranged from 5.27 to 18.03).
Table 3.
Potential risk factors for cholangiocarcinoma (unadjusted)
| HR (95% CI) | P value | |
|---|---|---|
| Age (years) | 1.17 (0.93–1.46) | 0.17 |
| Gender (male) | 1.14 (0.57–2.27) | 0.70 |
| Smoking history | 0.89 (0.27–2.92) | 0.85 |
| IBD present | 1.93 (0.75–4.96) | 0.17 |
| Duration of IBD (years) | 1.03 (1.00–1.06) | 0.06 |
| Duration of PSC (years) | 1.01 (0.96–1.06) | 0.65 |
| Fever | 1.11 (0.39–3.13) | 0.84 |
| Abdominal pain | 1.10 (0.52–2.34) | 0.80 |
| Weight loss | 1.44 (0.66–3.15) | 0.36 |
| Pruritus | 1.42 (0.69–2.94) | 0.34 |
| Jaundice | 1.73 (0.89–3.37) | 0.10 |
| Suspicious imaging | 0.88 (0.45–1.71) | 0.71 |
| Prior abnormal FISHa or atypical/suspicious cytology | 3.22 (1.67–6.21) | <0.001 |
| Worsening labs | 0.84 (0.43–1.64) | 0.61 |
| UDCA | 0.96 (0.51–1.84) | 0.91 |
| CA 19-9 ≥129 U/ml | 2.84 (1.43–5.65) | <0.01 |
| Alkaline phosphatase U/l | 0.99 (0.98–1.01) | 0.45 |
| Total bilirubin mg/dl | 1.01 (0.98–1.03) | 0.64 |
| MELD score | 0.98 (0.91–1.05) | 0.55 |
| Mayo risk score | 1.01 (0.79–1.30) | 0.95 |
| Child Pugh score | 0.90 (0.70–1.15) | 0.39 |
| Number of areas brushed | 2.32 (1.51–3.58) | <0.001 |
| Dominant stricture | 2.01 (1.04–3.88) | 0.04 |
| Negative cytology | Reference | |
| Atypical cytology | 5.92 (1.73–20.27) | <0.01 |
| Suspicious cytology | 26.31 (8.63–80.24) | <0.001 |
| Negative FISH | Reference | |
| Trisomy/tetrasomy | 2.68 (0.64–11.20) | 0.18 |
| UFP | 13.27 (3.32–53.08) | <0.001 |
| MFP | 82.42 (24.50–277.31) | <0.001 |
| Serial polysomy | 3.95 (1.47–18.16) | <0.001 |
| Proportion of cells positive for polysomy | 1.03 (1.01–1.05) | <0.01 |
CA 19-9, carbohydrate antigen 19-9; IBD, inflammatory bowel disease; MELD score, model for end-stage liver disease; MFP, multifocal polysomy; PSC, primary sclerosing cholangitis; UDCA, ursodeoxycholic acid; UFP, unifocal polysomy.
Abnormal FISH includes trisomy/tetrasomy or UFP among patients who developed MFP.
Figure 4.
