Abstract
Objective
To study the outcomes of microdissection testicular sperm extraction (microTESE) among men with pure Sertoli cell only histology on diagnostic testicular biopsy.
Design
Retrospective cohort study.
Setting
Tertiary referral center.
Patients
640 patients with pure Sertoli cell only histology on testicular biopsy who underwent microTESE by a single surgeon.
Intervention
MicroTESE.
Main Outcome Measure
Sperm retrieval rates.
Results
Overall, 44.5% of patients with Sertoli cell-only had sperm retrieved with microTESE. No difference was noted in sperm retrieval rates based on testis volume (≥ 15cc versus <15cc, 35.3% versus 46.1%, respectively). Patients with ≥ 15cc testicular volume and FSH 10-15 mU/mL had the worst prognosis, with a sperm retrieval rate of 6.7%.
Conclusions
Patients with previous testicular biopsy demonstrating Sertoli cell only histology can be counseled that they have a reasonable likelihood of sperm retrieval with the contemporary delivery of microTESE. Given this finding, the utility of testicular biopsy prior to microTESE is further questioned.
Keywords: Azoospermia, male infertility, testicular failure, sperm retrieval
INTRODUCTION
Azoospermia due to spermatogenic failure, or non-obstructive azoospermia (NOA), affects approximately one percent of the general population, and ten to fifteen percent of men seeking an infertility evaluation.(1, 2) Since the introduction of intracytoplasmic sperm injection in 1992, the management of NOA patients has focused on surgical sperm retrieval, as these patients will frequently demonstrate isolated foci of spermatogenesis within their testes. (3, 4) Sperm retrieval techniques, including conventional testicular sperm extraction (TESE) and microTESE can lead to successful retrieval as a result of this proposed heterogeneity within the testis of the NOA patient.
Predictive factors for the presence of spermatozoa within the testis have been actively studied, including serum follicle stimulating hormone (FSH) and inhibin-B levels, testicular volume and testicular histopathology.(5-9) These studies, however, have failed to allow definitive prediction of sperm retrieval in men undergoing surgical sperm extraction. Men with Sertoli cell only pattern on diagnostic testis biopsy were initially thought to have a very poor prognosis for successful treatment with lower sperm retrieval rates (19 to 43%) compared to patients with hypospermatogenesis and maturation arrest. (10-14) Although the likelihood of retrieval is lower, paternity remains a real possibility in the Sertoli cell only patients, as it has been noted that even men with one to four previous testis biopsies demonstrating Sertoli cell only pattern and no spermatozoa can have sperm retrieval rate (SRR) ranging from 11 to 37% with the use of microTESE. (15) This further demonstrates the heterogeneity within the testis and the likely sensitivity of microTESE in detecting focal spermatogenesis in these patients. Given this increased sensitivity, the role of diagnostic testis biopsy prior to microTESE is further called into question, as it is unlikely to provide prognostic information. To provide better characterization and allow prognostic information for specific patients, we report the outcomes of microTESE obtained at Weill Cornell Medical Center in Sertoli cell only pattern NOA patients.
MATERIALS AND METHODS
Patient Population
A retrospective review was performed of the charts of the last 1373 consecutive patients with NOA who underwent microTESE by a single surgeon from 1999 through 2013. The study protocol was approved by the New York Presbyterian/Weill Cornell institutional review board. Azoospermia was confirmed by analysis of 2 different centrifuged semen samples according to WHO criteria. All patients had an additional semen sample evaluated on the day of planned microTESE that confirmed azoospermia following extended sperm preparation. Karyotype and Y chromosomal microdeletion analysis were performed on all patients. Patients with complete AZFa or AZFb microdeletions were excluded.
Patient Evaluation
Testis volume was measured by use of physical examination with an orchidometer, with mean volume of both testes analyzed for patients undergoing bilateral microTESE. Testicular histology was determined based upon results of testicular biopsies done prior to microTESE or on the results of the intraoperative testicular exploration during microTESE. Patients with strictly Sertoli cell only histology (no other histologic patterns) on testis biopsy were included in this analysis. Serum FSH levels were obtained without any hormonal medical therapy within 2 months prior to microTESE. Clinical pregnancy in female partners was determined through identification of at least one gestational sac and one fetal heartbeat on transvaginal ultrasonography performed 6 to 7 weeks after embryo transfer.
