Abstract
Background
Methods for gene transfer to the cornea that yield high-level expression without inflammation or trauma are currently lacking. Because electroporation has proven effective for gene transfer in other tissues in terms of expression levels and safety, this study quantitatively evaluated its use in the cornea.
Methods
To evaluate the use of electroporation in the mouse cornea, plasmids expressing either luciferase or green fluorescent protein were injected intracorneally or subconjunctivally and square-wave electric pulses were immediately applied to the eyes. Gene expression was quantified at later times and trauma and inflammation were monitored visually and by measuring interleukin-6 (IL-6) production.
Results
The application of electric pulses to eyes injected with plasmid resulted in nanogram levels of gene product expression. At an optimal field strength of 200 V/cm, no trauma, corneal edema or inflammation was observed. However, at higher field strengths, corneal damage was detected. Compared with injection of DNA alone, up to 1000-fold more gene product was produced using electroporation. Expression was detected as early as 6 h post-electroporation, remained high for 3 days, and decreased by 7 days. Gene expression was detected over the entire surface of the cornea in both epithelial and stromal layers.
Conclusions
These results demonstrate that electroporation is an excellent method for delivering genes to multiple cell layers within the mouse cornea and that it results in extremely high levels of gene expression with little, if any, inflammatory response or tissue damage, making this a very useful technique for corneal gene transfer.
Keywords: gene therapy, gene transfer, non-viral vector, electroporation, plasmid
Introduction
In recent years, the potential to replace defective genes or to use altered genes to combat disease in humans has been realized [1,2]. At present, gene therapy protocols are being developed and used in clinical trials to treat or prevent genetic diso8rders, cancers, and infectious diseases [3]. Gene therapy is also being pursued to treat diseases in the eye. While one study has reported a strategy for treating proliferative vitreoretinopathy, most of the current work on gene therapy in the eye is focused on vector development and establishment of transfer techniques. One reason for this is that at present, only a few genes have been identified that cause known ocular diseases, most of which are manifested in the retina. However, many disease states of the anterior chamber of the eye have a genetic component. These include several forms of glaucoma [4,5], cataracts [6–8], congenital hereditary endothelial dystrophy, and Fuch’s corneal dystrophy. Thus, gene therapy will be a viable treatment option within the near future as the molecular defects causing these diseases are characterized. Additionally, gene therapy holds great promise for the treatment of infectious disease, including bacterial, fungal, and viral-induced stromal keratitis. Since the genetic components of these diseases are being characterized and gene therapy approaches are being developed, we must have ways to deliver the genes of interest once we are ready.
Numerous viral and non-viral approaches have been proposed and developed for transferring genes to cells but all have serious limitations. Inefficiency of gene transfer, immunological responses, and non-specificity of cell targeting are just a few of the problems associated with the current viral vectors. For example, although adeno-virus appears to be the vector in vogue for ocular gene therapy in the laboratory and has gene transfer efficiencies of almost 95% in vitro, the values are usually much less in vivo and require upwards of 108–109 pfu injected into the anterior chamber of the mouse eye [9,10]. Consequently, cell damage and inflammation are common. Unlike their viral counterparts, there is very little inflammation or pathology associated with non-viral vectors (e.g. plasmid DNA) [11]. Thus, multiple administrations of vector can be given with no decrease in activity. Unfortunately, the efficiency of transfer of DNA alone or plasmid–liposome complexes to the eye has been very low, of the order of 1% [12–15]. Thus, for non-viral vectors to be of clinical use in the eye, their ability to transfect cells in vivo must be increased.
Electroporation uses electric fields (voltage over a surface area) to create transient ‘holes’ in the plasma membrane of cells to allow the high-level entry of DNA or other molecules into the cytoplasm. While this technique is routinely used in most laboratories to transform/transfect bacteria, yeast, and mammalian cells in culture [16], it has only been applied in the last 2 years to tissues in living animals. In studies using skeletal muscle [17–19], liver [20,21], cardiac tissue [22], and blood vessels [23], the increase in gene expression seen by simply applying an electric field to tissue injected with DNA is of the order of 100- to 1000-fold compared with DNA alone without an electric pulse. This technique has recently been applied to the rabbit cornea and has been shown to increase gene targeting and expression in the corneal endothelium [24,25]. When a plasmid expressing β-galactosidase was injected into the anterior chamber and electric pulses were applied, qualitative gene expression was detected histologically in endothelial cells in the area that received the electric field. In the cornea, as in all other tissues studied to date, the use of field strengths below 200 V/cm resulted in little trauma or pathology to the tissue, but as the field strengths increased, significant damage was induced [25].
In the present study, electroporation was evaluated for the efficient transfer of DNA to the corneal epithelium and stroma using quantitative and qualitative measurements. Our results establish that electroporation yields levels of gene transfer and expression higher than previously seen with viral or non-viral techniques and results in no trauma or inflammation, making it a very useful method.
Materials and methods
Plasmids
The plasmids pCMV-Lux-DTS (5.8 kbp [26]) and pEGFP-N1 (4.7 kbp; Clontech, Palo Alto, CA, USA) express firefly luciferase and green fluorescent protein (GFP), respectively, from the CMV immediate early promoter/enhancer. Plasmids were grown in Escherichia coli and purified using Qiagen Giga-prep kits, as described by the manufacturer (Qiagen, Chatsworth, CA, USA). The concentration of plasmid was adjusted to 2 mg/ml in 10 mM Tris, pH 8.0, 1 mM EDTA, and 140 mM NaCl, unless otherwise indicated. Agarose gel electrophoretic analysis demonstrated that more than 80% of the purified DNA was present in the supercoiled form, and no RNA was detected.