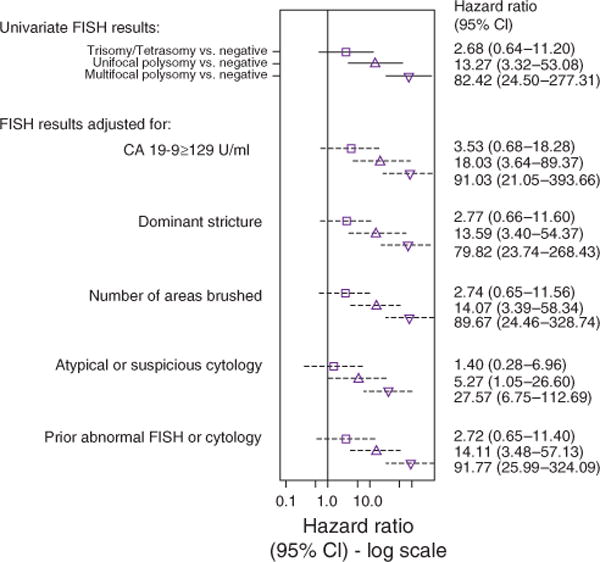
Forest plot illustrating the association of multifocal polysomy, unifocal polysomy, and trisomy/tetrasomy (compared with those with a negative FISH value) with the probability of being diagnosed with cholangio carcinoma after adjusting for other potential risk factors. The figure shows the point estimate (hazard ratio) and 95% confidence interval (CI) of a trisomy/tetrasomy, unifocal, and multifocal polysomy (unadjusted and adjusted for each individual covariate). The cytology adjustment includes two variables representing suspicious cytology vs. negative and atypical cytology vs. negative. A prior history of an abnormal FISH result or cytology included trisomy/tetrasomy or unifocal polysomy (among multifocal polysomy patients) and suspicious or atypical cytology results. Serial polysomy and the proportion of cells with polysomy are excluded from the adjustments since patients with a negative FISH and trisomy/tetrasomy are unable to have polysomy-specific covariates. CA 19-9, carbohydrate antigen 19-9; FISH, fluorescence in situ hybridization.
When only patients with polysomy were included in the multi-variable analysis, MFP (compared with UFP) remained a predictor of CCA even after adjusting for polysomy-specific covariates such as serial polysomy and the proportion of cells positive for polysomy (Figure 5). Of the 28 patients with serial poly somy, 0% (0/8) and 75% (15/20) patients with UFP and MFP (respectively) were diagnosed with CCA.
Figure 5.
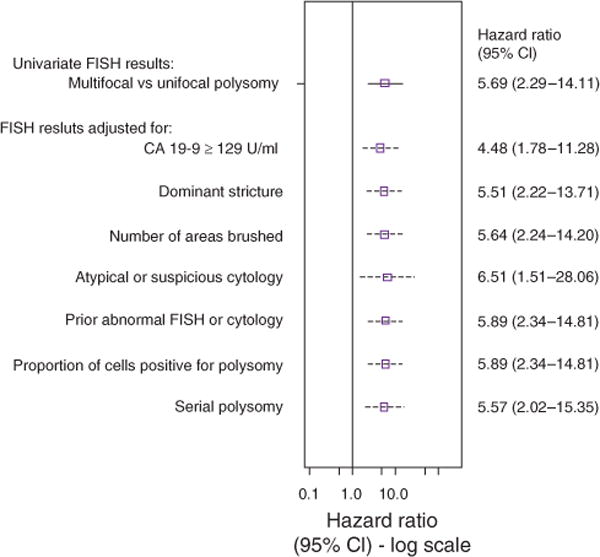
Forest plot illustrating the association of multifocal polysomy (compared with those with unifocal polysomy) with the probability of being diagnosed with cholangiocarcinoma after adjusting for other potential risk factors. The figure shows the point estimate (hazard ratio) and 95% confidence interval (CI) of MFP (unadjusted and adjusted for each individual covariate). The cytology adjustment includes two variables representing suspicious cytology vs. negative and atypical cytology vs. negative. A prior history of an abnormal FISH result or cytology included trisomy/tetrasomy or unifocal polysomy (among multifocal patients) and suspicious or atypical cytology results. CA 19-9, carbohydrate antigen 19-9; FISH, fluorescence in situ hybridization.
Among the 41 patients with suspicious cytology at the time of the index FISH, 14 (34%) were diagnosed with CCA. In turn, suspicious cytology (vs. negative cytology), remained predictive of CCA after adjustment for FISH (HR, 5.72; 95% CI, 1.48–22.05; P=0.01). In contrast, other covariates, which were significant in the univariable analysis were not associated with CCA after adjustment for FISH status: prior abnormal FISH or cytology (HR, 0.40; 95% CI 0.40–1.63), CA 19-9 ≥129 U/ml (HR, 1.78; 95% CI, 0.87–3.60), the number of areas sampled when the index FISH was collected (HR, 0.92; 95% CI, 0.55–1.54), dominant stricture (HR, 1.67; 95% CI, 0.85–3.25), atypical cytology (HR, 2.34; 95% CI, 0.68–9.20), serial polysomy (HR, 1.40; 95% CI, 0.46–4.26), and proportion of cells positive for polysomy (HR, 0.92; 95% CI, 0.55–1.54).