Surgical Technique
The technique of microTESE has been previously described in detail.(16) Briefly, the testis was delivered through a midline scrotal incision, and the tunica vaginalis was opened to directly examine the tunica albuginea and the testis in its entirety. The tunica albuginea was then opened in an equatorial plane under an operative microscope, taking care to avoid injury to the testicular vasculature. Following the initial wide incision and exposure of the testis, a random biopsy sample representative of the overall gross appearance of the testicular parenchyma and similar in size to a standard diagnostic testis biopsy performed through a traditional testicular window incision was taken from the seminiferous tubules and placed in Bouin’s solution. If a biopsy was performed preoperatively (done in approximately 40% of the patient population), pathology was reviewed at our institution by an experienced pathologist when tissue was available. The testicular parenchyma was directly examined under 12X to 20X magnification. Small samples of the larger, more opaque tubules identified during dissection were dropped in sperm wash medium and minced extensively until they could be passed in solution through a 24 gauge angiocatheter. The sample was then immediately examined by an experienced embryologist in the operating room for the presence of sperm. The procedure was terminated when spermatozoa were retrieved or all areas of the testis had been extensively examined without jeopardizing the testicular blood supply. If no spermatozoa were seen intraoperatively, the testicular tissue was digested and thoroughly examined for the presence of sperm cells in the embryology laboratory. (17)
Statistical Analysis
Microsoft Excel 2007® software was used for statistical analysis. Tests were considered statistically significant if p < 0.05. χ2 analysis was used to compare successful and failed sperm retrieval at the time of mTESE. Fisher’s exact test was used if n < 10 for any variable in the contingency table.
RESULTS
Overall Patient Population
A total of 640 patients with pure Sertoli cell only pathology were identified. The mean age was 34.0 ± 6.5 years (standard deviation). Mean partner age (female age) was 31.0 ± 5.3 years. The mean testicular volume of the testis undergoing mTESE was 8.3 ± 4.7 cc. Mean serum FSH was 25.2 ± 14.2 mU/mL. Etiology or associated conditions, if identifiable in the study population included: varicoceles (19%), Klinefelter syndrome (13%), cryptorchidism (10%), cancer diagnosis with a history of chemotherapy (10%), Y chromosomal microdeletions (4%), congenital adrenal hyperplasia, idiopathic hypogonadotrophic hypogonadism, and bilateral mumps orchitis (<1% combined). The overall SRR was 44.5%, with a clinical pregnancy rate of 43.1%.
Sub-Population Analysis
SRR were compared for men with normal volume testes (≥ 15cc) compared to men with smaller volume testes (<15cc). Potential clinical conditions associated with azoospermia among the two testis volume groups are presented in Table 1. There were no men with Klinefelter diagnosis in the normal volume testis group. SRR with a diagnosis of Klinefelter syndrome was 71%. There was no significant difference between SRR for small volume versus normal volume testes (46.1% versus 35.3%, respectively; p = 0.09). Excluding the men with Klinefelter diagnosis from the small volume group, there was no difference in SRR for small volume versus normal volume testes (41.7% versus 35.3%, respectively; p = 0.31).
Table 1.
Non-obstructive azoospermia etiologies stratified by testicular volume.
| Testis Volume <15cc (572 patients) | Testis Volume ≥ 15cc (68 patients) | |||
|---|---|---|---|---|
| N | % | n | % | |
| Varicocele | 114 | 20.0 | 15 | 22.1 |
|
Klinefelter’s
Syndrome |
90 | 15.7 | 0 | - |
| Cryptoorchidism | 62 | 10.8 | 7 | 10.3 |
|
History of
chemotherapy |
55 | 9.6 | 9 | 13.2 |
|
Y chromosomal
microdeletions |
23 | 4.0 | 2 | 2.9 |
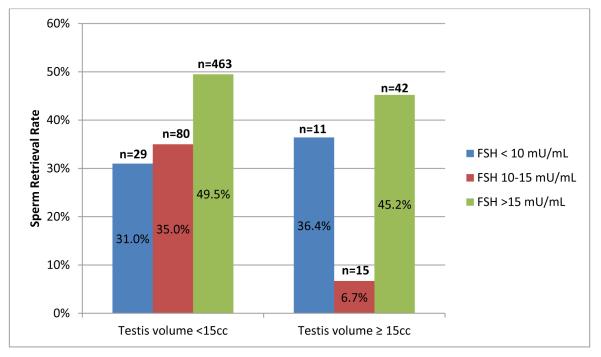
SRR stratified by FSH levels and testicular volumes are presented in Figure 1. Men with FSH of 10-15 mU/mL and normal volume testes had a uniquely poor prognosis, with a sperm retrieval rate of only 6.7%. On post-hoc analysis, among men with normal volume testes, this was significantly less than the SRR among men with FSH ≥ 15 mU/mL (p = 0.01), but not significantly different from men with FSH <10 mU/mL (p = 0.13). SRR in the small volume group excluding the Klinefelters patients did not show a significant difference in retrieval rates as FSH levels increased up to 15 mU/mL: FSH <10 mU/mL, 28.6%; FSH 10-15 mU/mL, 34.2%; FSH ≥ 15 mU/mL, 44.4%; p =0.09).