Injections and electroporation
Plasmids were injected into the cornea or the sub-conjunctival area of Nembutal-anesthetized Balb/c mice (13–17 g; n=130) using a 50 μl Hamilton syringe and a WPI syringe pump (WPI, Sarasota, FL, USA). Three microliters (subconjunctival) or 1 μl (intracorneal) of DNA was injected over a 3 or 1 s period, respectively, as previously described [27]. Within 2 min of injection, the eyes were electroporated using gold-plated Genetrode electrodes (Genetronics, San Diego, CA, USA) that were placed on either side of the protosed eye (typically 3 mm apart). A square-wave electroporator (Genetronics ECM830) was used to deliver eight pulses of 10 ms duration each to the eye at varying field strengths. Immediately after electroporation, the mice were allowed to recover and returned to the vivarium. At the end of the experiments, the mice were euthanized by Nembutal overdose and the eyes were removed. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for Care and Use of Laboratory Animals. Animal protocols were approved by the Institutional Animal Care and Use Committees of the University of South Alabama and Northwestern University.
Measurement of luciferase activity
After removal of the eyes, the lenses were removed and the eyes were snap-frozen in liquid nitrogen. The eyes were ground on a bed of frozen Promega Luciferase Lysis Buffer (Promega, Madison, WI, USA) using a drill press equipped with frozen bits, as previously described [28]. The ground tissue was thawed in the lysis buffer and subjected to three freeze–thaw cycles before the particulate matter was removed by centrifugation. Luciferase activity in the supernatant was assayed using the Promega Luciferase Assay kit in a Turner luminometer. Purified recombinant luciferase (Promega) was used to produce a standard curve for each experiment.
Histological analysis
Upon removal from the euthanized animals, eyes to be used for histological analysis were frozen in OCT tissue embedding medium and sectioned (6 μm) on a cryostat. Sections were placed on polylysine-coated slides, fixed in acetone for 30 s, and GFP expression was observed directly by fluorescence microscopy using an Olympus BMAX-40 microscope and photographed with an Optronics CCD camera. After GFP visualization, slides were stained with hematoxylin and eosin to assess the structure and health of the treated eyes. GFP expression was also detected by immunohistochemistry, using a rabbit polyclonal antibody against GFP (1: 50; Clontech) and a peroxidase-labeled secondary antibody (1: 50; Vector Laboratories, Burlingame, CA, USA). DAB was used as the substrate for the enzyme-linked secondary antibody and the slides were counter-stained with hematoxylin (Vector Laboratories), according to the manufacturer’s instructions.
Cytokine assays
The levels of cytokines were measured using Quantikine ELISA kits (R & D Systems, Minneapolis, MN, USA) in ocular extracts prepared by homogenization in RPMI, using a tissue tearor (Biospec Products, Bartlesville, OK, USA) after removal of lenses, as previously described [27].
Statistical analysis
Mann–Whitney U- or ANOVA tests were performed to determine statistical significance, using Instat 2.03 software (GraphPad Software, San Diego, CA, USA).
Results
Use of electroporation for plasmid delivery and expression in the eye
The eye is an extremely attractive tissue in which to develop methods for gene delivery because it is amenable to topical administration of vectors as well as several direct routes of injection. In order to obtain substantial levels of gene expression in the eye, we tested whether electroporation of plasmids would lead to increased amounts of produced gene product. To follow gene expression, we chose two gene products, luciferase and GFP, for quantitative and qualitative analysis, respectively. The ability to measure luciferase over a 105 concentration range makes it an excellent choice for quantitative measurements, while the direct detection of GFP by fluorescence microscopy makes it useful for analyzing the distribution of gene expression in the tissues.
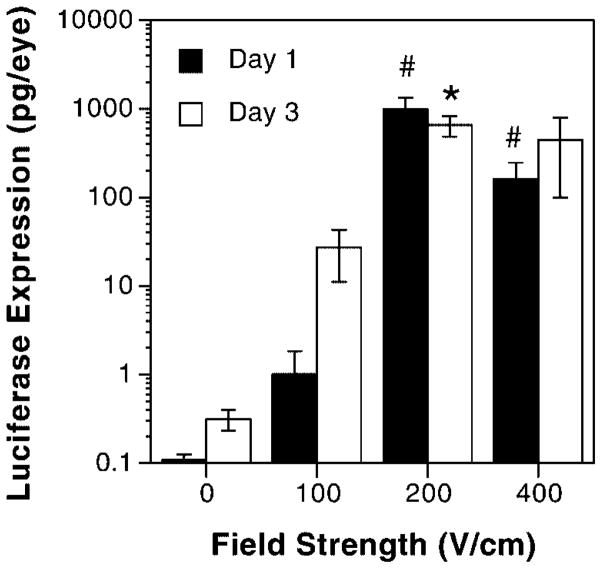
Three microliters of luciferase-expressing plasmid (5 μg total DNA) suspended in 10 mM Tris, pH 8, 1 mM EDTA, and 140 mM NaCl were injected into the subconjunctival region of the left eye, followed by a similar injection into the right eye of Balb/c mice. Immediately following the injection into the right eye (within 2 min of injection into the left), a series of eight electric pulses of 10 ms duration at varying field strengths was applied to the protosed eyes. The initial parameters tested were those established for other tissues, including skeletal muscle [17–19] and the vasculature [23]. Figure 1 shows the resulting gene expression at either 1 day (closed bars) or 3 days (open bars) post-delivery as a function of increasing field strength. No luciferase expression was detected at 24 h in the absence of an applied field (0 V/cm) or at a field strength of 100 V/cm. However, at 200 V/cm, approximately 1 ng/eye of luciferase was expressed. Expression was not as robust at 400 V/cm, but was still statistically significant when compared with the expression seen without electroporation. A similar expression profile was seen at 3 days post-delivery and electroporation, but slightly higher expression was detected at 0 and 100 V/cm. No trauma, edema, or tissue damage was detected after injection and electroporation using 0, 100, or 200 V/cm, but some of the animals did show corneal edema when exposed to a field strength of 400 V/cm (data not shown). Because substantial gene expression was detected with no resulting trauma, a field strength of 200 V/cm was used for all subsequent experiments.
