Abstract
IMPORTANCE
There are no treatments available to slow or prevent the progression of Parkinson disease, despite its global prevalence and significant health care burden. The National Institute of Neurological Disorders and Stroke Exploratory Trials in Parkinson Disease program was established to promote discovery of potential therapies.
OBJECTIVE
To determine whether creatine monohydrate was more effective than placebo in slowing long-term clinical decline in participants with Parkinson disease.
DESIGN, SETTING, AND PATIENTS
The Long-term Study 1, a multicenter, double-blind, parallel-group, placebo-controlled, 1:1 randomized efficacy trial. Participants were recruited from 45 investigative sites in the United States and Canada and included 1741 men and women with early (within 5 years of diagnosis) and treated (receiving dopaminergic therapy) Parkinson disease. Participants were enrolled from March 2007 to May 2010 and followed up until September 2013.
INTERVENTIONS
Participants were randomized to placebo or creatine (10 g/d) monohydrate for a minimum of 5 years (maximum follow-up, 8 years).
MAIN OUTCOMES AND MEASURES
The primary outcome measure was a difference in clinical decline from baseline to 5-year follow-up, compared between the 2 treatment groups using a global statistical test. Clinical status was defined by 5 outcome measures: Modified Rankin Scale, Symbol Digit Modalities Test, PDQ-39 Summary Index, Schwab and England Activities of Daily Living scale, and ambulatory capacity. All outcomes were coded such that higher scores indicated worse outcomes and were analyzed by a global statistical test. Higher summed ranks (range, 5–4775) indicate worse outcomes.
RESULTS
The trial was terminated early for futility based on results of a planned interim analysis of participants enrolled at least 5 years prior to the date of the analysis (n = 955). The median follow-up time was 4 years. Of the 955 participants, the mean of the summed ranks for placebo was 2360 (95% CI, 2249–2470) and for creatine was 2414 (95% CI, 2304–2524). The global statistical test yielded t1865.8 = −0.75 (2-sided P = .45). There were no detectable differences (P < .01 to partially adjust for multiple comparisons) in adverse and serious adverse events by body system.
CONCLUSIONS AND RELEVANCE
Among patients with early and treated Parkinson disease, treatment with creatine monohydrate for at least 5 years, compared with placebo did not improve clinical outcomes. These findings do not support the use of creatine monohydrate in patients with Parkinson disease.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00449865
Parkinson disease is a progressive neurodegenerative disorder that affects approximately 6 million people worldwide and more than one-half million individuals in the United States.1 Parkinson disease–associated morbidity and mortality in the United States contribute $6 billion to health care costs annually.2 Incidence of Parkinson disease is expected to increase over the next decade, but neither a cure nor a treatment is available that has been proven to slow progression. Identification and development of effective therapies for slowing progression of Parkinson disease is a research priority.
In 2001, the National Institute of Neurological Disorders and Stroke (NINDS) created the NINDS Exploratory Trials of Parkinson Disease (NET-PD) program to evaluate therapies to slow the progression of disability. The sponsor used 3 major advisory groups, the Committee to Identify Neuroprotective Agents for Parkinson (CINAPS),3 an Oversight Board, and an independent data and safety monitoring board (DSMB) to guide the operational elements of the NET-PD program. The program consisted of multiple operational groups: a statistical coordinating center, a clinical coordinating center, and a network of 45 clinical investigative sites in the United States and Canada (academic medical centers and Parkinson disease specialty centers). NET-PD investigators and the advisory groups applied CINAPS criteria (preclinical criteria for predicted safety, tolerability, and efficacy)3 to select 4 compounds for study. Futility trials,4–6 which identify compounds unlikely to have therapeutic benefit, were used to narrow the list of candidate compounds for future efficacy trials and to reduce resource commitments.7
Of the 4 compounds, only creatine monohydrate (creatine) was not found to be futile, based on a modified futility analysis of 2 clinical trials.4–6 The NINDS recommended that the NET-PD program evaluate creatine in a large, long-term trial (Long-term Study 1 [LS-1]) of individuals with early, stable Parkinson disease receiving dopaminergic therapy, testing the hypothesis that 5 years of creatine (10 g/d) would slow the rate of clinical disease progression by 1 year, as compared with placebo.
Methods
LS-1 was a multicenter, double-blind, parallel-group, placebo-controlled, 1:1 randomized efficacy trial. Participants were randomized to creatine or placebo within each site (45 total sites). Randomly chosen block sizes were used to approximately balance treatment assignments over time. Randomization lists were generated by the Statistical Coordination Center and provided to the central drug processing unit, which used the list to sequentially process unit packages of drug or placebo for distribution to sites. Sites remained blinded to treatment assignment through the use of a central computer-based randomization module to match drug kit with a randomized participant. Complete details on the trial rationale, eligibility criteria, outcome measures, sample size justification, and approach to analyses have been published8; the study protocol is available in Supplement 1. A summary is presented below.
Intervention
Participants received creatine monohydrate (5 g) or placebo, dispensed as identical 7-g sachets, and the contents were mixed with food and taken twice a day. Investigators, those collecting the data, and participants were unaware of the treatment assignment. All investigators were blinded to creatinine levels and estimated glomerular filtration rate (eGFR) (only the DSMB had access to actual values).
Study Recruitment and Retention
Enrollment occurred from March 13, 2007, to May 28, 2010. Eligible participants were fewer than 5 years from Parkinson disease diagnosis (defined as asymmetric features including bradykinesia plus resting tremor, rigidity, or both) and had taken levodopa or a dopamine agonist for at least 90 days but not longer than 2 years. Continuation of other prescribed Parkinson disease therapy was allowed. Participants were to be followed up for a minimum of 5 years or until the end of the trial (a maximum of 8 years for the first enrolled participants) and encouraged to remain in the study even if they discontinued study drug. Adjustments of Parkinson disease medication were permitted during the trial. The institutional review board(s) approved the study, the study protocol, and the informed consent process and documentation. All patients provided written informed consent.
Primary Outcome Measure
Comparison of clinical decline between treatment groups used a global statistical test (GST)8,9 to analyze 5 measures of Parkinson disease progression. The global outcome combined information on change from baseline in the Modified Schwab and England Activities of Daily Living Scale,10 39-Item Parkinson’s Disease Questionnaire (PDQ-39) Summary Index (PDSI),11,12 ambulatory capacity (the sum of 5 questions from the Unified Parkinson Disease Rating Scale [UPDRS]),8,13 Symbol Digit Modalities Test,14 and the modified Rankin Scale15 at 5 years in a single analysis outcome. The measures of function, activities of daily living, ambulation, cognition, and quality of life were chosen because they are generally thought to be relatively resistant to dopaminergic therapy and are the hallmarks of worsening Parkinson disease.