Figure 1.
Sperm retrieval rates stratified by testicular volume and serum FSH levels.
Note: Difference in sperm retrieval rates for patients with testis volume <15cc, p = 0.025 (χ2 for all three FSH groups), and for patients with testis volume ≥ 15cc, p = 0.023 (Fisher’s exact test for all three FSH groups).
Pregnancy rates were not significantly different across FSH groups according to testis volume. For patients with small volume testes, the pregnancy rates among patients with sperm retrieved were 6/9 (67%), 17/28 (61%) and 101/229 (44%) for FSH <10 mU/mL, FSH 10-15 mU/mL and FSH ≥ 15 mU/mL, respectively (p = 0.12 for difference between three groups). For patients with normal volume testes, the pregnancy rates among patients with sperm retrieved were 3/4 (75%), 0/1 (0%) and 7/19 (37%) for FSH <10 mU/mL, FSH 10-15 mU/mL and FSH ≥ 15 mU/mL, respectively (p = 0.26 for difference between three groups).
DISCUSSION
Overall, 44.5% of men with Sertoli cell only pattern identified on testicular histology successfully had sperm retrieved at the time of microTESE. The SRR noted in this patient population is above that previously reported for most men with complete Sertoli cell only pattern (19-43%), and represents the largest experience with microTESE in these men.(10-14, 18) It should be noted that many prior studies reporting retrieval rates in Sertoli cell-only have encompassed men with a predominant Sertoli cell pattern on histology as opposed to the pure histology as we have reported herein. Sperm retrieval with microTESE is dependent on the most advanced pattern of spermatogenesis, so it is critical to consider this most advanced, not the predominant pattern of spermatogenesis on a diagnostic biopsy. Although SRR varied in the current study according to testis size and serum FSH levels, the data reported herein can be used to counsel men with previous diagnostic testis biopsy demonstrating Sertoli cell only pattern.
Similar SRR were noted among men with Sertoli cell only histology and normal volume testes (35.3%) versus small volume testes (46.1%). Previous data in men undergoing conventional TESE for NOA has reported lower SRR in those men with smaller testes, although these studies have not reported a clear predictive value to testicular volume for sperm retrieval.(6, 14, 19) In addition, a recent report looking at microTESE for a large series of NOA patients has shown that testis volume has no effect on successful sperm retrieval.(20)
We have clearly identified a subset of men in this patient population with a poor prognosis for sperm retrieval, as men with abnormal serum FSH in the intermediate range (10-15 mU/mL) and a normal testis volume in this population had the worst performance with microTESE, with SRR of 6.7%. Obviously, these men had a universal pattern of Sertoli cell-only throughout the testis predicted with a high degree of probability by the clinical characteristics of FSH and testis volume. Neither testis volume nor FSH alone could be used in this patient population to give patients prognostic information. Overall patient characteristics are critical to identify preoperatively and discuss with potential candidates for sperm retrieval. Among men undergoing conventional TESE, FSH has been previously investigated as a predictor of sperm retrieval in men with conflicting results.(6, 9, 21, 22) Serum FSH has additionally been evaluated as a predictor of success for microTESE, and it has been noted that, paradoxically, men with higher FSH values (>15 mU/mL) have a higher likelihood of successful retrieval compared to lower FSH levels (<15 mU/mL), with continued success noted even among men with the highest FSH levels (>46 mU/mL).(23)
This observation reflects two findings: the heterogeneity of histology and therefore sperm production within the testis, and the success of the surgical procedure in finding those sites of sperm production. Obviously, having a high FSH does not imply better testicular function – indeed, men with smaller testes and higher FSH overall have worse testicular function. However, the ability to retrieve sperm is dependent on the most advanced pattern of spermatogenesis. The fact that sperm retrieval rates are maintained in men with small volume testes (20) and men with elevated FSH (23) reflect the fact that these men are still likely to have heterogeneous areas of histology within the testis.