Figure 1.
Voltage dependence of gene transfer. Eyes (n=8) were injected subconjunctivally with 5 μg of pCMV-Lux-DTS and varying electric field strengths were applied. In all cases, eight pulses of 10 ms duration each were used. The levels of gene expression were measured either 1 day (closed bars) or 3 days (open bars) post-treatment. The mean expression is shown ±SEM. Mann–Whitney U-tests were performed to determine statistical significance. *p<0.05 compared with 0 or 100 V/cm; #p<0.0005 compared with 0 or 100 V/cm. Based on these results, a field strength of 200 V/cm was used in all subsequent experiments
A time course was followed to determine the pattern of gene expression after electroporation (Figure 2, closed bars). Five micrograms of the luciferase-expressing plasmid was injected subconjunctivally and immediately electroporated at a field strength of 200 V/cm. Gene expression, above background levels, was detected as early as 6 h post-delivery and increased to a maximum at 24 h post-electroporation (982±355 pg/eye, mean±SEM, n=8). Expression remained high for 3 days, but by 7 days after DNA addition, gene expression had dropped to 5% of that seen on day 1. By contrast, subconjunctival injection of DNA without electroporation gave less than 1 pg/eye of expressed gene product at all time points (Figure 2, open bars), confirming the need for an applied electric field for high-level gene expression.
Figure 2.

Time course of gene expression. Eyes (n=8) were injected subconjunctivally with 5 μg of pCMV-Lux-DTS and electroporated at 200 V/cm. At the indicated times, the animals were euthanized and luciferase activity was measured in cell extracts prepared from the excised eyes. The mean expression is shown ±SEM. Mann–Whitney U-tests were performed to determine statistical significance. *p<0.01 compared with time 0; #p<0.001 compared with time 0
To determine the optimal concentration of DNA to deliver to the eye, a dose-dependence experiment was performed (Figure 3). Four mice (both eyes) were subconjunctivally injected with either no DNA or increasing concentrations of plasmid and immediately electroporated at 200 V/cm as described in the Materials and methods section. Three days later, the eyes were harvested and luciferase activities were determined. The limit of detection of this assay was 0.8±1.1 pg (mean±SEM, n=8), based on the level of activity detected in murine eyes that had received no DNA. When 0.1 μg of DNA was delivered to the eyes, the level of expression was almost identical to that seen without addition of DNA. However, as the DNA concentration was increased to 0.3 μg and above, statistically significant differences were observed. Gene expression increased in a dose-dependent fashion, with maximal expression being detected at the highest dose delivered, 10 μg. Because only 3 μl of DNA was delivered subconjunctivally, this was the highest dose capable of being delivered; injections became technically impossible at higher concentrations of DNA, due to the greatly increased viscosity of the plasmid solution. Based on our results, electroporation proved to be a reproducible method for gene transfer to the cornea, resulting in the production of nanogram levels of gene product, with no trauma under the optimum conditions for gene transfer.
Figure 3.
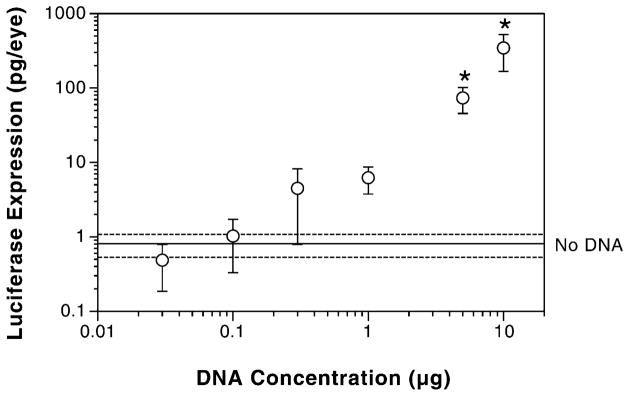
Dose dependence of gene expression. Eyes were injected subconjunctivally with varying concentrations of pCMV-Lux-DTS and electroporated at 200 V/cm. Eight eyes were used for each of the DNA concentrations. Three days later, luciferase activities were measured as described. The dotted lines represent the limit of assay sensitivity (mean±SEM, n=8) as measured by assaying naïve eyes for luciferase expression. Mann–Whitney U-tests were performed to determine statistical significance. *p<0.0005 compared with no DNA
Comparison of electroporation with DNA delivery by liposomes
Previous studies aimed at delivering plasmids to the eye have focused on liposome formulations as carriers for the DNA [12,13]. Although different liposome formulations provide varying in vivo gene transfer efficiencies, cationic liposomes appear to give the best transfection. One widely used cationic liposome formulation for both in vitro and in vivo applications is lipofectin [29]. To determine the efficiency of DNA electroporation relative to the standard liposome delivery, we injected the same amount (5 μg) of naked DNA, or DNA–lipofectin complexes, or DNA followed by electroporation into the subconjunctiva of mice (Figure 4). The lipofectin–DNA complexes were formed and tested in vitro on cells in culture to ensure that the complexes had indeed formed and were capable of transfection. When added to cultured human corneal epithelial cells as previously described [30], luciferase gene expression was detected (data not shown). When the same complexes were tested in vivo, very little gene expression was detected at 3 days post-delivery (Figure 4). In fact, the amount of gene expression was indistinguishable from that obtained by naked DNA injection alone, or from untreated eyes. By contrast, injection of the plasmid followed by electroporation gave almost 30-fold higher expression than that obtained with lipofectin.
