Abstract
Langerhans cells (LCs) are a specialized subset of epidermal dendritic cells. They represent one of the first cells of immunological barrier and play an important role during the inflammatory phase of acute wound healing. Despite considerable progress in our understanding of the immunopathology of diabetes mellitus and its associated co-morbidities such as diabetic foot ulcers (DFUs), considerable gaps in our knowledge exist. In this study, we utilized the human ex vivo wound model and confirmed the increased epidermal LCs at wound edges during early phases of wound healing. Next, we aimed to determine differences in quantity of LCs between normal human and diabetic foot skin and to learn if the presence of LCs correlates with the healing outcome in DFUs. We utilized immunofluorescence to detect CD207+ LCs in specimens from normal and diabetic foot skin and DFU wound edges. Specimens from DFUs were collected at the initial visit and 4 weeks at the time when the healing outcome was determined. DFUs that decreased in size by >50% were considered to be healing, while DFUs with a size reduction of <50% were considered non-healing. Quantitative assessment of LCs showed a higher number of LCs in healing when compared to non–healing DFU’s. Our findings provide evidence that LCs are present in higher number in diabetic feet than normal foot skin. Healing DFUs show a higher number of LCs compared to non-healing DFUs. These findings indicate that the epidermal immune barrier plays an important role in the DFU healing outcome and may offer new therapeutic avenues targeting LC in non-healing DFUs.
Keywords: Langerhans Cells, Epidermis, Diabetic Foot Ulcers, Chronic Wound
INTRODUCTION
Dendritic cells, a potent group of antigen-presenting cells (APCs), are considered the initiators of the skin immune response [1]. Present in the epidermis, they serve to continuously survey their surroundings for danger signals. A specialized subset of epidermal dendritic cells, Langerhans cells (LCs) form a sophisticated surveillance network in the epidermal layer of the skin and are considered first-line host defenders. Derived from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages [2], adult LCs are maintained through self-renewal or circulating hematopoietic precursor cells [3]. They are recognized under electron microscopy by tennis racket-shaped Birbeck granules. The formation of these unique Birbeck granules is induced by the binding of Langerin, a C-type lectin endocytic receptor encoded by the CD207 gene [4]. In inflamed or injured skin, inflammatory signals produced by various cell types can promote LC activation and maturation. Activated LCs then migrate towards regional lymph nodes where they elicit a primary immune response [5]. Consequently, dendritic cells have been recognized for their role in bridging innate and adaptive immunity.[6] During their migration, LCs proceed through a maturation process, acquiring the surface phenotype of a mature dendritic cell marked by induction of CCR7 and upregulation of CD40, CD83, CD86, MHC and other molecules associated with antigen presentation [7]. The development of antigen-presenting immune cells is mediated by TGFβ1, as well as inhibitor of DNA binding (ID2), and runt-related transcription factor 3 (RUNX3). Mice deficient in these transcription factors demonstrate a loss of LCs [8–10].
LCs have gained recognition for their immunologic role during wound healing. Wound healing is an elegantly executed process orchestrated to restore the integrity of injured tissue. Acute wound healing can be divided into four sequentially overlapping phases: hemostasis, inflammation, proliferation, and remodeling [11]. This well-coordinated sequence of events is regulated by a variety of cells. Immediately following wounding, resident immune cells including dendritic cells, T cell subsets, and mast cells are activated, subsequently releasing signaling molecules to recruit other immune cells[12, 13]. Neutrophils, macrophages and leukocytes then migrate to the site of injury, initiating the robust, but controlled inflammatory response required for successful wound healing. Increasing evidence underscores an important role for immune cells in the process of wound repair. LCs represent an important immune cellular component during the initial stages of acute wound healing. Repopulation of the epidermis with LCs has been shown to occur during the course wound re-epithelialization in murine and porcine models [14, 15]. While our understanding of LC involvement during acute wound healing has advanced considerably in recent years, much remains to be elucidated regarding their function in chronic wounds such as DFUs.