Secondary outcome measures included change in the total UPDRS score,13 UPDRS subscores, Scales for Outcomes in Parkinson Disease–Cognition,16 EuroQOL instrument,17 Total Functional Capacity,18 Beck Depression Inventory,19 levodopa equivalent daily dose,20 and body mass index.
Sample Size
As described in the design article,8 860 participants per group with 5 years of follow-up would provide 99% power to detect a 1-year difference in clinical progression using a GST,8 if such a difference existed. The prespecified difference of interest detectable with the GST was a global treatment effect9 of 0.1189, which approximately aligned with a difference of a 1-year delay in disability between the 2 treatment groups.8,21 LS-1 had 80% to 85% power for secondary analyses of the individual outcome measures of clinical progression.
Analysis
All analyses were conducted according to the intention-to-treat (ITT) principle. To compute the GST, all measures were coded such that higher values are worse (reverse coding for Modified Schwab and England Activities of Daily Living Scale and Symbol Digit Modalities Test). Next, the summed ranks for a participant were computed by ranking each participant on each measure (across both treatment groups) and then summing the ranks for each participant, such that the summed rank could range from 5 to 5 × N. The mean summed ranks for the 2 treatment groups were compared by fitting a linear mixed model of the summed ranks (dependent variable), adjusting for site as a random effect. The GST has a t-distribution. To describe trends across time, the GST was computed for each year of the trial. Secondary efficacy outcomes were reported as the difference in means (or proportions) between treatment groups, with 95% CIs. Analyses were conducted using SAS version 9.3 (SAS Institute Inc).
Analysis of Efficacy and Futility
Two planned interim analyses for efficacy were conducted after approximately 25% and 50% of the trial participants were eligible for 5 years of follow-up, adjusted for multiple testing using O’Brien-Fleming–type stopping boundaries to constrain the type I error rate at .05 (2-sided).
Interim analyses for futility were conducted using a B-statistic to compute conditional power.22 The smaller the computed value, the lower the probability of rejecting the null hypothesis (declaring a treatment benefit) at the end of the study. A computed value of less than or equal to 20% was prespecified as grounds for consideration of stopping the trial23 based on the projected effect size for the GTE8,9 and on the observed trend.
At the second interim analysis participants were classified into 2 cohorts (cohort 1 [n = 955, participants randomized at least 5 years before July 2013] and cohort 2 [n = 786, participants randomized less than 5 years before July 2013]). Participants who died were included in cohort 1 or cohort 2 based on date of randomization. The second interim analysis was performed on cohort 1.
Missing Values
All randomized participants in cohort 1 were included in the second interim analyses. Participants in either cohort who died prior to 5 years were given the worst possible value (Symbol Digit Modalities Test = 0, modified Rankin Scale = 6, PDSI = 100, ambulatory capacity = 20, Schwab and England Activities of Daily Living = 0), as recommended by the DSMB and sponsor (and included in the final statistical analysis plan). Remaining missing values were imputed using a multivariate method.24 Secondary efficacy outcomes are presented without imputation.
Sensitivity Analyses
Three sensitivity analyses were performed using cohort 1: (1) a per-protocol analysis among cohort 1 participants receiving treatment for at least 4 years (80% of the 5-year study time) and who completed a 5-year visit; (2) a “completers” analysis among cohort 1 participants who completed a 5-year visit regardless of treatment adherence; and (3) an ITT analysis with all missing values (including deaths) imputed using multiple imputation.
Safety Analyses
The DSMB reviewed safety analyses of all trial participants semiannually. In September 2008, the DSMB noted increasing creatinine levels and reductions in eGFR and became concerned that creatine was affecting the reliability of creatinine as a means of monitoring adverse trends in eGFR. Entry criteria were changed to exclude new participants with a baseline eGFR less than 50 mL/min per 1.7 m2. Study drug was also discontinued if participants reached eGFR less than 30 mL/min per 1.7 m2 or if creatinine levels doubled from the baseline value.
Adverse events were classified into modified body systems.8 For the final safety analysis, proportions of adverse events in each modified body system were compared between treatment groups using a χ2 test or Fisher exact test and P value, with P < .01 considered significant. The difference in proportions of deaths between treatment groups was continually tested using a triangular test25 with overall type I error of .05.
Results
The enrollment goal of 860 participants per treatment group was attained, with a total of 1741 enrolled and a total of 1328 (≈ 75%) actively observed participants at study close. At the time of the first interim analysis (September 17, 2012), 28% of the total cohort had reached eligibility for the 5-year follow-up visit. The median follow-up time was 4 years (interquartile range, 3–4.9 years). The conditional power under the planned effect size specified in the original design8 was 0.94 and under the observed 5-year-trend was 0.04. Given conflicting results for futility and the lack of safety concerns at that time, the trial was continued.
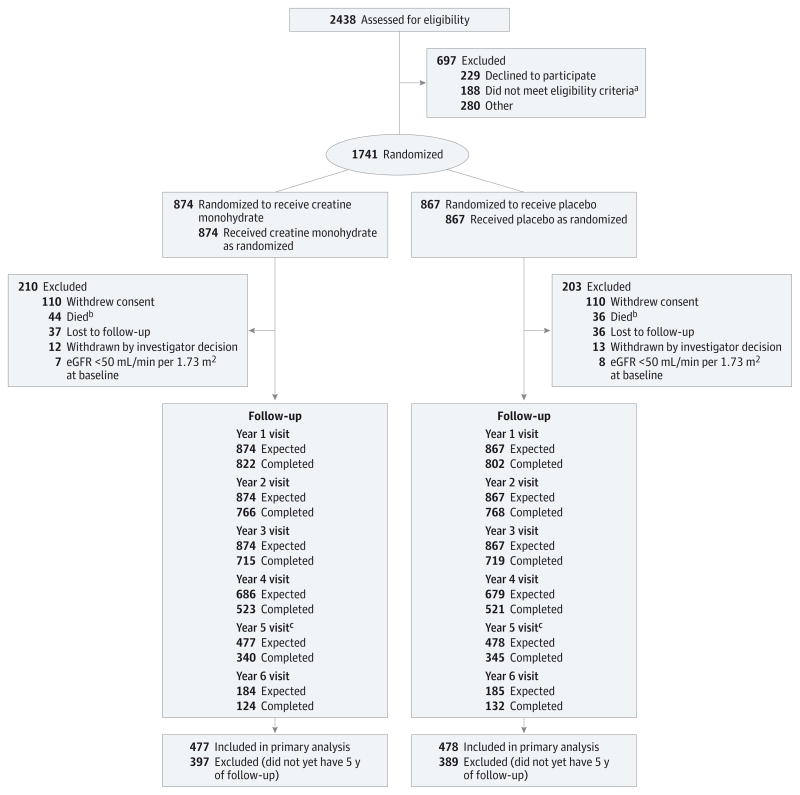
The second interim analysis was conducted on July 17, 2013, after 55% (n = 955; cohort 1) of the participants were eligible for a 5-year follow-up visit. The conditional power under the original design was 0.19 and under the observed 5-year trend was 0.001. Both assumptions met the prespecified stopping criteria (≤0.20). The observed global treatment effect was −0.02. The DSMB reviewed the data on August 27, 2013, and recommended termination of LS-1 for futility. The NINDS accepted the recommendation and notified site investigators and study participants on September 11, 2013. No additional efficacy data were collected beyond this point. The Figure depicts the CONSORT diagram for LS-1 at the time of the second interim analysis.