Figure 4.

Comparison of gene delivery methods. Eyes (n=8) were either electroporated with no DNA (‘No DNA’), injected subconjunctivally with 5 μg of pCMV-Lux-DTS (‘DNA only’), injected with 5 μg of pCMV-Lux-DTS complexed with 5 μg of lipofectin (Life Technologies; ‘Lipofectin’), or injected with 5 μg of pCMV-Lux-DTS and electroporated at 200 V/cm (‘Electroporation’). Three days later, luciferase activities were measured as described. The mean expression is shown ±SEM. Mann–Whitney U-tests were performed to determine statistical significance. *p<0.05 compared with DNA only
Gene expression as a function of the injection site
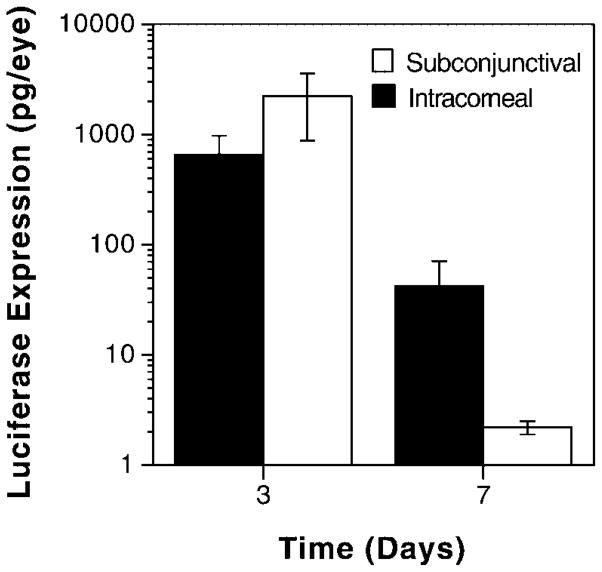
The preceding results clearly establish electroporation of subconjunctivally injected DNA as a method for obtaining high levels of gene expression in the eye. Although substances injected into the subconjunctiva can diffuse to the cornea as well as the limbus, the gene expression detected in these experiments could be due to expression in both tissues. In some instances, it may be desirable to deliver genes only to the cornea or even to the distinct layers of the cornea by direct corneal injection. To compare the expression efficacy of intracorneally injected and electroporated DNA with that of subconjunctivally delivered DNA, 1 μl of plasmid (2.5 μg) was injected into the stromal layer of the cornea or into the subconjunctiva, and a series of electric pulses were delivered to the protosed eye (Figure 5). Surprisingly, injection directly into the cornea resulted in slightly increased levels of gene expression compared with subconjunctival delivery, although the difference was not statistically significant. As seen in subconjunctival injections, very little expression was obtained by intracorneal DNA injection without electroporation (0.16±0.05 pg luciferase/eye, n=34). Similarly, expression decayed over the same time frame as for subconjunctivally injected and electroporated eyes; by 7 days, less than 1% of the expression seen at day 3 remained present (Figure 5). These results demonstrate that the largely non-dividing cells of the cornea are capable of taking up the DNA and expressing it to a high level.
Figure 5.
Intracorneal vs. subconjunctival DNA delivery. Eyes (n=8) were injected either intracorneally or subconjunctivally with 2.5 μg of pCMV-Lux-DTS and electroporated at 200 V/cm. Three days later, luciferase activities were measured as described. The mean expression is shown ±SEM. A Mann–Whitney U-test revealed that there was no statistical significance between the sites of injection
Localization of gene expression
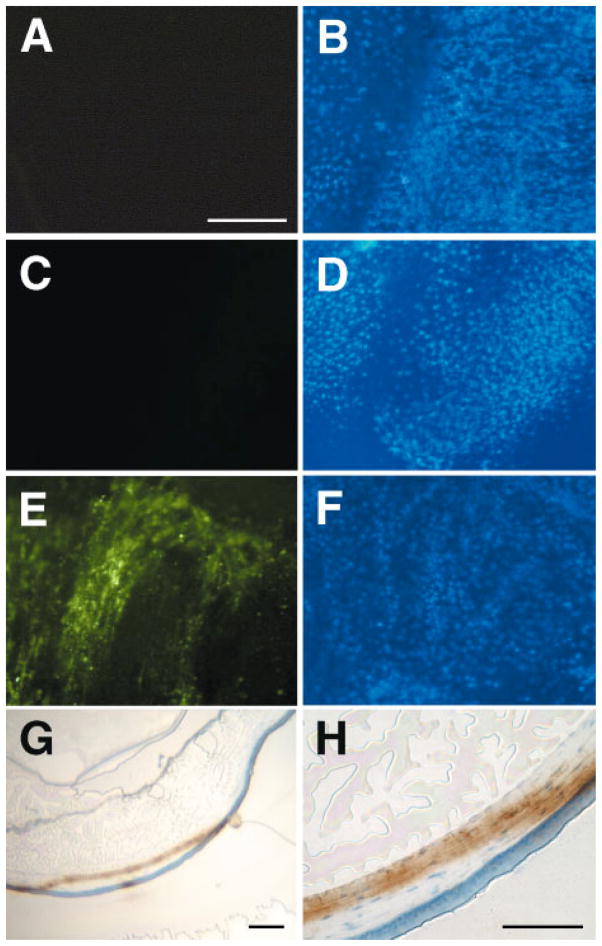
In order to determine where gene expression was localized in the electroporated eyes, both whole corneas and histological sections were observed. A plasmid expressing green fluorescent protein (GFP) was injected subconjunctivally, electroporated, and 3 days later, GFP expression was detected directly by fluorescence microscopy (Figures 6A–6D). When saline alone was injected (i.e. no DNA) and the eyes were electroporated, no gene expression was detected by fluorescence microscopy of the flattened cornea and subconjunctival area 3 days post-treatment (Figure 6A), although a large number of cells are shown in the field, as determined by DAPI staining of the nuclei (Figure 6B). Similarly, when DNA was injected subconjunctivally but without subsequent electroporation, no GFP expression was observed (Figures 6C and 6D). By contrast, when the GFP-expressing plasmid was delivered to the eye by subconjunctival injection and electroporation, a large percentage of the cells in the cornea and subconjunctiva expressed GFP (Figures 6E and 6F). Similar patterns and percentages of GFP-expressing cells were detected when the DNA was delivered intracorneally, but expression was more centralized in the cornea itself (Figures 6G and 6H, and data not shown). Seven out of eight injected and electroporated eyes showed GFP expression, with five eyes having levels of expression similar to that shown in Figure 6E; the remaining two eyes showed expression in fewer cells (not shown).