It is important to consider the extent of microTESE and its effects on sperm retrieval. It is an obvious observation that a single diagnostic biopsy can easily miss sperm that can be detected on subsequent dissection of the testis, since 45% of men with no sperm – and no germ cells – on diagnostic biopsy will have sperm found with microTESE at our institution. Similarly, we have found that the use of an operating microscope alone will miss sperm in up to 1/3 of men with spermatogenesis unless a detailed microdissection process is performed with the operating microscope. (24)
Counter-intuitively, in our population with Sertoli cell only pathology, those men with FSH 10-15 mU/mL had a lower chance of having sperm present than those with lower or higher FSH levels. Conceptually, among Sertoli cell only patients, those with normal range FSH levels may have a larger number of Sertoli cells, often in association with a larger volume testis providing feedback to the hypothalamic-pituitary-gonadal (HPG) axis that relatively suppresses FSH secretion. Those patients with higher FSH may represent men with increased heterogeneity within the testis and therefore a better possibility of detecting sperm through microTESE. On the other hand, some men with lower FSH values (<10 mU/mL) may have more feedback to the HPG axis as a result of complete spermatogenesis.
As expected, there were no patients with Klinefelter syndrome and normal volume testes. Patients with Klinefelters have previously been described as a group of men with better outcomes with microTESE since they often have sperm production despite a predominant pattern of Sertoli only, sclerotic tubules and Leydig cell hyperplasia. A recent article evaluating the performance of a nomogram predicting successful sperm retrieval with microTESE, based on retrospective review of 1,026 patients, reported the diagnosis of Klinefelter syndrome as the single most important identifiable variable to predict sperm retrieval on multivariate analysis.(8) Controlling for the presence of Klinefelters in the small volume testis group did not change our overall comparative SRR results for men with serum FSH levels between 10-15 mU/mL after adjusting for testis size.
Our study is limited by its retrospective nature and its representation of a single surgeon experience that may not translate to every surgeon’s experience. In addition, surgeon decision making as to the site of testis biopsy serves as a potential point of bias, although efforts were made to select a random, representative section of the overall testicular parenchyma. However, given the large volume of patients evaluated, the results overall are promising for men with Sertoli cell only histology undergoing microTESE.
CONCLUSIONS
These observations support our management of men with NOA, where we avoid a diagnostic testis biopsy prior to definitive microTESE, as nearly half of the patients who “fail” with diagnostic biopsy are able to have sperm retrieval with a dedicated microTESE. A combination of testicular volume and serum FSH levels among patients with known Sertoli cell only pathology may further aid the practitioner with patient counseling, with a poor prognosis noted among the subset of men with normal volume testes and intermediately abnormal FSH levels.
Acknowledgments
Study funding: James Buchanan Brady Foundation and the Reeves Foundation of New York Presbyterian Hospital, as well as the CTSC of Weill Cornell Medical College provided support for this study.
REFERENCES
- 1.Willott GM. Frequency of azoospermia. Forensic science international. 1982;20:9–10. doi: 10.1016/0379-0738(82)90099-8. [DOI] [PubMed] [Google Scholar]
- 2.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. The Journal of urology. 1989;142:62–5. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel PN, Palermo GD, Goldstein M, Menendez S, Zaninovic N, Veeck LL, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology. 1997;49:435–40. doi: 10.1016/S0090-4295(97)00032-0. [DOI] [PubMed] [Google Scholar]
- 4.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 5.Bohring C, Schroeder-Printzen I, Weidner W, Krause W. Serum levels of inhibin B and follicle-stimulating hormone may predict successful sperm retrieval in men with azoospermia who are undergoing testicular sperm extraction. Fertility and sterility. 2002;78:1195–8. doi: 10.1016/s0015-0282(02)04259-0. [DOI] [PubMed] [Google Scholar]
- 6.Ezeh UI, Taub NA, Moore HD, Cooke ID. Establishment of predictive variables associated with testicular sperm retrieval in men with non-obstructive azoospermia. Human reproduction. 1999;14:1005–12. doi: 10.1093/humrep/14.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Su LM, Palermo GD, Goldstein M, Veeck LL, Rosenwaks Z, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. The Journal of urology. 1999;161:112–6. [PubMed] [Google Scholar]
