Abstract
Background
The effect of increasing negative margin width following breast conserving therapy (BCT) on local recurrence (LR) is controversial. LR rates vary by subtype, with the highest rates seen in triple negative breast cancer (TNBC). This study examined LR rates in relationship to margin width in TNBC treated with BCT.
Methods
Women with TNBC who underwent BCT between 1999-2009 were identified. Margins were defined as positive (ink on tumor), 0.1-2.0, and 2 mm. Patients with positive margins were excluded. Statistical comparisons were by t tests, Fishers exact test and Wilcoxon rank sum test. Cumulative incidence of LR was compared using competing-risks methodology.
Results
Of 535 cancers, 71 had margins ≤2mm and 464 had margins >2mm. At median follow-up of 84 months (range 8-165 months) there were 37 local, 18 regional, and 77 distant recurrences or deaths as first events. 10 patients had a locoregional recurrence prior to planned radiation therapy and were excluded from cumulative incidence analyses. The cumulative incidence of LR at 60 months for margins ≤2mm was 4.7% (95% CI 0, 10.0), and for >2mm 3.7% (1.8, 5.5), p=0.11. After controlling for chemotherapy and tumor size, there was no difference in LR between the two margin groups (p=.06). A difference in the risk of distant recurrence or death was not observed (p=.53).
Conclusions
Margin width greater than 2mm was not associated with reduced LR rates. This data supports a negative margin definition of no ink on tumor, even in this high risk TNBC cohort.
Keywords: triple negative breast cancer, local recurrence, margin, breast conserving therapy
Introduction
Breast-conservation therapy (BCT) is a well established treatment modality for breast cancer, with equivalent survival to mastectomy.1 Although rates of local recurrence (LR) have decreased since the initial prospective randomized trials of BCT2, significant controversy exists regarding the optimal negative margin width. Positive margins, defined as ink on tumor, are definitively associated with increased rates of LR compared to negative margins3, but the optimal amount of normal breast tissue which constitutes a negative margin remains uncertain. Both surgeons and radiation oncologists use widely varying definitions of what margin width precludes the need for re-excision4-6, in spite of the findings of a meta-analysis of 21 published studies which found no significant improvement in rates of LR with increasing negative margin width.7 Recent data indicate that rates of LR vary by breast cancer subtype as approximated by estrogen receptor (ER), progesterone receptor (PR), and HER2/neu, with patients with triple negative breast cancer (TNBC) having higher LR rates compared to the non-triple negative subtypes.8 Given the higher reported rates of LR in TNBC, we sought to examine the impact of margin status in this subgroup to determine if margins greater than 2 mm were associated with decreased rates of LR in this population.
Methods
Following approval by the institutional review board at Memorial Sloan-Kettering Cancer Center, a prospectively maintained database was used to identify women with TNBC who underwent BCT at our institution between 1999 and 2009. TNBC was defined as ER and PR positivity of < 1%, and HER2/neu negativity (0, 1+ staining) by immunohistochemistry and/or no amplification by FISH. All patients underwent lumpectomy and had planned whole breast radiation therapy (RT). Patients receiving partial breast radiation and patients not undergoing whole breast radiation (due to refusal or medical recommendation) were excluded. Because the population of interest was women who completed standard treatment (i.e., lumpectomy and RT), women who recurred prior to RT were also excluded from estimates and comparisons of cumulative incidence of LR, regional recurrence, distant metastasis, and death. Margin status was defined as positive (ink on tumor), 0.1-2.0 mm, or > 2.0 mm. 46 patients with positive margins were excluded from analysis. One woman had a contralateral TNBC three years after her initial diagnosis; these cancers were treated as independent for all analyses. Data on patient, tumor, and treatment characteristics were collected: age at surgery; tumor size; lymphovascular invasion (present or absent); ER and PR status; HER 2/neu status; receipt of adjuvant whole breast radiation, including a boost to the tumor bed or regional nodal irradiation; and receipt of adjuvant chemotherapy. Patient charts were reviewed for date of last follow-up, status at last follow-up (no evidence of disease; alive with disease; died of disease; died other cause; or died unknown cause). Recurrence events and dates were captured for local (ipsilateral breast), regional (ipsilateral axillary, internal mammary, or supraclavicular nodal basins), or distant metastasis. Group characteristics were compared using t-tests and Wilcoxon rank-sum tests for age and tumor size, respectively, and Fisher's exact test for categorical covariates. Time to first event (LR, regional recurrence, distant metastasis/death, or end of follow-up) was calculated from date of definitive surgery, and competing risks methodology was used to estimate the cumulative incidence of LR, regional recurrence, and distant recurrence/death. Nine women had a bilateral prophylactic mastectomy during follow-up and were censored at the time of the procedure. Gray's test and Fine and Gray regression were used to test associations between clinical factors and LR in univariate and multivariate settings, respectively. Multivariate models were built to investigate the effect of margin status after adjusting for covariates with p < 0.15 for either margin status or LR on univariate analysis; radiation was not included in these models as patients who recurred prior to RT were excluded. All statistical analysis was performed in SAS 9.2 (SAS Institute, Cary, NC) or R 2.3.1 (R Foundation for Statistical Computing, Vienna, Austria), and the cmprsk package was used. P-values < 0.05 were considered significant.