Figure 6.
GFP expression in electroporated eyes. Eyes (n=8) were injected subconjunctivally with saline (A and B) or 5 μg of pEGFP-N1 (C–F) and electroporated at 200 V/cm. Three days later, they were removed and either flattened on microscope slides (A–D) or embedded in OCT and sectioned (10 μm thick, E and F) and observed for GFP expression. GFP expression was detected by direct fluorescence in A–D, or by immunohistochemistry using an antibody against GFP followed by deposition of DAB and counter-staining with hematoxylin (brown color, E and F). Eyes in A–D were stained with DAPI (B and D) to visualize nuclei and the same fields that were photographed for GFP expression (A and C). Panels A–D are all at the same magnification; bar=200 μm. Bar=200 μm in G and 50 μm in H
To determine which cells in the cornea were responsible for the observed gene expression, intracorneally injected and electroporated eyes were removed 3 days post-treatment and embedded in OCT medium to produce thin sections for immunohistochemistry. Using an antibody immunoreactive against GFP, we were able to identify the cells that were expressing the protein (Figures 6G and 6H). Upon intracorneal injection, both stromal and epithelial cells stained for GFP, with areas of intense immunoreactivity near the center of the cornea in the epithelial layer, and across most of the cornea in the stromal layer. Thus, electroporation of eyes receiving DNA either intracorneally or subconjunctivally caused gene expression in a significant number of cells throughout the cornea.
Production of the pro-inflammatory cytokine IL-6 in treated eyes
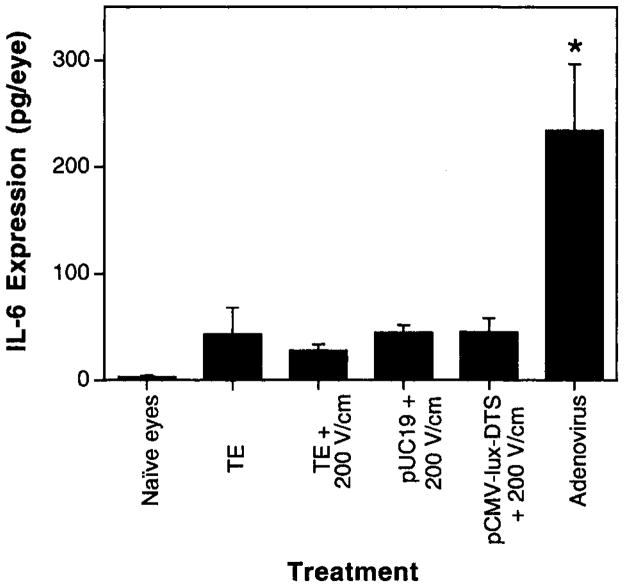
Although direct injection of DNA into the cornea and subsequent electroporation resulted in high levels of gene expression throughout the cornea with no apparent trauma or edema in the eye, it remained to be seen whether an inflammatory response was induced by the treatment. To determine this, we chose to look at the production of the pro-inflammatory cytokine IL-6, which often serves as an indicator of cell damage and severity of immune response against both viral and bacterial pathogens [31,32]. Three days after treatment, eyes were removed and IL-6 levels were measured by ELISA in whole eye extracts (Figure 7). Whereas untreated, naïve eyes expressed no detectable IL-6, all eyes that received subconjunctival injections of either buffer alone or DNA, with or without electroporation, had slightly elevated levels of IL-6, as has been seen previously by others [33], but still lower than seen in an inflammatory response. The levels of IL-6 produced in electroporated eyes injected with buffer alone, or two different plasmids, one expressing a gene product and one containing no expressed genes, were virtually identical to those produced in eyes injected with saline but not electroporated. This is in contrast to the production of IL-6 seen when the eyes were injected with recombinant replication-deficient adenovirus particles (5×108 pfu). Thus, unlike gene delivery by adenoviral vectors, electroporation did not induce any significant IL-6 production.
Figure 7.