Diabetic foot ulcers, with or without the presence of neuropathy, contribute to a significant number of hospitalizations and amputations and frequently cause morbidity. An estimated 15% of diabetic patients are afflicted with foot ulcers [16, 17], with 84% of amputations are preceeded by ulceration [18]. Ten year mortality rates are increased 50% in patients with DFUs compared to patients without ulceration [19]. Recent studies have revealed the presence of increased skin inflammation in human diabetic skin.[20] Additionally, the correlation of an increased number of skin LCs in patients with neuropathy and diabetes compared to those with diabetes alone suggested a possible role of LCs in the generation and maintenance of neuropathic pain [21]. Although significant differences in the cellular infiltrate of acute, healing wounds versus chronic, non-healing wounds have been reported, [22–24]the correlation with a healing outcome has not been studied yet. Therefore, in the present study, we employed immunofluorescence to correlate epidermal LC presence with DFUs healing outcomes.
MATERIALS AND METHODS
Skin specimens
After Institutional Review Board approval, normal and diabetic foot skin specimens in addition to healthy skin specimens were obtained as discarded tissue from patients 46–72 years of age undergoing elective plastic surgery. Skin specimens derived from non-healing edges of DFUs were collected after University of Miami IRB approval was obtained. Skin specimens from non-healing edges of 12 patients with DFUs presenting to the clinic were collected. DFU specimens were collected from discarded tissue after surgical debridement procedures as described previously [25] at the patient’s initial presentation to the wound healing clinic at week 0 (W0) and 4 weeks after the standard of care (W4). Patients presenting with DFUs were ≥18 years of age (mean age 54 years), had Type 1 or 2 diabetes with a history of neuropathy, and an ulcer greater than 0.5 cm2 with Wagner grade 1 or 2. Wound duration was an average of 16.6 months. Patients that had a hemoglobin A1c ≥ 12%, compromised arterial supply as measured by an ankle brachial index (ABI) less than 0.7 or greater than 1.3, or revascularization to the ipsilateral lower extremity in 6 weeks before presenting to the Wound Clinic were excluded from the study. In addition, the use of investigational wound therapies a month prior to enrollment and/or active bone, soft tissue, skin and/or wound infection at the screening visit were considered exclusion criteria.
Wound size measurement
Wound size was measured weekly by planimetry [26]. Patients with DFUs that showed reduction in size, by >50% at W4 of implementation of standard of care were considered to be “healing DFUs”, while patients with DFUs displaying a size reduction of <30% were considered to be “non-healing DFUs”. This assessment was based on the ability to predict healing outcomes by week 4 using healing status as a surrogate marker[27]. In the current study, we utilized specimens from 6 healing and 6 non-healing DFU’s as determined by planimetry at W4.
Ex Vivo Wound Healing Model
Healthy skin samples obtained from reduction surgeries were used to generate wounds as previously described [28–32]. Briefly, the skin was cleaned and a 3mm punch biopsy was used to create a wound by removing epidermis and papillary dermis. Untreated wounds were maintained at an air-liquid interface and collected at the time of wounding and after 1, 2, 3, 4and 7 days. The acute wound sets, derived from three donors, demonstrating complete healing at day 7 were used for further analysis.
Histology and Immunohistochemistry
Both normal skin and DFU specimens were fixed in formalin and routinely processed for paraffin embedding. Seven-μm sections were cut and stained with hematoxylin and eosin to assess DFU tissue morphology. Healing in the ex-vivo wounds were assessed using routine hematoxylin and eosin staining as previously described[29].
After de-paraffinisation and antigen retrieval, 5μm thick sections were blocked and incubated overnight using Langerin specific antibody (CD207) (Abcam/Dendritics, Cambridge, MA) diluted in DAKO antibody diluent (DAKO, Carpinteria, CA). Positive staining was visualized using a secondary fluorescein isothiocyanate anti-mouse IgG antibody (Invitrogen, Grand Island, NY). All sections were mounted with mounting media containing propidium iodide (Vector Laboratories, Burlingame, CA) to help visualization of the nuclear staining. All negative controls were prepared by omission of the primary antibody. The sections were analyzed using a Nikon Eclipse E800 microscope, and digital images were collected using the NIS Elements camera advanced program.