Figure. CONSORT Diagram of Long-term Study 1 Trial Enrollment Status From Initiation of Screening to the Time of the Interim Analysis on July 17, 2013.
aTaking exclusionary medications (n = 67), not taking dopaminergic therapy (n = 34), diagnosis uncertain (n = 24), medical condition (n = 22), Parkinson disease too advanced (n = 22), enrolled in another study (n = 4), inability to consent (n = 2), non–English-speaking (n = 2), or failure to meet other inclusion criteria (n = 11).
bNine additional deaths occurred after participants withdrew consent (creatine monohydrate, n = 4; placebo, n = 5).
cCohort (cohort 1) used in the interim efficacy analyses.
Table 1 reports the demographic characteristics and baseline clinical measures of all LS-1 participants by treatment group. eTable 1 in Supplement 2 reports these same variables for cohorts 1 (initial 55% enrolled) and 2 (participants enrolled later). Participants in cohort 1 were, on average, 2 years older and had been diagnosed 0.3 years earlier than those in cohort 2. Participants in cohort 1 had lower scores on the baseline Symbol Digit Modalities Test and PDSI. No other significant baseline differences were detected between cohorts.
Table 1.
Baseline Characteristics of All LS-1 Participants (n=1741) by Treatment Groupa
| Demographics | Placebo
|
Creatine
|
||
|---|---|---|---|---|
| No.b | Mean (SD) | No.b | Mean (SD) | |
| Age, y | 867 | 61.5 (9.6) | 874 | 62.1 (9.7) |
|
| ||||
| Men, No. (%) | 867 | 554 (64) | 874 | 569 (65) |
|
| ||||
| Non-Hispanic whites, No. (%) | 867 | 783 (90) | 874 | 788 (90) |
|
| ||||
| Parkinson disease characteristicsc | ||||
|
| ||||
| Time since diagnosis, y | 867 | 1.6 (1.1) | 874 | 1.5 (1.1) |
|
| ||||
| Duration of symptoms, y | 867 | 3.3 (2.2) | 874 | 3.2 (2.2) |
|
| ||||
| Duration of symptomatic therapy, y | 866 | 0.8 (0.7) | 873 | 0.8 (0.7) |
|
| ||||
| Total daily LEDD, mg | 866 | 376 (247) | 872 | 391 (241) |
|
| ||||
| UPDRS | ||||
|
| ||||
| Total | 864 | 25.9 (11) | 868 | 26.5 (11.7) |
|
| ||||
| Mental | 867 | 1.3 (1.4) | 874 | 1.3 (1.4) |
|
| ||||
| ADL | 867 | 7.0 (3.8) | 873 | 7.3 (4.1) |
|
| ||||
| Motor | 864 | 17.6 (8.1) | 869 | 17.9 (8.6) |
|
| ||||
| Ambulatory capacity | 866 | 1.7 (1.5) | 873 | 1.7 (1.5) |
|
| ||||
| Modified Rankin scored | 867 | 1.2 (0.5) | 874 | 1.2 (0.5) |
|
| ||||
| No. (%) | ||||
|
| ||||
| 0 | 12 (1.4) | 11 (1.3) | ||
|
| ||||
| 1 | 680 (78.4) | 664 (76.0) | ||
|
| ||||
| 2 | 163 (18.8) | 182 (20.8) | ||
|
| ||||
| 3 | 12 (1.4) | 17 (2.0) | ||
|
| ||||
| PDQ-39 Summary Index | 865 | 13 (10.7) | 873 | 13.5 (10.6) |
|
| ||||
| Schwab and England Activities of Daily Living | 867 | 91.4 (6.3) | 873 | 90.9 (6.6) |
|
| ||||
| Symbol Digit Modalities Test | 863 | 44.5 (11.6) | 873 | 44.4 (11.8) |
|
| ||||
| Total Functional Capacity | 867 | 12.1 (1.4) | 872 | 12.0 (1.5) |
|
| ||||
| Scales for Outcomes in Parkinson Disease–Cognition | 863 | 30.5 (5.3) | 868 | 30.0 (5.4) |
|
| ||||
| EQ-5D | 867 | 0.8 (0.2) | 874 | 0.8 (0.2) |
|
| ||||
| BDI score | 867 | 6.9 (5.5) | 869 | 6.8 (5.6) |
|
| ||||
| BDI score >17, No. (%) | 867 | 37 (4) | 869 | 46 (5) |
|
| ||||
| BMIe | 863 | 27.9 (5.4) | 868 | 27.9 (8.1) |
Abbreviations: ADL, activities of daily living; BDI, Beck Depression Inventory; BMI, body mass index; EQ-5D, EuroQOL instrument; LEDD, levodopa equivalent daily dose; LS-1, Long-term Study 1; PDQ-39, 39-Item Parkinson’s Disease Questionnaire; UPDRS, Unified Parkinson Disease Rating scale.
Data as of May 5, 2014, final locked database.
Differences in number of participants attributed to missing data.
A higher score indicates a better outcome for Schwab and England, Symbol Digit Modalities, Total Functional Capacity (TFC), Scales for Outcomes in Parkinson Disease–Cognition (SCOPA-Cog), and the EuroQOL instrument (EQ-5D). For all other measures, a higher score indicates a worse outcome. Range of possible scores for each measure: UPDRS Total: 0-176; UPDRS Mental: 0-16; UPDRS ADL: 0-52; UPDRS Motor: 0-108; Ambulatory Capacity: 0-20; Schwab and England Activities of Daily Living: 0%–100%; PDQ-39 Summary Index: 0–100; Symbol Digit Modalities Test: 0-110; TFC: 0-13; SCOPA-COG: 0-43; EQ-5D: 0-1; BDI: 0-63; Modified Rankin: 0-6.
A score of 0 indicates no significant symptoms; 1, no significant disability despite symptoms; 2, slight disability; 3, moderate disability.
Calculated as weight in kilograms divided by height in meters squared.