DNA injection and electroporation do not induce IL-6 expression. Eyes (n=8) were either untreated (‘naïve eyes’), injected subconjunctivally with TE/NaCl (‘TE’), injected with 5×108 pfu of recombinant replication-deficient adenovirus (‘Adenovirus’), or injected subconjunctivally and electroporated using TE/NaCl (‘TE+200 V/cm’), 5 μg of pUC19 (‘pUC19+200 V/cm’), or 5 μg of pCMV-Lux-DTS (‘pCMV-Lux-DTS+200 V/cm’). Three days later, IL-6 levels were measured by ELISA. The mean expression is shown ±SEM. ANOVA with Tukey–Kramer post-test analysis revealed p<0.0001, comparing adenovirus with all other conditions
Discussion
In this study we have shown that delivery of plasmid DNA using electroporation is a simple and highly effective method to direct gene transfer and expression in the cornea. By injecting limited amounts of naked DNA into either the subconjunctival region or the cornea itself and then delivering a series of eight 10 ms pulses to the eye at an optimal field strength of 200 V/cm, up to 5 ng of gene product could be detected by 3 days post-treatment. Gene expression was detected as early as 6 h post-treatment and lasted for 3 days, after which time expression levels dropped. By 7 days, the level of gene expression was reduced to 5% of that seen at day 1, but was still significantly higher than at time 0. Compared with the delivery with liposomes or DNA injection in the absence of electric fields, electroporation gave over 30-fold higher expression levels. An added benefit of this approach to gene delivery in the eye is that no trauma or inflammatory response was induced, as determined visually and histologically as well as by cytokine quantitation. Finally, both epithelial cells and keratocytes took up the foreign DNA and expressed gene product. Thus, delivery of plasmids to the cornea by electroporation is a simple, rapid, and highly efficient procedure of transferring genes to the multiple cell types within the cornea.
Although multiple approaches, both viral and non-viral, for gene delivery to the eye have been developed, all have potentially serious drawbacks that may limit their usefulness except under certain conditions. Viral vectors, including adenoviruses [9,10,34–36], adeno-associated viruses [37], retroviruses [38,39], lentiviruses [40], herpes simplex viruses [37], and even polyoma viruses [41], can all be used to deliver genes to the cornea and produce gene products. However, one of the main drawbacks of using any of these viral vectors is that inflammatory and immune responses are mounted, limiting their usefulness. By contrast, delivery of plasmids with cationic and neutral lipids, or with other polymers such as dendrimers or polyethyleneimine, generates little inflammatory response but also results in low levels of gene expression [12–15,42]. One non-viral technique that has generated intermediate levels of gene expression in the cornea is biolistic delivery [43]. However, this approach results in gene expression in only several layers of epithelial cells at the surface of the cornea, due to the delivery method.
The advantage of delivery of DNA using electric fields is that high-level gene expression is obtained with little inflammatory response. Indeed, when 5 μg of plasmid was injected into the subconjunctiva and the eye was electroporated, over 1 ng of gene product was typically produced in the cornea within 2 days. Further, there was no significant expression of IL-6, a general indicator of inflammation, caused by this method of gene delivery when compared with eyes that had been injected in a similar manner with buffer alone, or injected and electroporated with either buffer or a non-expressing plasmid. It was noted, however, that IL-6 levels in all injected eyes were higher than in naïve eyes, suggesting that the slight increase in IL-6 was due to the injection and not the electric field. This is in stark contrast to the increase in IL-6 expression observed in eyes injected with a recombinant first-generation adenovirus seen by us as well as by others. Thus, electroporation-mediated gene delivery reproduces the best aspects of each type of vector.
Sakamoto and co-workers have also demonstrated that electroporation can be used in the cornea to deliver genes to the endothelial layer by injecting plasmid into the anterior chamber and then applying an electric field to the corneal surface [24,25]. One difference between their work and ours is the design of the electrodes: we used a pair of rod-like electrodes that were placed normal to the cornea, resulting in gene delivery to the entire surface of the cornea, whereas their group used a disc electrode with a point source in the middle to deliver the electric field to the area below the disc. Because of their electrode design, the field is delivered to a defined area less than that of the whole cornea, resulting in expression in 80% of cells within the field, but only 6% of cells over the corneal surface. By comparison, we are obtaining expression in a larger number of cells that are relatively equally dispersed throughout the cornea. That different cell types were transfected in our two studies appears to be a function of the plasmid distribution at the time of electroporation. Because their group injected DNA into the anterior chamber, endothelial cells were bathed on one side with the plasmids and were thus transfected. By contrast, we injected DNA directly into the subconjunctival region or the upper layers of the cornea itself, introducing the plasmids to keratocytes and epithelial cells.
Over the past 3 years, electroporation-mediated delivery of plasmids has been used successfully in a variety of tissues in vivo. These include skeletal muscle [17–19], liver [20,21], blood vessels [23], and the cornea [24,25]. What is surprising is that the optimal field strength for gene delivery and expression in all of these diverse tissue types is strikingly similar when millisecond square-wave pulses are used. Indeed, when gene expression is graphed as a function of the applied field strength, virtually superimposable figures are generated for all the above tissues, with no gene transfer at 0 V/cm, highly variable expression at 100 V/cm, reproducible high-level expression at 200 V/cm, and reduced expression at higher voltages. This is in contrast to the huge variation seen in protocols for the electroporation of mammalian cells in culture [44]. These results suggest that most tissues in vivo behave similarly to the effects of electric fields in terms of their ability to take up and express foreign DNA.