The remaining 334 patients without CCA were censored at the time of liver transplantation (n=59) or the last abdominal image or cholangiogram (n=275). The median (IQR) duration of followup among those who remained free of CCA was similar between FISH subgroups: 2.26 (0.78–5.87) years, 3.07 (1.54–5.28) years, 3.06 (1.01–4.73) years, and 1.89 (0.22–3.36) years for negative FISH, trisomy/tetrasomy, UFP, and MFP (respectively). Among the 26 patients with a UFP who were not diagnosed with CCA, eight had serial polysomy and underwent two or more ERCs after the index FISH, which did not detect CCA over the course of 2.20 (0.63–4.80) years of follow-up. Similarly, at least two ERCs were performed in those who did not develop serial polysomy after a diagnosis of UFP over a course of 1.87 (1.37–3.18) years. Hence it is unlikely that patients with UFP could have harbored an undiagnosed cancer at the time of censoring. Among the eight patients with MFP who were not diagnosed with CCA during this study period, one individual had high-grade dysplasia on a bile duct biopsy. The seven other individuals did not have a history of dysplastic bile duct tissue detected on bile duct biopsies. Of these seven patients, one had serial polysomy and suspicious cytology detected and underwent a liver transplant for end-stage liver disease (explant was negative for CCA), while another individual had suspicious cytology and a CA 19-9 of 778 U/ml and died (cause unknown) within a month of MFP detection. The median (IQR) duration of follow-up for the remaining five patients was 1.90 (0.32–4.71) years and all five patients underwent at least one ERC since the index FISH.
DISCUSSION
This study, the largest published cohort of PSC patients who underwent FISH testing to date, shows for the first time that patients with polysomy detected in multiple sites are more likely to be diagnosed with CCA compared to those with polysomy detected in a single location. Our results also indicate that patients with MFP are more likely to be diagnosed with CCA compared with patients with other FISH findings such as trisomy/tetrasomy and serial polysomy. Indeed, those with MFP were more likely to have a history of weight loss, suspicious cytology, an imaging study suspicious but indeterminate for CCA, or a greater proportion of cells positive for polysomy and serial polysomy when compared with patients with UFP. Our results also illustrate that UFP and suspicious cytology (regardless of the FISH subtype) both remain a concerning finding associated with CCA.
The observations of this study re-inforce the role of FISH in risk stratifying PSC patients and improve our ability to interpret the results of FISH studies when managing individuals with PSC. The primary benefit of performing FISH is improving the sensitivity of CCA detection when compared with routine cytology alone (14). In a prior study, the overall proportion of patients with polysomy with CCA was 55% (14). However, when polysomy was detected in the context of a dominant stricture or serial polysomy after 3 years of follow-up, the proportion of patients with CCA was 73 and 69%, respectively (14,15). By comparison, the 3-year cumulative incidence of CCA among patients with MFP detected on a single exam was 83% regardless if they had a dominant stricture or developed serial polysomy. An elevated CA 19-9 plus polysomy is suspicious for malignancy. For example, a prior study noted 100% (10/10) patients with polysomy, atypical or suspicious cytology, and a CA 19-9 ≥129 U/ml were diagnosed with CCA (8). However, an elevated CA 19-9 was not an independent predictor of CCA in the current study. Indeed, several studies have reported that nearly one-third of PSC patients (with and without polysomy) and a CA 19-9 >129 U/ml do not have underlying malignancy (10,11). In the present study, we focus on PSC patients with polysomy FISH result(s) when multiple biliary sites were sampled in a single exam. In this context, MFP was the strongest predictor of CCA. The increased proportion of other concerning features among MFP patients such as weight loss, suspicious cytology, indeterminate imaging findings, and serial polysomy also suggests that MFP differs from other FISH findings including UFP. Therefore, patients with MFP should be considered a distinct subgroup of PSC patients at higher risk for CCA. However, patients with UFP and suspicious cytology are also at an increased risk and should not be ignored.