Adherence
As of July 17, 2013, 668 (76%) of the 874 participants randomized to creatine and 669 (77%) of the 867 participants randomized to placebo remained in the study, but since participants were allowed to remain in the study while not receiving study drug, not all of these individuals were actively receiving treatment. Participants randomized to creatine were more likely to stop study drug (34%) vs participants randomized to placebo (26%). In some cases, stopping study drug was per protocol if participants reached eGFR less than 30 mL/min per 1.7 m2 or creatinine levels doubled (n = 8 in placebo, n = 41 creatine). There was a significant difference between groups regarding time to stopping study drug (P < .001 by log-rank test). eFigure 1 in Supplement 2 displays cumulative time receiving study drug as a percentage of cumulative participant-years of follow-up (71% for creatine vs 79% for placebo).
Interim Efficacy Analysis of Cohort 1
The interim analysis of cohort 1 (n = 955) determined that the mean of the summed ranks of the GST for placebo was 2360 (95% CI, 2249–2470) and for creatine was 2414 (95% CI, 2304–2524). Higher summed ranks indicate worse outcomes. The GST, adjusted for site, yielded t1865.8 = −0.75 (2-sided P = .45) and did not exceed the O’Brien-Fleming critical value of α = .0027. There was no detected benefit or harm attributable to creatine at the time of LS-1 termination.
Table 2 reports the 95% CIs for each of the 5 components of the global score that make up the GST for cohort 1 at 5 years.
Table 2.
Components of the Global Statistical Test by Treatment Group for LS-1 Cohort 1; Change From Baseline to Year 5a
| Components Included in the Computation of Global Outcome | Treatment Group, Mean (SD) | Difference, Mean (95% CI)b | |
|---|---|---|---|
| Placebo (n = 478) | Creatine (n = 477) | ||
| Ambulatory capacity score | 2.8 (5.0) | 3.1 (5.5) | −0.3 (−1.0 to 0.4) |
| Modified Rankinc | 2.1 (1.5) | 2.2 (1.6) | −0.1 (−0.3 to 0.1) |
| PDQ-39 Summary Index | 13 (23.2) | 14.2 (23.5) | −1.2 (−4.2 to 1.7) |
| Schwab and England ADLd | 14.8 (26.0) | 16.8 (28.3) | −2.0 (−5.5 to 1.5) |
| Symbol Digit Modalitiesd | 4.5 (16.8) | 4.9 (17.7) | −0.4 (−2.7 to 1.8) |
Abbreviations: ADL, activities of daily living; LS-1, Long-term Study 1; PDQ-39, 39-Item Parkinson’s Disease Questionnaire.
Cohort 1 includes those participants (n = 955) eligible for a 5-year follow-up visit at the time of interim analysis (July 17, 2013). Missing values are imputed.
Placebo-treatment as reference group.
Modified Rankin is the actual score at 5 years. All others outcomes are change from baseline to 5 years.
Reverse coded such that higher scores indicate worse outcomes. Higher raw values are worse for all outcomes.
eFigure 2 in Supplement 2 reports the GST test statistics by year of follow-up. Each time point includes any randomized participant from cohort 1 or 2 eligible for that visit. Comparing the GST to an approximate critical value of 1.96 (t distribution), unadjusted for multiple comparisons, a difference between groups was not detected for any year of follow-up. Missing data were imputed (see Methods).
Sensitivity Analyses
All sensitivity analyses for cohort 1 were consistent with the primary analysis results suggesting no detectable benefit of creatine (per-protocol analysis: GST = −0.11, P = .92; completers analysis: GST = −0.67, P = .50; multiple imputation of all data including deaths: GST = −0.44, P = .66).
Secondary Outcomes
Table 3 shows the mean at year 5 or the mean change from baseline to year 5 of the secondary outcomes for cohort 1 by treatment group and mean differences between treatment groups at 5 years with 95% CIs. No differences were detected in the total levodopa equivalent daily dose20 by treatment group (t720 = 1.5, 2-sided P = .14) in cohort 1. eFigure 3 in Supplement 2 shows the change in total UPDRS over time. Both groups display an improvement at 3 months, but the 95% CIs for each treatment group are overlapping at each time point.
Table 3.
Secondary Outcome Measures for Cohort 1a
| Outcomes | Placebo
|
Creatine
|
Difference, Mean (95% CI) | ||
|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | ||
| Total LEDD, (mean at year 5), mgb | 365 | 782 (408) | 366 | 738 (401) | 45 (−14 to 103) |
|
| |||||
| UPDRS (mean change)c | |||||
|
| |||||
| Total | 336 | 10.4 (13.8) | 330 | 11.3 (15.3) | −0.9 (−3.1 to 1.3) |
|
| |||||
| Mental | 339 | 1.1 (1.8) | 333 | 1.2 (1.9) | −0.1 (−0.4 to 0.1) |
|
| |||||
| ADL | 339 | 4.0 (5.1) | 333 | 4.5 (5.7) | −0.5 (−1.3 to 0.3) |
|
| |||||
| Motor | 336 | 5.3 (9.8) | 330 | 5.6 (10.2) | −0.2 (−1.8 to 1.3) |
|
| |||||
| Total functional capacity (mean change)c | 343 | −1.7 (2.4) | 334 | −1.9 (2.7) | 0.2 (−0.2 to 0.6) |
|
| |||||
| Scales for Outcomes in Parkinson disease–Cognition (mean change)c | 315 | −2.0 (4.9) | 309 | −1.9 (5.4) | −0.1 (−0.9 to 0.7) |
|
| |||||
| EQ-5D (mean change)c | 342 | −0.1 (0.2) | 334 | −0.1 (0.2) | 0.005 (−0.03 to 0.04) |
|
| |||||
| BDI score (mean at year 5)c | 335 | 8.5 (6.7) | 329 | 8.6 (6.3) | −0.1 (−1.1 to 0.9) |
|
| |||||
| BDI score >17 (at year 5), No. (%)b | 335 | 29 (8.7%) | 329 | 29 (8.8%) | 0.002 (−0.04 to 0.04) |
|
| |||||
| BMI, mean changec,d | 341 | −0.4 (3.3) | 338 | −0.1 (2.9) | −0.3 (−0.8 to 0.2) |
Abbreviations: ADL, activities of daily living; BDI, Beck Depression Inventory; BMI, body mass index; EQ-5D, EuroQOL instrument; LEDD, levodopa equivalent daily dose; UPDRS, Unified Parkinson Disease Rating scale.
Data reported from final interim analysis (July 17, 2013) with the exception of BMI and total LEDD, which are reported from the final locked database (May 5, 2014).
Values are means at year 5; BDI score greater than 17 is the difference in proportions at year 5.
Values are mean change from baseline to year 5.
Calculated as weight in kilograms divided by height in meters squared.