The plasmids delivered into the corneal epithelium and stroma expressed high levels of gene product for 3 days, but by 7 days post-transfer, the levels of gene expression dropped greatly. A similar course of expression was seen in the corneal endothelium by Oshima et al. after anterior chamber injection of DNA and electroporation [25]. Indeed, almost indistinguishable temporal patterns of expression have been seen in many studies in a wide variety of tissues, with the exception of skeletal muscle [17–19], when the same promoter (hCMViep) was used to drive gene expression of different reporter genes using various plasmid backbones [45,46]. This transient in vivo expression seen with plasmids is likely due to inactivation of this promoter, degradation and loss of the vector, or a combination of the two, although the exact mechanisms have not been elucidated. Current work in our laboratory and others is exploring methods to increase the duration of gene expression from plasmids by using various non-viral promoters and other cis-acting sequences to prolong transcription and stabilize the episomes within the nucleus. Thus, the CMV immediate early promoter and enhancer is well suited for short-term expression studies, but is not the choice for long-term expression.
In summary, our results demonstrate that electroporation is an excellent method for delivering genes to multiple cell layers within the murine cornea and results in extremely high levels of gene expression with little, if any, inflammatory response or tissue damage, making this a technique with potential clinical applications.
Acknowledgments
We thank Xiao-Tian Yan for teaching us the corneal injection methods, Troy Stevens for the gift of recombinant adenovirus, and Robert N. Lausch and John E. Oakes for stimulating discussions. This work was supported in part by grant EY12962 from the National Eye Institute of the NIH and by funds from the Lions/USA Eye Research Institute and the Lions International Foundation.
References
- 1.Anderson WF. Human gene therapy. Science. 1992;256:808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan RC. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 3.Kerr WG, Mulé JJ. Gene therapy: current status and future prospects. J Leukocyte Biol. 1994;56:210–214. doi: 10.1002/jlb.56.2.210. [DOI] [PubMed] [Google Scholar]
- 4.Sheffield VC, Stone EM, Alward WL, et al. Genetic linkage of familial open angle glaucoma to chromosome 1q21–q31. Nature Genet. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- 5.Wiggs JL, Haines JL, Paglinauan C, Fine A, Sporn C, Lou D. Genetic linkage of autosomal dominant juvenile glaucoma to chromosome 1q21–q31 in three affected pedigrees. Genomics. 1994;21:299–303. doi: 10.1006/geno.1994.1269. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez IR, Gonzalez P, Zigler JS, Jr, Borrás TA. A guinea pig hereditary cataract contains a splice site deletion in a crystallin gene. Biochim Biophys Acta. 1992;1180:44–52. doi: 10.1016/0925-4439(92)90025-i. [DOI] [PubMed] [Google Scholar]
- 7.Armitage MM, Kivlin JD, Ferrell RE. A progressive early onset cataract gene maps to chromosome 17q24. Nature Genet. 1995;9:37–40. doi: 10.1038/ng0195-37. [DOI] [PubMed] [Google Scholar]
- 8.Eiberg H, Lund AM, Warburg M, Rosenberg T. Assignment of congenital cataract Volkmann type (CCV) to chromosome 1p36. Hum Genet. 1995;9:33–38. doi: 10.1007/BF00214183. [DOI] [PubMed] [Google Scholar]
- 9.Borrás T, Tamm ER, Zigler JS. Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci. 1996;37:1282–1293. [PubMed] [Google Scholar]
- 10.Mashhour B, Couton D, Perricaudet M, Briand P. In vivo adenovirus-mediated gene transfer into ocular tissues. Gene Ther. 1994;1:122–126. [PubMed] [Google Scholar]
- 11.Parker SE, Vahlsing HL, Serfilippi LM, et al. Cancer gene therapy using plasmid DNA: safety evaluation in rodents and non-human primates [see comments] Hum Gene Ther. 1995;6:575–590. doi: 10.1089/hum.1995.6.5-575. [DOI] [PubMed] [Google Scholar]
- 12.Masuda I, Matsuo T, Yasuda T, Matsuo N. Gene transfer with liposomes to the intraocular tissues by different routes of administration. Invest Ophthalmol Vis Sci. 1996;37:1914–1920. [PubMed] [Google Scholar]
- 13.Matsuo T, Masuda I, Yasuda T, Matsuo N. Gene transfer to the retina of rat by liposome eye drops. Biochem Biophys Res Commun. 1996;219:947–950. doi: 10.1006/bbrc.1996.0326. [DOI] [PubMed] [Google Scholar]
- 14.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse BT. Suppression of ongoing ocular inflammatory disease by topical administration of plasmid DNA encoding IL-10. J Immunol. 1997;159:1945–1952. [PubMed] [Google Scholar]
- 15.Noisakran S, Campbell IL, Carr DJJ. Ectopic expression of DNA encoding IFN-a1 in the cornea protects mice from herpes simplex virus type 1-induced encephalitis. J Immunol. 1999;162:4184–4190. [PubMed] [Google Scholar]
- 16.Ausubel FM, Brent R, Kingston RE, et al., editors. Short Protocols in Molecular Biology. John Wiley; New York: 1999. [Google Scholar]