These findings have several clinical implications, which raise new questions. First, these data highlight the importance of obtaining brush cytology from the bile ducts of PSC patients who are undergoing an ERC for suspected CCA, even in those without a dominant stricture, and placing the specimens obtained from different locations into separate vials to determine if MFP is present. We observed that polysomy is frequently found in areas not associated with a dominant stricture and the presence of a dominant stricture was not more common in MFP patients or an independent predictor of CCA. This finding may be secondary to several factors. Although the presence of a dominant stricture should raise concern for CCA, cancer (particularly early stage) may not always appear as a dominant stricture on a cholangiogram. For example, of the 12 patients with a biliary cytology positive for adenocarcinoma detected at the time of the index FISH, only 42% had a dominant stricture. In addition, a previous study noted that only one-quarter of dominant strictures were associated with malignancy, which also suggests that many dominant strictures are benign (22). Thus, not all dominant strictures are malignant and not all cases of cancer present as a dominant stricture. However, CCA that appears as a dominant stricture may represent a more advanced stage of cancer. For example, in a prior study of 21 patients with CCA associated with a dominant stricture, all patients died of their disease and had a median survival of 6 months (22). Furthermore, the ability for an ERC to distinguish benign from malignant strictures in PSC is limited (23). Although a prior study noted an improved diagnostic performance when polysomy was detected in patients with a dominant stricture, many of the patients included in that study had a mass lesion suspicious for CCA at the time of the index FISH (14). This could increase the probability of having a malignant dominant stricture and would represent a different patient population than the present study, which did not include patients with definitive radiographic features of CCA at the time of the index FISH. This may be another reason why we did not observe a strong association with CCA and dominant strictures. Hence, these observations and our results suggest that it may be important to brush other areas of the central biliary tree, regardless of whether and where a dominant stricture is present, and place the specimens in separate vials among PSC patients suspected of having CCA but lack definitive features on imaging. Insurers such as Medicare have provided reimbursement for this assay (15).Future prospective studies should examine the utility and cost-effectiveness of this strategy to confirm whether this approach improves our ability to detect early-stage CCA when bile duct malignancy is suspected in patients with PSC.
Our findings also provide insight into the mechanistic processes of CCA development in PSC patients and lend credence to the hypothesis that CCA arises from a field defect. Field defect is the concept that larger areas of premalignant tissue may extend beyond the primary site of malignancy. These areas are likely to harbor molecular and chromosomal aberrations, but may appear histologically normal. In PSC, CCA develops in a background of chronic biliary tract inflammation. Chronic inflammation may mediate CCA development through the induction of DNA damage, enhanced cell proliferation and resistance to apoptosis (24,25). In addition, several studies have suggested that bile duct dysplasia beyond the malignant lesion is common in PSC and there are a greater number of ducts with dysplasia when CCA is present (26,27). Thus, sampling a larger surface area may improve the diagnostic performance of FISH testing, which could enhance our ability to detect MFP and cancer among PSC patients suspected of having CCA.