- 8.Ramasamy R, Padilla WO, Osterberg EC, Srivastava A, Reifsnyder JE, Niederberger C, et al. A comparison of models for predicting sperm retrieval before microdissection testicular sperm extraction in men with nonobstructive azoospermia. The Journal of urology. 2013;189:638–42. doi: 10.1016/j.juro.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Boitrelle F, Robin G, Marcelli F, Albert M, Leroy-Martin B, Dewailly D, et al. A predictive score for testicular sperm extraction quality and surgical ICSI outcome in non-obstructive azoospermia: a retrospective study. Human reproduction. 2011;26:3215–21. doi: 10.1093/humrep/der314. [DOI] [PubMed] [Google Scholar]
- 10.Kalsi J, Thum MY, Muneer A, Abdullah H, Minhas S. In the era of micro-dissection sperm retrieval (m-TESE) is an isolated testicular biopsy necessary in the management of men with non-obstructive azoospermia? BJU international. 2012;109:418–24. doi: 10.1111/j.1464-410X.2011.10399.x. [DOI] [PubMed] [Google Scholar]
- 11.Abdel Raheem A, Garaffa G, Rushwan N, De Luca F, Zacharakis E, Abdel Raheem T, et al. Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia. BJU international. 2013;111:492–9. doi: 10.1111/j.1464-410X.2012.11203.x. [DOI] [PubMed] [Google Scholar]
- 12.Hussein A. Evaluation of diagnostic testis biopsy and the repetition of testicular sperm extraction surgeries in infertility patients. Fertility and sterility. 2013;100:88–93. doi: 10.1016/j.fertnstert.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Gul U, Turunc T, Haydardedeoglu B, Yaycioglu O, Kuzgunbay B, Ozkardes H. Sperm retrieval and live birth rates in presumed Sertoli-cell-only syndrome in testis biopsy: a single centre experience. Andrology. 2013;1:47–51. doi: 10.1111/j.2047-2927.2012.00003.x. [DOI] [PubMed] [Google Scholar]
- 14.Tournaye H, Verheyen G, Nagy P, Ubaldi F, Goossens A, Silber S, et al. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Human reproduction. 1997;12:80–6. doi: 10.1093/humrep/12.1.80. [DOI] [PubMed] [Google Scholar]
- 15.Ramasamy R, Schlegel PN. Microdissection testicular sperm extraction: effect of prior biopsy on success of sperm retrieval. The Journal of urology. 2007;177:1447–9. doi: 10.1016/j.juro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Human reproduction. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 17.Ramasamy R, Reifsnyder JE, Bryson C, Zaninovic N, Liotta D, Cook CA, et al. Role of tissue digestion and extensive sperm search after microdissection testicular sperm extraction. Fertility and sterility. 2011;96:299–302. doi: 10.1016/j.fertnstert.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Colpi GM, Colpi EM, Piediferro G, Giacchetta D, Gazzano G, Castiglioni FM, et al. Microsurgical TESE versus conventional TESE for ICSI in non-obstructive azoospermia: a randomized controlled study. Reproductive biomedicine online. 2009;18:315–9. doi: 10.1016/s1472-6483(10)60087-9. [DOI] [PubMed] [Google Scholar]
- 19.Bromage SJ, Falconer DA, Lieberman BA, Sangar V, Payne SR. Sperm retrieval rates in subgroups of primary azoospermic males. European urology. 2007;51:534–9. doi: 10.1016/j.eururo.2006.08.032. discussion 9-40. [DOI] [PubMed] [Google Scholar]
- 20.Bryson CF, Ramasamy R, Sheehan M, Palermo GD, Rosenwaks Z, Schlegel PN. Severe Testicular Atrophy does not Affect the Success of Microdissection Testicular Sperm Extraction. The Journal of urology. 2013 doi: 10.1016/j.juro.2013.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CS, Chu SH, Lai YM, Wang ML, Chan PR. Reconsideration of testicular biopsy and follicle-stimulating hormone measurement in the era of intracytoplasmic sperm injection for non-obstructive azoospermia? Human reproduction. 1996;11:2176–9. doi: 10.1093/oxfordjournals.humrep.a019072. [DOI] [PubMed] [Google Scholar]
- 22.Jezek D, Knuth UA, Schulze W. Successful testicular sperm extraction (TESE) in spite of high serum follicle stimulating hormone and azoospermia: correlation between testicular morphology, TESE results, semen analysis and serum hormone values in 103 infertile men. Human reproduction. 1998;13:1230–4. doi: 10.1093/humrep/13.5.1230. [DOI] [PubMed] [Google Scholar]
- 23.Ramasamy R, Lin K, Gosden LV, Rosenwaks Z, Palermo GD, Schlegel PN. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertility and sterility. 2009;92:590–3. doi: 10.1016/j.fertnstert.2008.07.1703. [DOI] [PubMed] [Google Scholar]
- 24.Ramasamy R, Reifsnyder JE, Husseini J, Eid PA, Bryson C, Schlegel PN. Localization of sperm during microdissection testicular sperm extraction in men with nonobstructive azoospermia. The Journal of urology. 2013;189:643–6. doi: 10.1016/j.juro.2012.09.031. [DOI] [PubMed] [Google Scholar]