Safety
There was no significant difference in deaths (occurring prior to July 17, 2013) by treatment group (n = 44 creatine; n = 36, placebo) among all randomized participants. No stopping boundaries were crossed (triangular test, P = .036 by log-rank test). eTable 2 in Supplement 2 presents cause of death by body system. Among all randomized participants, 21 randomized to creatine and 28 randomized to placebo underwent deep brain stimulation surgery for Parkinson disease. There were no detectable differences (P < .01 to partially adjust for multiple comparisons) in adverse and serious adverse events by body system (Table 4). Heat maps by treatment group show an immediate increase in creatinine levels in the creatine group at the first post-baseline visit, followed by stabilization (eFigure 4 in Supplement 2). No notable changes by treatment group occurred in other laboratory values over time. Heat maps show no detectable differences from baseline to 5 years in BMI category by treatment group (eFigure 5 in Supplement 2). No treatment differences were detected in change in BMI from baseline to 5 years (t677 = −1.31, P = .20).
Table 4.
Frequency of Adverse Events and Serious Adverse Events by Modified Body System for all LS-1 Participants (n=1741)a
| Modified Body Systemb | Participants, No. (%) | P Value (2-Sided)c | |
|---|---|---|---|
| Placebo | Creatine | ||
| General disorders and administration site conditions | 522 (60) | 526 (60) | .99 |
| Nerve/muscle | 437 (50) | 478 (55) | .07 |
| Nervous system | 409 (47) | 458 (52) | .03 |
| Gastrointestinal | 406 (47) | 412 (47) | .90d |
| Infections and infestations | 368 (42) | 353 (40) | .38 |
| Renal and urinary | 368 (42) | 321 (37) | .02 |
| Psychiatric disorders | 322 (37) | 335 (38) | .61 |
| Injury, poisoning, and procedural complications | 316 (36) | 308 (35) | .60 |
| Respiratory | 305 (35) | 333 (38) | .21 |
| Vascular | 294 (34) | 254 (29) | .03 |
| Bone/joint | 233 (27) | 215 (25) | .28 |
| Metabolism and nutrition | 228 (26) | 233 (27) | .86 |
| Skin | 222 (26) | 226 (26) | .9 |
| Cardiac | 189 (22) | 172 (20) | .28 |
| Eye disorders | 155 (18) | 162 (19) | .72 |
| Reproductive | 143 (16) | 123 (14) | .16 |
| Neoplasm | 142 (16) | 133 (15) | .51 |
| Blood | 141 (16) | 145 (17) | .85 |
| Surgical and medical procedures | 131 (15) | 146 (17) | .36d |
| Endocrine | 69 (8.0) | 78 (8.9) | .47 |
| Hepatobiliary | 55 (6.3) | 61 (7.0) | .60 |
| Ear | 42 (4.8) | 41 (4.7) | .88 |
| Immune | 22 (2.5) | 32 (3.7) | .18 |
| Investigations | 12 (1.4) | 20 (2.3) | .16 |
| Congenital, familiale | 4 (0.5) | 0 | .06d |
| Pregnancy | 1 (0.1) | 0 | .50d |
Abbreviation: LS-1, Long-term Study 1.
Data reported from final interim analysis (July 17, 2013).
A single event may be classified in more than 1 modified body system.
By χ2 test, unless otherwise indicated.
By Fisher exact test.
The congenital body system includes congenital, familial, and genetic conditions affecting the participant, discovered during the course of the study. These include congenital coronary artery malformation, Gilbert syndrome, factor V deficiency, and diastematomyelia.
Discussion
LS-1, with 1741 participants, was one of the largest clinical trials for Parkinson disease to our knowledge. Creatine was initially considered because of evidence that it plays an important role in cellular energy production, which may be impaired in Parkinson disease. Deficits in complex I activity in platelets of patients with early Parkinson disease26,27 and in post mortem substantia nigra pars compacta tissue of patients with more advanced disease28 have been identified. Oral creatine supplementation in mice protected against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced dopamine depletion, suggesting a neuroprotective effect.29,30 Additionally, preclinical and clinical evidence suggested that creatine would be well tolerated. Based on these data, the NINDS, CINAPS, oversight board, and DSMB recommended starting a futility trial. The initial futility trial of creatine showed a possible benefit in terms of the UPDRS both at 1 year4 and 18 months.6 The analysis of 18-month futility data6 included all participants regardless of dopaminergic and other Parkinson disease therapies and showed a continuing benefit of creatine based on the total UPDRS. Yet despite the available preclinical and clinical evidence, creatine failed to slow the clinical progression of Parkinson disease as measured across 5 domains of Parkinson disease measured in the long-term clinical trial.
Although futility studies can eliminate therapies that are highly unlikely to be successful in an efficacy trial, futility studies are generally not designed with sufficient power to assess positive findings. Compounds evaluated under a mechanistic algorithm may also fail in subsequent adequately powered efficacy testing. Failure to find a treatment effect in this trial may have been related to the creatine dosage or to a change in the stage of Parkinson disease studied compared with the futility study (use of a de novo placebo group unexposed to any dopaminergic therapy in the futility study vs early in the course of Parkinson disease but requiring coadministered potent dopaminergic therapy).
Strengths of the Trial
We enrolled 1741 participants and were able to retain more than 76% in this 5-year trial at the time the study was stopped. We chose novel measures of Parkinson disease progression because we believed no single outcome measure captured the progressive disability in Parkinson disease and also used a GST to combine information from these outcomes, giving us greater than 99% power to detect treatment effects. The chosen creatine dosage of 10 g/d was generally well tolerated. Despite early concerns that creatine exposure could be associated with deterioration of renal function or weight gain, long-term creatine use did not appear to adversely affect renal function or body mass index. The stabilization in creatinine levels that followed the initial rise, in the setting of continued creatine use, suggests that the initial increase represents an artifact of treatment rather than a sudden onset of renal disease.
Limitations of the Trial
The observed annual rate of progression on the individual measures was slower than anticipated in our power analysis for Symbol Digit Modalities Test (1.5 points expected, 1 point observed) and PDSI (3 points expected, 2.5 points observed), and as expected for the other measures (Schwab and England Activities of Daily Living, 2–3 points per year; ambulatory capacity, 0.5 points per year; and Modified Rankin Scale, a doubling from 1 to 2 over 5 years). Variability in the rate of progression over 5 years was higher than anticipated,8 but the PDSI progression in another large Parkinson disease trial was similar.31 However, our failure to find a benefit was not attributable to reduced power, given the high power of the GST even in the presence of increased variability.