Quantification of the Langerin positive cells
Quantification of the Langerin positive cells was performed by three blinded lab members. Three to five images were taken per condition and used for manual counting at 20x magnification for total number of stained cells per 1mm of a tissue specimen as previously described[33]. Averages and standard deviations were calculated.
RESULTS
Langerhans Cells are Present During the Early Phases of Acute Wound Healing
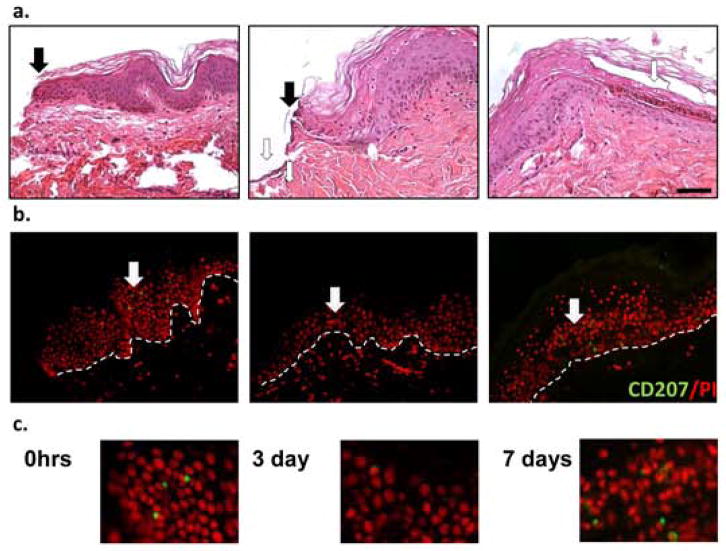
To analyze the presence of LCs during acute wound healing, the human ex vivo wound healing assay was employed. Wounds were created as previously described and maintained at an air-liquid interface for seven days [28–32]. Complete epithelialization was confirmed by hematoxylin and eosin staining (Figure 1a). Acute wound specimens were stained using a Langerin specific antibody. Epidermal localization of LCs at the wound edges was observed during the early phases of wound healing, between 0 and 48 hours after wounding (Figure 1b, c). These findings were consistent with the understanding that LC activation is one of the first events in the skin immune response, with LCs initiating a cascade of innate immune reactions in response to danger signals such as wounding [34]. After 3 days, LCs were significantly reduced in the epidermis of the wound edges (Figure 1b, c). The wounds were fully closed by day 7 post wounding, at the time when the new epithelial barrier begins to mature. At this time, LCs were again evident in the epidermis.
Fig. 1. Number of intra-epidermal LCs changes during a course of acute wound healing.
Hematoxylin and eosin staining of ex vivo human skin wounds at a time of wounding (0hrs) and 3 and 7 days post wounding. Full arrows demarcate a wound edge while empty ones point at migrating epithelial tongue at day 3 and newly formed multilayered epidermis on day 7 post wounding (n=3) (a). Same specimens were stained using CD207/Langerin antibody, a Langerhans cell marker. White arrows point at the positive LCs staining. Dotted line demarcates basement membrane (b). Enlargements of the CD207 positive staining are shown (c). Scale bar 50μm.