Our study has several limitations. First, this was a retrospective study conducted at a single academic center that included a select group of PSC patients where a majority of individuals underwent an ERC to evaluate for CCA. The selective nature of this cohort is reflected in the 3-year incidence of CCA for the entire group (11%) when compared with population-based studies, which reported the incidence of CCA among all PSC patients regardless of whether an ERC with FISH testing was performed (10% after 8 years) (28). Consequently, our results should be interpreted in the context of PSC patients where there is a clinical suspicion for underlying CCA and these findings would not be applicable to all PSC patients nor would the evidence support the use of ERC and FISH testing as a universal screening tool. Second, the reporting of a dominant stricture likely varies between practitioners and the inter-observer variability of dominant stricture identification is unknown. Third, despite having a large overall sample size, we had a limited number of patients with polysomy and the power of our study is limited by the few number of events. Because of this limitation and the number of comparisons made, our findings should be confirmed. Last, multiple endoscopists performed the sampling for biliary cytology and FISH specimens during the course of this study. However, the likelihood that sampling bias could explain the differences observed between the polysomy groups is small for several reasons: (i) the presence and number of dominant strictures was similar between those with UFP and MFP; (ii) although patients with MFP tended to have suspicious imaging features at the time of the index FISH, the number of areas brushed and placed in separate vials was similar between those with UFP and MFP and this was included in the multivariable analysis; and (iii) if sample overlap occurred when brushings were obtained from adjacent ductal segments (thereby potentially converting UFP to MFP), it is unlikely this was a significant con-founder as we observed numerous differences between the MFP and UFP groups, which suggest MFP patients are a high-risk subgroup.
In conclusion, the current study suggests that MFP is a very strong risk factor for CCA among PSC patients suspected of having biliary cancer but lack a mass lesion on imaging at the time of the initial assessment. Patients with MFP may already harbor CCA and patients without other definitive features of biliary cancer should have close interval follow-up. Brushing multiple areas of the biliary tree may improve the ability to detect MFP and CCA among a select group of PSC patients and this technique warrants further study. Optimizing the performance of ERC and FISH testing in PSC patients has the potential to facilitate the early diagnosis of CCA, which can in turn expand the pool of patients eligible for curative therapy.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Patients with PSC are at an increased risk for cholangio-carcinoma.
-
✓
Polysomy, particularly serial polysomy has been associated with cholangiocarcinoma in PSC. However, only a subset of patients with polysomy will be diagnosed with cholangio-carcinoma.
WHAT IS NEW HERE
-
✓
Compared with other prognostic features, multifocal polysomy is the strongest predictor of cholangiocarcinoma among PSC patients who lack definitive radiographic features of biliary cancer.
-
✓
Cholangiocarcinoma can arise from areas not associated with a dominant stricture.
-
✓
PSC patients with unifocal polysomy and suspicious cytology are also at an increased risk of cholangiocarcinoma.
Acknowledgments
Financial support: This work was supported by NIH grants: DK 84960 (K.N.L.), DK 59427 (G.J.G.).
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: John E. Eaton, MD.
Specific author contributions: John E. Eaton: study concept and design, acquisition of data, analysis, and interpretation of data; drafting of the manuscript and critical revision of the manuscript; Emily G. Barr Fritcher: acquisition and interpretation of data and critical revision of the manuscript; Gregory J. Gores: study design, interpretation of data, and critical revision of the manuscript; Elizabeth J. Atkinson: study design, analysis and interpretation of data, and critical revision of the manuscript; Mark D. Topazian: interpretation of data and critical revision of the manuscript; James H. Tabibian: analysis and interpretation of data and critical revision of the manuscript; Andrea A. Gossard: interpretation of data and critical revision of the manuscript; Kevin C. Halling: interpretation of data and critical revision of the manuscript; Benjamin R. Kipp: interpretation of data and critical revision of the manuscript; Konstantinos N. Lazaridis: study design, interpretation of data, and critical revision of the manuscript.
Potential competing interests: Kevin C. Halling and Mayo Clinic have a patent on and receive royalties from the sale of the FISH prove set (UroVysion). Kevin C. Halling and Benjamin R. Kipp receive grant funding from Abbott Molecular, the manufacturers of the UroVysion probe set. The remaining authors declare no conflict of interest.