Results
535 TNBCs treated with BCT during the study period were identified in 534 women. Of the 535 cancers, 464 had margins greater than 2 mm and 71 had margins of 2 mm or less. Mean age of the study population was 55.4 years. The median tumor size was 1.6 cm (range, 0.1-7.3), 29% were node positive, and 24% had lymphovascular invasion identified. 84% of the patients underwent adjuvant chemotherapy, 94% had a radiation boost to the lumpectomy bed, and 11% received regional nodal radiation. Patient, tumor, and treatment characteristics by margin status are shown in Table 1. No significant differences in age, tumor size, nodal status, presence of lymphovascular invasion, or radiation use between the ≤ 2 mm and > 2 mm margin groups were noted. There was a trend toward increased use of chemotherapy in the > 2 mm margin group (p = 0.05). During the study period, the use of chemotherapy regimens changed. In the initial year of the study (1999), cyclophosphamide, methotrexate, and fluorouracil (CMF) was the most frequent regimen used, with 44% percent of patients receiving this regimen. Between 2000 and 2009, an anthracycline with or without a taxane was increasingly used, and an anthracycline with a taxane became the most frequently used regimen by 2009 (75%). Among women who completed standard treatment, there was no difference in the rate of LR by year of surgery during the study period (p = 0.240) or when comparing patients treated during the first 5 years (1999-2003) to those treated in the last 5 years (2004-2009) (p = 0.25).
Table 1. Patient and treatment variable by margin status.
| Patient/Treatment Variable | ≤ 2 mm Margin (n = 71) |
> 2 mm Margin (n = 464) |
p-value |
|---|---|---|---|
| Age at Surgery (mean ± standard deviation) | 56.7 ± 12.2 | 55.2 ± 12.2 | 0.30 |
| Age > 50 years | 53 (75%) | 312 (67%) | 0.27 |
| Tumor size (median, IQR) | 1.5 (1, 2.2) | 1.6 (1.1, 2.3) | 0.27 |
| Tumor size > 2 cm | 22/69 (32%) | 165/457 (36%) | 0.59 |
| Lymphovascular invasion present | 19 (27%) | 110 (24%) | 0.55 |
| Nodal status positive | 25/71 (35%) | 130/463 (28%) | 0.26 |
| Chemotherapy | 54 (76%) | 398 (86%) | 0.05 |
| Whole breast radiation therapy* | 68 (96%) | 457 (98%) | 0.14 |
| Tumor bed boost** | 59/63 (94%) | 380/405 (94%) | 1.00 |
| Regional radiation** | 10/62 (16%) | 41/404 (10%) | 0.19 |
Denominators are included in cases where not all patients could be included due to missing data.
The only patients who did not receive RT were those who recurred prior to planned RT
within the subset of patients treated with RT
IQR interquartile range
For the entire population, at median follow-up of 84 months (range, 8-165 months), there were 37 local recurrences, 18 regional recurrences, and 77 distant recurrences or deaths as first events. Ten patients had an early locoregional recurrence (LRR) following lumpectomy but prior to planned RT (9 local, 1 regional). 4% (3 of 71) of patients with less than or equal to 2mm margins experienced LRR before planned RT, compared to 1.5 % (7 of 464) of patients with margins greater than 2 mm (p = 0.14). All 10 patients with an LRR prior to planned RT developed distant metastasis, and at date of last follow-up, 8 had died. The 10 patients with an LRR prior to planned RT were not included in analyses of cumulative incidence of LR, regional recurrence, and distant metastasis/death.