Diabetic Foot Skin Shows Higher Number of Langerhans Cells Than Normal Foot Skin
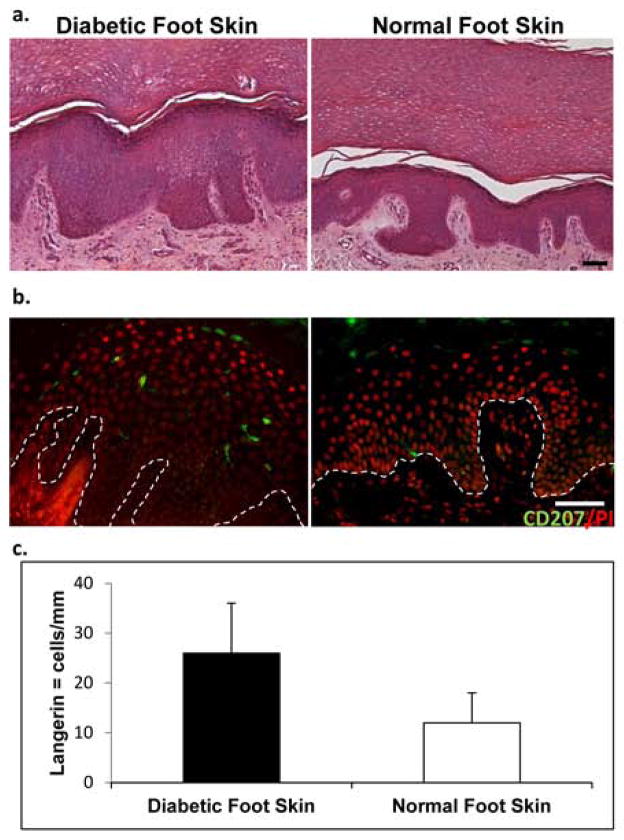
It has been reported that the number of LCs vary in the skin depending on the anatomical location [35]. To assess if the number of LCs differs in diabetic foot skin and normal foot skin, we utilized specimens derived from plantar skin in the presence or absence of diabetes. Morphology of the specimens demonstrated a thick cornified layer, a well-known characteristic of plantar skin (Figure 2a). Specimens were than stained and the number of Langerin-positive cells per mm of tissue was quantified (Figure 2b, c). We observed an increased number of LCs in diabetic foot skin (26±9) when compared to normal foot skin (12± 6).
Fig. 2. Human diabetic plantar foot skin shows increased presence of LCs.
Histology of diabetic and normal plantar foot skin showing thick epidermis and cornified layer (a) Number of 207/Langerin positive cells is increased in diabetic (n=5) when compared to normal foot skin (n=3). (b) A bar graph depicts number of Langerin positive cells quantified per mm of tissue. Data are presented as means ± S.D (c). Scale bar 50μm.
The number of Langerhans Cells can be Correlated with the Healing Outcome of Diabetic Foot Ulcer Patients
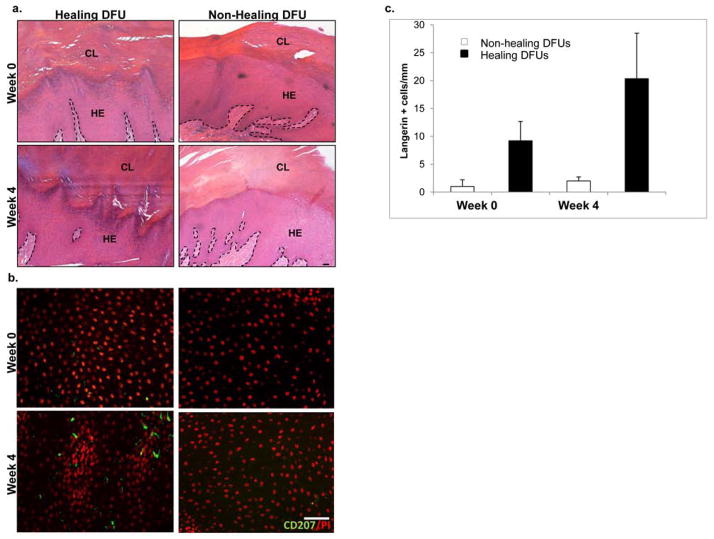
To characterize the phenotype of DFUs and ensure adequate epidermis from which to quantify LCs, we first analyzed the morphology of chronic wounds from patients at time they presented to the clinic at week 0 and four weeks later. Consistent with our previous findings in chronic ulcers [28, 25], DFU specimens harvested at both time points showed epidermal hyperplasia with the presence of hyperkeratosis and parakeratosis (Figure 3a). To analyze the immune response in DFU patients, we determined the presence of LCs by immunofluorescence staining using a Langerin antibody (Figure 3b). Localization of LCs was quantified in healing and non-healing DFUs as determined by planimetry at week 4. We observed that the healing DFUs had higher numbers of LCs when matched to non-healing DFUs. At week 0, healing DFUs showed a higher number of LCs per mm (9±6). At week 4, the number of LCs per mm (20±6) in the healing group was significantly increased compared to all other groups (Figure 3c). These findings may suggest that patients with a healing ulcer phenotype have the ability to shift from a chronic wound phenotype towards that of an acute wound after administration of standard of care, including offloading, removal of non-viable tissue and surgical debridement. Additionally, LCs in healing DFUs resumed dendritic morphology as evidenced in non-wounded tissue.