Second, although futility testing eliminated 3 other interventions, with only creatine demonstrating sufficient promise to go forward, creatine still did not show a benefit. We used the futility trial clinical screening approach, rather than a continued focus on assessment in animal models. A mechanistic approach would attempt to confirm that an agent engages its known molecular target and has an intended effect on downstream biology or pharmacology. We proceeded directly to a clinical approach (futility studies) because the mechanisms and molecular targets in Parkinson disease remain unclear. Until such targets are well established, the screening of compounds with futility studies without prior mechanistic studies is useful to identify clearly futile compounds. Future futility studies in cohorts of patients with early Parkinson disease may consider testing new treatments against a background of other nondopaminergic therapies such as monoamine oxidase inhibitors to raise the threshold for success required to take a new treatment forward to a long-term trial.
It is also possible that the study used too low a dose of creatine. Because the loss of the reliability of serum creatinine as a marker of kidney function is a likely adverse consequence of creatine use in clinical practice with older adults, we studied a total dosage of creatine (10 g/d) used in the futility study. Another dosage could have different beneficial or harmful effects; however, concerns about tolerability and masking of adverse kidney consequences limited the dosage used in the study.
Initial short-term futility studies were conducted in participants with early, untreated Parkinson disease. The initial futility studies did not enroll treated participants, because the slow rate of functional change while receiving dopaminergic treatment requires a large sample size and long follow-up, not feasible for a short-term futility study. In our trial we studied early, treated Parkinson disease because most patients with Parkinson disease would require early treatment during the course of a 5-year trial, thus making it more difficult to observe differences between groups over time. The treated phase is often associated with the most disability, and demonstrating a treatment effect during this phase would have a greater clinical and public health benefit. We also did not preclude the concurrent use of other Parkinson disease treatments, relying on randomization to provide some balance between treatment groups.
With respect to adherence, at the time of analysis 34% of participants randomized to creatine had stopped medication and 5% had stopped per protocol. Only 26% of those randomized to placebo stopped medication, and less than 1% stopped per protocol. A completers’ analysis of the subset continuing to take their medication for at least 4 years and with a 5-year visit gave results similar to analysis of the ITT cohort.
Conclusions
Among participants with early Parkinson disease and treated with background dopaminergic therapy, treatment with creatine monohydrate for at least 5 years, compared with placebo, did not improve clinical outcomes. These findings do not support the use of creatine monohydrate in such patients with Parkinson disease.
Supplementary Material
Acknowledgments
Funding/Support: Financial support for the LS-1 study was provided by National Institute of Neurological Disorders and Stroke (NINDS) grant U01NS43128.
Role of the Funder/Sponsor: The NINDS had input as to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication (required prior review by the NINDS data and safety monitoring board).
Affiliations of Authors/Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators
University of Rochester, Rochester, New York (Kieburtz, A. H. Augustine, E. U. Augustine, Kamp); University of Texas Health Science Center at Houston (Tilley, Luo, Pérez, Rajan); Medical University of South Carolina, Charleston (Elm); National Institutes of Health, Bethesda, Maryland (Babcock); University of South Florida, Tampa (Hauser); Pacific Health Research and Education Institute, Honolulu, Hawaii (Ross, Petrovitch); University of California, San Francisco (Aminoff, Christine, Roth); State University of New York Downstate Medical Center, Brooklyn (Bodis-Wollner); University of Vermont, Burlington (Boyd); University of Kentucky, Lexington (Cambi); University of Michigan, Ann Arbor (Chou); University of Maryland School of Medicine, Baltimore (Cines); University of Pennsylvania, Philadelphia (Dahodwala, Reichwein); University of Calgary, Calgary, Alberta, Canada (Derwent); University of Texas Southwestern Medical Center, Dallas (Dewey); University of Southern California, Los Angeles (Hawthorne, Lew); Ochsner Medical Center, New Orleans, Louisiana (Houghton); University of Colorado Denver, Aurora (Leehey); The Parkinson’s Institute and Clinical Center, Sunnyvale, California (Liang, Tanner); Johns Hopkins University, Baltimore, Maryland (Mari); Georgia Regents University, Augusta (Morgan); Struthers Parkinson’s Center, Golden Valley, Minnesota (Parashos); Thomas Jefferson University, Philadelphia, Pennsylvania (Schneider); Rush University Medical Center, Chicago, Illinois (Shannon); Beth Israel Deaconess Medical Center, Boston, Massachusetts (Simon); Northwestern University, Chicago, Illinois (Simuni, Williams); University of Miami, Miami, Florida (Singer); Brigham and Women’s Hospital, Boston, Massachusetts (Sudarsky, Umeh, Wills).
NET-PD Steering Committee
Karl Kieburtz, MD, MPH (principal investigator, coordination center), University of Rochester, Rochester, New York; Barbara Tilley, PhD (principal investigator, statistical center), University of Texas, Houston; Debra Babcock, PhD, MD, and Wendy Galpern, MD, PhD, National Institutes of Health, Bethesda, Maryland; Robert Hauser, MD, University of South Florida, Tampa; Connie Kawai, RN, BSN, CCRC, University of Southern California, Los Angeles; Brad A. Racette, MD, Washington University School of Medicine, St Louis, Missouri; Bernard Ravina, MD, MSCE, Voyager Therapeutics Inc, Cambridge, Massachusetts; Sue Reichwein, CCRC, University of Pennsylvania, Philadelphia; G. Webster Ross, MD, Pacific Health Research and Education Institute, Honolulu, Hawaii; Kathleen M. Shannon, MD, Rush University Medical Center, Chicago, Illinois; Oksana Suchowersky, MD, University of Calgary, Alberta, Canada; Caroline M. Tanner, MD, PhD, The Parkinson’s Institute, Sunnyvale, California; Jessie Tatsuno Roth, RN, BSN, University of California San Francisco. NET-PD Statistical Center (University of Texas Health Science Center at Houston): Keith Burau, PhD; Jordan Elm, PhD; Rong Ye, MS; and Adriana Pérez, MS, PhD. NET-PD Clinical Trials Coordination Center Staff (University of Rochester, Rochester, New York): Debbie Baker, AAS; Liana Baker, MPH; Susan Bennett, AAS; Lisa DeBlieck, MPA, CCRC; Debbie Frasier, BS; Irenita Gardiner, RN; Jennifer Harman, PhD, CCRP, CCRC; Cornelia Kamp, MBA; Laith Khadim, MD; Gina Lau, BS; Beverly Olsen, BA; Saloni Sharma, MD; David Shprecher DO; Ann Stoutenburg, CCRC; Christine Weaver, CCRP; and Renee Wilson, MA. NET-PD Consultants: Christopher Goetz, MD, Rush University Medical Center, Chicago, Illinois; David Ploth, MD, Medical University of South Carolina, Charleston. Data and Safety Monitoring Board: Cynthia R. Gross, PhD (chair), University of Minnesota, Minneapolis; Karen L. Bell, MD, Columbia University, New York, New York; Donna T. Chen, MD, MPH, University of Virginia Health System, Charlottesville; Robert Foley, MD, United States Renal Data System Coordinating Center, Minneapolis, Minnesota; David E. Levy, MD, Weill Cornell Medical College, New York, New York; Robert L. Rodnitzky, MD, University of Iowa College of Medicine, Iowa City. Oversight Board: K. Michael Welch, MD (chair), Rosalind Franklin University of Medicine and Science, North Chicago, Illinois; M. Flint Beal, MD, Weill Medical College of Cornell University, New York, New York; Jeffrey L. Cummings, MD, University of California, Los Angeles, Alzheimer Disease Center; Diane DiEuliis, PhD, Health and Human Services, Washington, DC; David J. Edwards, PharmD, Wayne State University, Detroit, Michigan; Stanley Fahn, MD, and Bruce Levin, PhD, Columbia University, New York, New York; Russell G. Katz, MD, US Food and Drug Administration, Rockville, Maryland; Deborah B. Marin, MD, and C. Warren Olanow, MD, Mount Sinai School of Medicine, New York, New York; Jeffrey C. Martin, Esq, Goodwin Proctor LLP, Rockville, Maryland; Steven Piantadosi, MD, PhD, Cedars-Sinai Medical Center, Los Angeles, California; William J. Powers, MD, University of North Carolina School of Medicine, Chapel Hill; Alison Wichman, MD, National Institutes of Health, Bethesda, Maryland. NIH (National Institute of Neurological Disorders and Stroke [NINDS] Bethesda, Maryland): Debra Babcock, PhD, MD; Wendy Galpern, MD, PhD; John Marler, MD; Claudia Moy, PhD; Joanne Odenkirchen, MPH.