- 17.Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- 18.Mir LM, Bureau MF, Gehl J, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci U S A. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nature Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 20.Heller R, Jaroszeski M, Atkin A, et al. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996;389:225–228. doi: 10.1016/0014-5793(96)00590-x. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Shin BC, Fujikura K, Matsuzaki T, Takata K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett. 1998;425:436–440. doi: 10.1016/s0014-5793(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 22.Harrison RL, Byrne BJ, Tung L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998;435:1–5. doi: 10.1016/s0014-5793(98)00987-9. [DOI] [PubMed] [Google Scholar]
- 23.Martin JB, Young JL, Benoit JN, Dean DA. Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res. 2000;37:372–380. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto T, Oshima Y, Nakagawa K, Ishibashi T, Inomata H, Sueishi K. Target gene transfer of tissue plasminogen activator to cornea by electric pulse inhibits intracameral fibrin formation and corneal cloudiness. Hum Gene Ther. 1999;10:2551–2557. doi: 10.1089/10430349950016889. [DOI] [PubMed] [Google Scholar]
- 25.Oshima Y, Sakamoto T, Yamanaka I, Nishi T, Ishibashi T, Inomata H. Targeted gene transfer to corneal endothelium in vivo by electric pulse. Gene Ther. 1998;5:1347–1354. doi: 10.1038/sj.gt.3300725. [DOI] [PubMed] [Google Scholar]
- 26.Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Ther. 1999;6:1006–1014. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan XT, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39:1854–1862. [PubMed] [Google Scholar]
- 28.Manthorpe M, Hartikka J, Vahlsing HL, Sawdey M. Quantification of plasmid DNA expression in vivo. In: Ferre F, editor. Gene Quantification. Burkhaüser; Cambridge, MA: 1996. [Google Scholar]
- 29.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dean BS, Byrd JN, Jr, Dean DA. Nuclear targeting of plasmid DNA in human corneal cells. Curr Eye Res. 1999;19:66–75. doi: 10.1076/ceyr.19.1.66.5344. [DOI] [PubMed] [Google Scholar]
- 31.Kernacki KA, Berk RS. Characterization of the inflammatory response induced by corneal infection with Pseudomonas aeruginosa. J Ocul Pharmacol. 1994;10:281–288. doi: 10.1089/jop.1994.10.281. [DOI] [PubMed] [Google Scholar]
- 32.Staats HF, Lausch RN. Cytokine expression in vivo during murine herpetic stromal keratitis. J Immunol. 1993;151:277–283. [PubMed] [Google Scholar]
- 33.Yan XT, Zhuang M, Oakes JE, Lausch RN. Autocrine action of IL-10 suppresses proinflammatory mediators and inflammation in the HSV-1-infected cornea. J Leukoc Biol. 2001;69:149–157. [PubMed] [Google Scholar]
- 34.Arancibia-Carcamo CV, Oral HB, Haskard DO, Larkin DF, George AJ. Lipoadenofection-mediated gene delivery to the corneal endothelium: prospects for modulating graft rejection. Transplantation. 1998;65:62–67. doi: 10.1097/00007890-199801150-00012. [DOI] [PubMed] [Google Scholar]
- 35.Oral HB, Larkin DF, Fehervari Z, et al. Ex vivo adenovirus-mediated gene transfer and immunomodulatory protein production in human cornea. Gene Ther. 1997;4:639–647. doi: 10.1038/sj.gt.3300443. [DOI] [PubMed] [Google Scholar]
- 36.Larkin DF, Oral HB, Ring CJ, Lemoine NR, George AJ. Adenovirus-mediated gene delivery to the corneal endothelium. Transplantation. 1996;61:363–370. doi: 10.1097/00007890-199602150-00005. [DOI] [PubMed] [Google Scholar]
- 37.Hudde T, Rayner SA, De Alwis M, et al. Adeno-associated and herpes simplex viruses as vectors for gene transfer to the corneal endothelium. Cornea. 2000;19:369–373. doi: 10.1097/00003226-200005000-00022. [DOI] [PubMed] [Google Scholar]
- 38.Seitz B, Baktanian E, Gordon EM, Anderson WF, LaBree L, McDonnell PJ. Retroviral vector-mediated gene transfer into keratocytes: in vitro effects of polybrene and protamine sulfate. Graefes Arch Clin Exp Ophthalmol. 1998;236:602–612. doi: 10.1007/s004170050129. [DOI] [PubMed] [Google Scholar]
- 39.Seitz B, Moreira L, Baktanian E, et al. Retroviral vector-mediated gene transfer into keratocytes in vitro and in vivo. Am J Ophthalmol. 1998;126:630–639. doi: 10.1016/s0002-9394(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Appukuttan B, Ott S, et al. Efficient and sustained transgene expression in human corneal cells mediated by a lentiviral vector. Gene Ther. 2000;7:196–200. doi: 10.1038/sj.gt.3301075. [DOI] [PubMed] [Google Scholar]
- 41.Krauzewicz N, Cox C, Soeda E, Clark B, Rayner S, Griffin BE. Sustained ex vivo and in vivo transfer of a reporter gene using polyoma virus pseudocapsids. Gene Ther. 2000;7:1094–1102. doi: 10.1038/sj.gt.3301219. [DOI] [PubMed] [Google Scholar]
- 42.Hudde T, Rayner SA, Comer RM, et al. Activated polyamidoamine dendrimers, a non-viral vector for gene transfer to the corneal endothelium. Gene Ther. 1999;6:939–943. doi: 10.1038/sj.gt.3300886. [DOI] [PubMed] [Google Scholar]
- 43.Tanelian DL, Barry MA, Johnston SA, Le T, Smith G. Controlled gene gun delivery and expression of DNA within the cornea. Biotechniques. 1997;23:484–488. doi: 10.2144/97233st06. [DOI] [PubMed] [Google Scholar]
- 44.Knutson JC, Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal Biochem. 1987;164:44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- 45.Yew NS, Wysokenski DM, Wang KX, et al. Optimization of plasmid vectors for high-level expression in lung epithelial cells. Hum Gene Ther. 1997;8:575–584. doi: 10.1089/hum.1997.8.5-575. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler CJ, Felgner PL, Tsai YJ, et al. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proc Natl Acad Sci U S A. 1996;93:11454–11459. doi: 10.1073/pnas.93.21.11454. [DOI] [PMC free article] [PubMed] [Google Scholar]