Fig. 3. Number of Langerhans cells is increased in the epidermis of healing DFUs.
Hematoxylin and eosin staining of healing and non-healing diabetic foot ulcers showing distinctive chronic wound morphology characterized by hyperproliferative epidermis (HE) and thick cornified layer (CL) with a presence of nuclei. Dotted line represents epidermal- dermal junction (a) The most representative images of immunofluorescent staining of CD207/Langerin in non-healing (n=6) and healing (n=6) DFUs at week 0 and 4 weeks after shows increase in number of LCs in healing DFUs at both weeks (b) Quantification of CD207/Langerin positive cells in healing and non-healing DFUs per mm of tissue is shown (c). Scale bar 50μm.
DISCUSSION
The finding of the present study indicates there is an increased number of LCs in the epidermis of non-ulcerated plantar skin derived from diabetic patients. Interestingly, skin derived from wound edges of DFUs show fewer LCs present in the epidermis compared to non- ulcerated plantar skin at the time of initial presentation to the clinic (week 0). However, when correlated to healing outcomes, measured by planimetry at week 4, healing DFUs show increased numbers of LCs compared to week 0 and to non-healing ones at both weeks. This is, to the best of our knowledge, the first study to report an increased number of LCs in non-ulcerated plantar epidermis of patients suffering from diabetes and a decreased number of LCs in DFU wound edge tissue collected at initial presentation to the wound clinic. However, an increased number of LCs found at the wound edges of healing DFUs demonstrates a correlation between LC numbers and DFU healing outcomes, four weeks of implementation of standard of care.
Langerhans cells play an important role in epidermal homeostasis and protect the skin and host against foreign invaders by providing an elaborate epidermal immune surveillance network. It is well known that their activities range from ingesting foreign molecules to priming naive T cells [26]. An understanding of the function of these immature dendritic cells during wound healing is still incomplete. Our findings are consistent with previous reports, suggesting a critical role for LCs in acute wound healing [14, 15, 36]. It is also reported that relative frequency of LCs is inversely related to rate of keratinocyte proliferation[37]. We found a prominent epidermal presence of Langerin positive cells between 0 hours and 48 hours following wounding in the ex-vivo human wound model. This accumulation resolved by 72 hours, when proliferation of keratinocytes ensures, indicating that LC contribution is likely most critical during earlier stages of wound healing. LC repopulation of the wounded area occurred by 7 days post-wounding, after full closure of the denuded wound area which is in agreement with findings reported in a porcine model [15].