Author Contributions
Drs Elm and Tilley had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kieburtz, Tilley, Elm, Babcock, Hauser, Dewey, Kamp, Simon, Tanner.
Acquisition, analysis, or interpretation of data: Kieburtz, Elm, Hauser, Ross, A. Augustine, E. Augustine, Aminoff, Bodis-Wollner, Boyd, Cambi, Chou, Christine, Cines, Dahodwala, Derwent, Dewey, Hawthorne, Houghton, Kamp, Leehey, Lew, Liang, Luo, Mari, Morgan, Parashos, Perez, Petrovitch, Rajan, Reichwein, Roth, Schneider, Shannon, Simon, Simuni, Singer, Sudarsky, Tanner, Umeh, Williams, Wills.
Drafting of the manuscript: Kieburtz, Tilley, Elm, A. Augustine, E. Augustine, Aminoff, Hawthorne, Liang, Mari, Perez, Williams.
Critical revision of the manuscript for important intellectual content: Kieburtz, Elm, Babcock, Hauser, Ross, A. Augustine, E. Augustine, Aminoff, Bodis-Wollner, Boyd, Cambi, Chou, Christine, Cines, Dahodwala, Derwent, Dewey, Houghton, Houghton, Kamp, Leehey, Lew, Liang, Luo, Morgan, Parashos, Petrovitch, Rajan, Reichwein, Roth, Schneider, Shannon, Simon, Singer, Simuni, Sudarsky, Tanner, Umeh, Wills.
Statistical analysis: Tilley, Elm, Luo, Perez.
Obtained funding: Tilley, Ross, Boyd, Dewey, Kamp, Leehey, Petrovitch, Shannon, Simon.
Administrative, technical, or material support: Kieburtz, Babcock, A. Augustine, E. Augustine, Aminoff, Kamp, Mari, Perez, Williams.
Study supervision: Kieburtz, Tilley, Bodis-Wollner, Dewey, Kamp, Luo, Mari.
Footnotes
Additional Contributions: We would like to acknowledge the long-term efforts and commitment made by the LS-1 participants and their caregivers.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Kieburtz reported serving as a consultant for National Institutes of Health (NIH) (National Institute of Neurological Disorders and Stroke [NINDS]), the US Food and Drug Administration (FDA), the US Veterans Administration, Acorda, Astellas Pharma, AstraZeneca, Auspex, Biotie, Britannia, Cangene, CHDI Foundation, Civitas, Clintrex, Cynapsus, INC Research, Intec, Isis, Lilly, Lundbeck, Medavante, Medivation, Melior Discovery, Neuroderm, Omeros, Otsuka, Pharm2B, Prothena/Neotope/Elan Pharmaceutical, Roche/Genentech, Sage Bionetworks, Serina, Stealth Peptides, Synagile, Teikoku Pharma, Titan, Upsher-Smith, US WorldMeds, Vaccinex, Voyager, and Weston Brain Institute; and receiving grants or research support from the NIH (National Eye Institute, NINDS, National Institute on Aging [NIA], Eunice Kennedy Shriver National Institute of Child Health and Human Development), the Michael J. Fox Foundation, and Teva. Dr Tilley reported receiving grant support from NINDS and receiving travel funding from the CHDI Foundation. Dr Elm reported receiving research grant support from NINDS and serving as a consultant for Teva. Dr Hauser reported receiving honoraria or payments for consulting AbbVie, Allergan, AstraZeneca, Biotie Therapeutics, Ceregene, Chelsea Therapeutics, Cleveland Clinic, Eli Lilly, GE Healthcare, Impax Laboratories, Neurocrine, Indus, Ipsen Biopharmaceuticals, Lundbeck, Merck/MSD, Noven Pharmaceuticals, Pfizer, Straken Pharmaceuticals, Targacept, Teva Pharmaceuticals Industries Ltd, Teva Neuroscience, Upsher-Smith Laboratories, GLG, UCB Pharma SA, University of Houston, US WorldMeds, Xenoport, Zambon Company, SpA, Pricespective LLC, HealthLogix, and Gerson Lehrman Group Inc. Dr Ross reported receiving funding support from NINDS. Dr E. U. Augustine reported receiving funding from NINDS. Dr Aminoff reported receiving research support from NINDS (U10 NS044460; AAV-hAADC-2-003 [site principal investigator]) and the National Parkinson Foundation (NPF). Dr Boyd reported receiving personal fees from Lundbeck and Auspex and receiving grants from Auspex, the Michael J. Fox Foundation, Parkinson Disease Foundation, AbbVie, CHDI Foundation, and NINDS. Dr Chou reported receiving research support from the NIH (NS44504-08) and serving as a consultant to Medtronic. Dr Dahodwala reported receiving grants from NINDS, NIA, Teva, and the Parkinson Council. Dr Dewey reported serving as a consultant for Teva Pharmaceuticals, US WorldMeds, Lundbeck, Acadia, Merz, Xenoport, and GE Healthcare and receiving speakers fees from Teva Pharmaceuticals, US WorldMeds, and UCB. Ms Kamp reported receiving a grant from the University of Rochester. Dr Leehey reported receiving a grant from the NIH and receiving personal fees from Guidepoint Global, Gerson Lehman Group, MEDAcorp, and the Health Practices Institute. Dr Lew reported serving as a consultant for Teva Neurosciences, Baxter, Auspex, UCB, Impax, US WorldMeds, and Ipsen. Dr Liang reported receiving a grant from NINDS to the Parkinson’s Institute; receiving research support from Impax Pharmaceuticals, Teva Neuroscience, the Michael J. Fox Foundation, Novartis, NPF, Merck-Serono, Merck, Adamas, Kyowa, Chelsea Therapeutics, Berg Pharma, and Acadia; receiving an honorarium from Teva Neuroscience; and that she is currently employed by, and holds stock options in, Neurocrine Biosciences. Dr Luo reported receiving grants from the NIH, the Movement Disorder Society, and the CHDI Foundation. Dr Mari reported that his institution has received grant support from the NIH, NPF, Michael J. Fox Foundation, AVID, and AbbVie. Dr Morgan receiving grant support from the NIH and NPF; receiving speaking fees from NPF and Teva; and serving as a consultant for Teva, Impax Labs, Veloxis, Lundbeck, UCB, and Noven. Dr Morgan reported receiving grants from the NIH and the NPF and receiving speaking and consulting fees from Teva. Dr Pérez reported receiving grant support from the NIH. Dr Petrovich reported receiving grant support from NINDS. Dr Simon reported receiving grant support from NINDS. Dr Simuni reported receiving research support from NPF, Teva, Impax, Synosia, Auspex, Serono, Phytopharm, NIH, Michael J. Fox Foundation, the Dixon Foundation, and the Parkinson Associated Risk Study; receiving consulting fees from Acadia, AbbVie, Allergan, Boehringer Ingelheim, GE Medical, Eli Lilly, Harbor, Ibsen, Impax, Lundbeck, Merz, NPF, Navidea, Teva, UCB, and US WorldMeds; receiving honoraria from Allergan, GE Medical, Ibsen, Teva, and UCB; and receiving educational grant support from GE Medical and Teva. Dr Sudarsky reported receiving a grant from the NIH. Dr Tanner reported receiving grant support from NINDS and serving as a consultant for Adamas (fees paid to institution), Impax (fees paid to institution), and Pfizer. No other authors reported disclosures.