References
- 1.Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–36. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein C, Bernstein H, Payne CM, et al. Field defects in progression to gastrointestinal tract cancers. Cancer Lett. 2008;260:1–10. doi: 10.1016/j.canlet.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangio-carcinoma at 12 US centers. Gastroenterology. 2012;143:88–98. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trikudanathan G, Navaneethan U, Njei B, et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2013;79:783–9. doi: 10.1016/j.gie.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangio-carcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–17. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 7.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangiti. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 8.Barr Fritcher EG, Voss JS, Jenkins SM, et al. Primary sclerosing cholangitis with equivocal cytology: fluorescence in situ hybridization and serum CA 19-9 predict risk of malignancy. Cancer Cytopathol. 2013;121:708–17. doi: 10.1002/cncy.21331. [DOI] [PubMed] [Google Scholar]
- 9.Levy C, Lymp J, Angulo P, et al. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–40. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 10.Sinakos E, Saenger AK, Keach J, et al. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9:434–9. doi: 10.1016/j.cgh.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh PG, Navaneethan U, Shen B, et al. Increased serum levels of carbohydrate antigen 19-9 and outcomes in primary sclerosing cholangitis patients without cholangiocarcinoma. Dig Dis Sci. 2013;58:850–7. doi: 10.1007/s10620-012-2401-3. [DOI] [PubMed] [Google Scholar]
- 12.Azeem N, Gostout CJ, Knipschield M, et al. Cholangioscopy with narrow-band imaging in patients with primary sclerosing cholangitis undergoing ERCP. Gastrointest Endosc. 2014;79:773–9. doi: 10.1016/j.gie.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Bergquist A, Tribukait B, Glaumann H, et al. Can DNA cytometry be used for evaluation of malignancy and premalignancy in bile duct strictures in primary sclerosing cholangitis? J Hepatol. 2000;33:873–7. doi: 10.1016/s0168-8278(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 14.Bangarulingam SY, Bjornsson E, Enders F, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51:174–80. doi: 10.1002/hep.23277. [DOI] [PubMed] [Google Scholar]
- 15.Barr Fritcher EG, Kipp BR, Voss JS, et al. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am J Gastroenterol. 2011;106:2023–28. doi: 10.1038/ajg.2011.272. [DOI] [PubMed] [Google Scholar]
- 16.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688–94. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 17.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 18.Lucey MR, Brown KA, Everson GT, et al. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628–37. doi: 10.1002/lt.500030613. [DOI] [PubMed] [Google Scholar]
- 19.Renshaw AA, Madge R, Jiroutek M, et al. Bile duct brushing cytology: statistical analysis of proposed diagnostic criteria. Am J Clin Pathol. 1998;110:635–40. doi: 10.1093/ajcp/110.5.635. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MB, Wittchow RJ, Johlin FC, et al. Brush cytology of the extra hepatic biliary tract: comparison of cytologic features of adenocarcinoma and benign biliary strictures. Mod Pathol. 1995;8:498–502. [PubMed] [Google Scholar]
- 21.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–18. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 22.Chapman MH, Webster GJ, Bannoo S, et al. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051–58. doi: 10.1097/MEG.0b013e3283554bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergquist A, Glaumann H, Persson B, et al. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27:311–16. doi: 10.1002/hep.510270201. [DOI] [PubMed] [Google Scholar]
- 24.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangio-carcinoma. Gastroenterology. 2013;145:1215–29. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi S, Werneburg NW, Bronk SF, et al. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–65. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Bergquist A, Glaumann H, Stal P, et al. Biliary dysplasia, cell proliferation and nuclear DNA-fragmentation in primary sclerosing cholangitis with and without cholangiocarcinoma. J Intern Med. 2001;249:69–75. doi: 10.1046/j.1365-2796.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JT, Talwalkar JA, Rosen CB, et al. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangio-carcinoma. Am J Surg Pathol. 2010;34:27–34. doi: 10.1097/PAS.0b013e3181bc96f9. [DOI] [PubMed] [Google Scholar]
- 28.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–55. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