Patients with DM are relatively immunocompromised and are thereby susceptible to infectious complications [38–40]. Skin inflammation is also increased in the skin of individuals suffering from diabetes [20]. Previous studies report a difference in accumulation of LCs between normal epidermis from plantar foot skin compared to normal calf skin. Consequently, it is not surprising that we observed a change in epidermal dendritic cell presence in non-ulcerated and ulcerated diabetic foot skin. LCs, specifically, have been shown to accumulate at the edge of DFUs, while reduced numbers are found at the margins of chronic venous ulcers [35]. This is the first study, to our knowledge, correlating the presence of LCs with DFU healing outcomes. We sought to evaluate the patients’ immunologic status by characterizing and quantifying LCs in healing and non-healing DFUs. Our data demonstrate that a decreased presence of epidermal Langerin positive cells was associated with poorer healing outcomes in DFUs. In accordance with our acute ex vivo wound findings, these results suggest that patients with healing DFUs may be able to shift their epidermal immune response towards that of an acute wound. This immunologic phenotype may assist the healers in the wound repair process. Since an increased number of LCs was inversely correlated to the proliferative rate of basal keratinocytes, [37] one can speculate that due to increased LC presence in the healing DFUs, keratinocytes stop proliferating and revert to a normal, differentiating phenotype. A recent study showed a correlation, in skin biopsies derived from above the lateral malleolus, between increased numbers of LCs and diabetes associated with small fiber neuropathy [21]. All of our diabetic wound tissue was taken from patients with neuropathies, however a lower number of langerin positive cells was observed. This discrepancy from the previous report may be due to a difference in anatomical location of skin sampling. Given the aforementioned variability in LCs in different body sites, we compared DFU wound edges with plantar foot skin in attempts to decrease the influence of potential confounding factors.
Our lab has shown that keratinocytes at the non-healing edges of chronic wounds differ significantly from their healthy counterparts. Not only do they display delayed migration and hyperproliferation, but they are also ineffective in their ability to perform cross-talk and signaling with other cells [41, 42]. The production of keratinocyte-derived monocyte chemoattractant protein-1 (MCP-1) by keratinocytes is particularly critical in the recruitment of Langerhans cells to the skin.[43] It may be that our healing DFUs are able to normalize keratinocyte function to produce enough MCP-1 to recruit LCs, while non-healing DFUs cannot. Moreover, a deficiency of MCP-1, produced by keratinocytes at the wound edge, has been shown to result in delayed wound re-epithelialization, which is a characteristic feature of chronic wounds[44].
The implications of an LC deficiency in chronic wounds have not yet been determined. However, we can speculate, based on in vivo studies [7, 45], that the consequences of such an immune-deficiency are significant. LCs have both immune-regulatory and immune-stimulatory functions. While it has been proposed that LCs are potent stimulators of CD4 and CD8 T-cell responses [46, 47], LCs have also been shown to inhibit the proliferation of CD4/CD8 T cells or enhance T regulatory response [7, 48]. LCs have been shown to interact directly with CD4-positive T cells in lymph nodes, secreting IL-10 and thus inhibiting the initiation of T cell activity [7]. Thus, it is probable that, for an optimal functioning immune system, there needs to be a delicate balance in the activity of LCs. The limitation of the current study is the small number of specimens, thus no statistical differences were reached when numbers of LCs were compared between skin specimens.
Taken together, the insufficient population of LCs in DFUs may represent yet another contributing factor to their pathogenesis. Furthermore, an abundance of LCs in DFUs may be used as a predictive biomarker to reliably identify ulcers disposed to heal, while a decreased presence of LCs might help to predict wounds with poorer prognoses. Further studies are needed to expand our understanding of immune cell function in the context of chronic wounds and develop therapies designed to correct or attenuate a deregulated immune system.
Acknowledgments
We thank Dr Anthony LaBruna and Dr Thomas Zwick for providing skin specimens, Shailee Patel and members of the Wound Healing Clinical Research Team for technical assistance. This work is supported by National Institutes of Health (DK086364; NR013881) to MT-C.