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28(3):311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 3.Ravina BM, Fagan SC, Hart RG, et al. Neuroprotective agents for clinical trials in Parkinson’s disease: a systematic assessment. Neurology. 2003;60(8):1234–1240. doi: 10.1212/01.wnl.0000058760.13152.1a. [DOI] [PubMed] [Google Scholar]
- 4.NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66(5):664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 5.NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology. 2007;68(1):20–28. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 6.NINDS NET-PD Investigators. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31(3):141–150. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilley BC, Palesch YY, Kieburtz K, et al. NET-PD Investigators. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66(5):628–633. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 8.Elm JJ NINDS NET-PD Investigators. Design innovations and baseline findings in a long-term Parkinson’s trial: the National Institute of Neurological Disorders and Stroke Exploratory Trials in Parkinson’s Disease Long-Term Study-1. Mov Disord. 2012;27(12):1513–1521. doi: 10.1002/mds.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang P, Goetz CG, Woolson RF, et al. Parkinson Study Group. Using global statistical tests in long-term Parkinson’s disease clinical trials. Mov Disord. 2009;24(12):1732–1739. doi: 10.1002/mds.22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwab R, England A. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham F, Donaldson I, editors. Third Symposium on Parkinson’s Disease. Edinburgh, Scotland: E & S Livingstone; 1969. pp. 152–157. [Google Scholar]
- 11.Bushnell DM, Martin ML. Quality of life and Parkinson’s disease: translation and validation of the US Parkinson’s Disease Questionnaire (PDQ-39) Qual Life Res. 1999;8(4):345–350. doi: 10.1023/a:1008979705027. [DOI] [PubMed] [Google Scholar]
- 12.Jenkinson C, Fitzpatrick R, Norquist J, Findley L, Hughes K. Cross-cultural evaluation of the Parkinson’s Disease Questionnaire: tests of data quality, score reliability, response rate, and scaling assumptions in the United States, Canada, Japan, Italy, and Spain. J Clin Epidemiol. 2003;56(9):843–847. doi: 10.1016/s0895-4356(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Elton R. UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Marsden S, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 14.Smith A. Symbol Digit Modalities Test Manual. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 16.Marinus J, Visser M, Verwey NA, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61(9):1222–1228. doi: 10.1212/01.wnl.0000091864.39702.1c. [DOI] [PubMed] [Google Scholar]
- 17.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 18.Shoulson I, Kurlan R, Rubin A, et al. Assessment of functional capacity in neurodegenerative movement disorders: Huntington’s disease as a prototype. In: Munsat T, editor. Quantification of Neurologic Deficit. Boston, MA: Butterworths; 1989. pp. 271–283. [Google Scholar]
- 19.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 21.Huang P, Tilley BC, Woolson RF, Lipsitz S. Adjusting O’Brien’s test to control type I error for the generalized nonparametric Behrens-Fisher problem. Biometrics. 2005;61(2):532–539. doi: 10.1111/j.1541-0420.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan KK, Wittes J. The B-value: a tool for monitoring data. Biometrics. 1988;44(2):579–585. [PubMed] [Google Scholar]
- 23.Ellenberg SS, Fleming TR, DeMets DL. Data Monitoring Committees in Clinical Trials: A Practical Perspective. London, United Kingdom: John Wiley & Sons; 2002. [Google Scholar]
- 24.Luo S, Lawson AB, He B, Elm JJ, Tilley BC. Bayesian multiple imputation for missing multivariate longitudinal data from a Parkinson’s disease clinical trial. Stat Methods Med Res. doi: 10.1177/0962280212469358. published online December 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitehead J. The Design and Analysis of Sequential Clinical Trials. Chichester, United Kingdom: John Wiley & Sons; 1997. Rev 2nd ed. [Google Scholar]
- 26.Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH Royal Kings and Queens Parkinson Disease Research Group. Platelet mitochondrial function in Parkinson’s disease. Ann Neurol. 1992;32 (6):782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- 27.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989;26 (6):719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 28.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54(3):823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 29.Klivenyi P, Gardian G, Calingasan NY, Yang L, Beal MF. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Mol Neurosci. 2003;21(3):191–198. doi: 10.1385/jmn:21:3:191. [DOI] [PubMed] [Google Scholar]
- 30.Matthews RT, Ferrante RJ, Klivenyi P, et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157(1):142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 31.Gray R, Ives N, Rick C, et al. PD Med Collaborative Group. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384(9949):1196–1205. doi: 10.1016/S0140-6736(14)60683-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.