REFRENCES
- 1.Gallo PM, Gallucci S. The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity. Frontiers in immunology. 2013;4:138. doi: 10.3389/fimmu.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. The Journal of experimental medicine. 2012;209(6):1167–81. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nature immunology. 2002;3(12):1135–41. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12(1):71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Strbo N, Yin N, Stojadinovic O. Innate and Adaptive Immune Responses in Wound Epithelialization. Advances in Wound Care. 2013 doi: 10.1089/wound.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igyarto BZ, Kaplan DH. The evolving function of Langerhans cells in adaptive skin immunity. Immunology and cell biology. 2010;88(4):361–5. doi: 10.1038/icb.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. The Journal of experimental medicine. 1998;187(6):961–6. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nature immunology. 2003;4(4):380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 10.Fainaru O, Woolf E, Lotem J, Yarmus M, Brenner O, Goldenberg D, et al. Runx3 regulates mouse TGF-beta-mediated dendritic cell function and its absence results in airway inflammation. The EMBO journal. 2004;23(4):969–79. doi: 10.1038/sj.emboj.7600085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer AJ, Clark RA. Cutaneous wound healing. The New England journal of medicine. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 12.Jameson JM, Sharp LL, Witherden DA, Havran WL. Regulation of skin cell homeostasis by gamma delta T cells. Frontiers in bioscience : a journal and virtual library. 2004;9:2640–51. doi: 10.2741/1423. [DOI] [PubMed] [Google Scholar]
- 13.Noli C, Miolo A. The mast cell in wound healing. Veterinary dermatology. 2001;12(6):303–13. doi: 10.1046/j.0959-4493.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 14.Juhasz I, Simon M, Jr, Herlyn M, Hunyadi J. Repopulation of Langerhans cells during wound healing in an experimental human skin/SCID mouse model. Immunology letters. 1996;52(2–3):125–8. doi: 10.1016/0165-2478(96)02596-5. [DOI] [PubMed] [Google Scholar]
- 15.Helfman T, Streilein JW, Eaglstein WH, Mertz PM. Studies on the repopulation of Langerhans cells in partial-thickness wounds. Air exposed and occlusively dressed. Archives of dermatology. 1993;129(5):592–5. [PubMed] [Google Scholar]
- 16.Reiber GE. The epidemiology of diabetic foot problems. Diabetic medicine : a journal of the British Diabetic Association. 1996;13(Suppl 1):S6–11. [PubMed] [Google Scholar]
- 17.Reiber GE, Ledous WE. Epidemiology of diabetic foot ulcers and amputations: evidence for prevention. In: Williams R, Herman W, A-LK, editors. The evidence base for diabetes care. London: John Wiley and Sons; 2002. pp. 641–65. [Google Scholar]
- 18.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes care. 1990;13(5):513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 19.Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabetic medicine : a journal of the British Diabetic Association. 1996;13(11):967–72. doi: 10.1002/(SICI)1096-9136(199611)13:11<967::AID-DIA266>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Tellechea A, Kafanas A, Leal EC, Tecilazich F, Kuchibhotla S, Auster ME, et al. Increased skin inflammation and blood vessel density in human and experimental diabetes. The international journal of lower extremity wounds. 2013;12(1):4–11. doi: 10.1177/1534734612474303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casanova-Molla J, Morales M, Planas-Rigol E, Bosch A, Calvo M, Grau-Junyent JM, et al. Epidermal Langerhans cells in small fiber neuropathies. Pain. 2012;153(5):982–9. doi: 10.1016/j.pain.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Rosner K, Ross C, Karlsmark T, Petersen AA, Gottrup F, Vejlsgaard GL. Immunohistochemical characterization of the cutaneous cellular infiltrate in different areas of chronic leg ulcers. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 1995;103(4):293–9. doi: 10.1111/j.1699-0463.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 23.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. The Journal of investigative dermatology. 1998;111(5):850–7. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 24.Galkowska H, Wojewodzka U, Olszewski WL. Low recruitment of immune cells with increased expression of endothelial adhesion molecules in margins of the chronic diabetic foot ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13(3):248–54. doi: 10.1111/j.1067-1927.2005.130306.x. [DOI] [PubMed] [Google Scholar]
- 25.Stojadinovic O, Landon JN, Gordon KA, Pastar I, Escandon J, Vivas A, et al. Quality assessment of tissue specimens for studies of diabetic foot ulcers. Experimental dermatology. 2013;22(3):216–8. doi: 10.1111/exd.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger CL, Vasquez JG, Shofner J, Mariwalla K, Edelson RL. Langerhans cells: mediators of immunity and tolerance. The international journal of biochemistry & cell biology. 2006;38(10):1632–6. doi: 10.1016/j.biocel.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes care. 2003;26(6):1879–82. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 28.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. The American journal of pathology. 2005;167(1):59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee B, Vouthounis C, Stojadinovic O, Brem H, Im M, Tomic-Canic M. From an enhanceosome to a repressosome: molecular antagonism between glucocorticoids and EGF leads to inhibition of wound healing. Journal of molecular biology. 2005;345(5):1083–97. doi: 10.1016/j.jmb.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Stojadinovic O, Lee B, Vouthounis C, Vukelic S, Pastar I, Blumenberg M, et al. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. The Journal of biological chemistry. 2007;282(6):4021–34. doi: 10.1074/jbc.M606262200. [DOI] [PubMed] [Google Scholar]
- 31.Tomic-Canic M, Mamber SW, Stojadinovic O, Lee B, Radoja N, McMichael J. Streptolysin O enhances keratinocyte migration and proliferation and promotes skin organ culture wound healing in vitro. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15(1):71–9. doi: 10.1111/j.1524-475X.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 32.Olivera S, Tomic-Canic M. Human ex vivo wound healing model. Methods in molecular biology. 2013;1037:255–64. doi: 10.1007/978-1-62703-505-7_14. [DOI] [PubMed] [Google Scholar]
- 33.Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M, et al. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PloS one. 2011;6(4):e18907. doi: 10.1371/journal.pone.0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asahina A, Tamaki K. Role of Langerhans cells in cutaneous protective immunity: is the reappraisal necessary? Journal of dermatological science. 2006;44(1):1–9. doi: 10.1016/j.jdermsci.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Galkowska H, Olszewski WL, Wojewodzka U. Expression of natural antimicrobial peptide beta-defensin-2 and Langerhans cell accumulation in epidermis from human non-healing leg ulcers. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2005;43(3):133–6. [PubMed] [Google Scholar]
- 36.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nature reviews Immunology. 2008;8(12):935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 37.Potten CS, Allen TD. A model implicating the Langerhans cell in keratinocyte proliferation control. Differentiation; research in biological diversity. 1976;5(1):43–7. doi: 10.1111/j.1432-0436.1976.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 38.Hostetter MK. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes. 1990;39(3):271–5. doi: 10.2337/diab.39.3.271. [DOI] [PubMed] [Google Scholar]
- 39.McMahon MM, Bistrian BR. Host defenses and susceptibility to infection in patients with diabetes mellitus. Infectious disease clinics of North America. 1995;9(1):1–9. [PubMed] [Google Scholar]
- 40.Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2012;31(4):379–88. doi: 10.1007/s10096-011-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surgical technology international. 2008;17:105–12. [PubMed] [Google Scholar]
- 42.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura K, Williams IR, Kupper TS. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. The Journal of investigative dermatology. 1995;105(5):635–43. doi: 10.1111/1523-1747.ep12324061. [DOI] [PubMed] [Google Scholar]
- 44.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, et al. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. The American journal of pathology. 2001;159(2):457–63. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23(6):611–20. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone AM, Fathman CG, et al. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. The Journal of experimental medicine. 1989;169(3):1169–78. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, et al. Langerhans cells cross-present antigen derived from skin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7783–8. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutz MB, Dohler A, Azukizawa H. Revisiting the tolerogenicity of epidermal Langerhans cells. Immunology and cell biology. 2010;88(4):381–6. doi: 10.1038/icb.2010.17. [DOI] [PubMed] [Google Scholar]