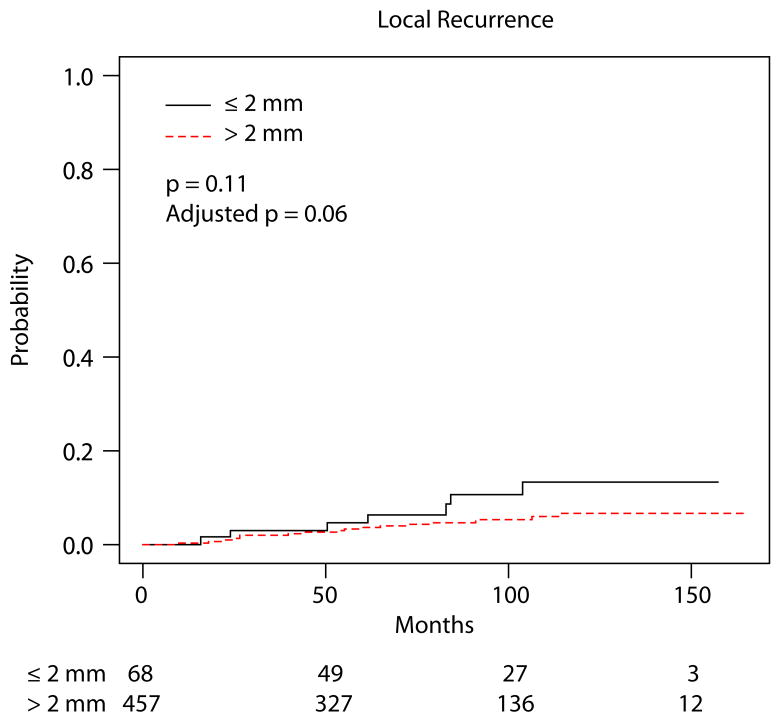
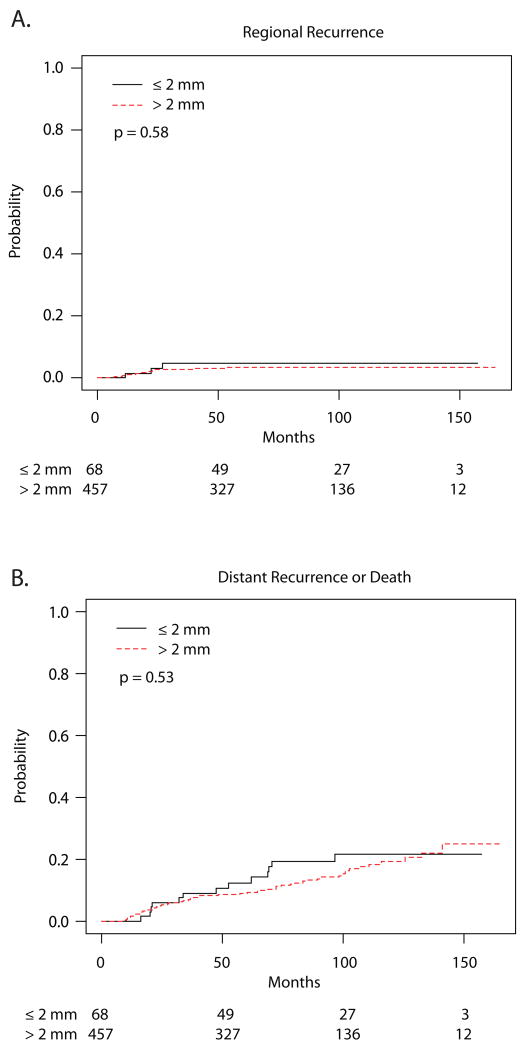
Among the remaining 525 patients who completed RT, the cumulative incidence of LR at 60 months for patients with margins of 2 mm or less was 4.7% (95% confidence interval [CI], 0-10.0) and 3.7% (1.8-5.5) for those with margins greater than 2 mm (p = 0.11) (Table 2, Figure 1). After controlling for the use of adjuvant chemotherapy and tumor size, there remained no significant difference in LR between the margin groups (p = 0.06, subdistribution hazard ratio = 2.23 for margins 2 mm or less compared to greater than 2 mm with 95% CI: 0.97-5.14). No significant differences in incidence of LR were observed based on age, tumor size, LVI, nodal status, chemotherapy, or radiation boost. There were no observed differences in the risk of regional recurrence (p = 0.58) or distant recurrence/death (p = 0.53) between the margin groups (Figure 2).
Table 2. Univariate analysis of patient and treatment factors, and risk of local recurrence.
| Factor | Cumulative Incidence of LR at 60 months (95% confidence interval) |
p-value |
|---|---|---|
| Margin status ≤ 2 mm | 4.7% (0-10.0) | 0.11 |
| Margin status > 2 mm | 3.7% (1.8-5.5) | |
| Age at surgery > 50 years | 3.8% (1.7-5.8) | 0.60 |
| Age at surgery ≤ 50 years | 3.9% (0.8-7.0) | |
| Tumor size > 2 cm | 6.4%(2.5-10.3) | 0.11 |
| Tumor size ≤ 2 cm | 2.6% (0.8-4.3) | |
| LVI present | 4.3% (0.6-8.1) | 0.79 |
| LVI absent | 3.6% (1.7-5.6) | |
| Nodal status positive | 5.3% (1.4-9.2) | 0.65 |
| Nodal status negative | 3.2% (1.3-5.1) | |
| Chemo | 4.0% (2.1-5.9) | 0.83 |
| No chemo | 2.7% (0-6.3) | |
| Boost | 4.3% (2.3-6.4) | 0.48 |
| No boost | 3.4% (0-10.2) |
LR local recurrence LVI lymphovascular invasion
Fig. 1.
Cumulative incidence of local recurrence.
Fig. 2.
Cumulative incidence curves for a regional recurrence b distant recurrence or death.
Discussion
Our study does not support the idea that wider surgical margins improve LR rates in patients with TNBCs. These data add to the growing literature that obtaining a margin more widely clear than no ink on tumor in BCT for invasive breast cancer does not impact local control.7,9,10 A meta-analysis by Houssami et al of 14,571 women found no significant decrease in LR with increasing negative margin distance, although positive margins were highly correlated with LR.7 However, this meta-analysis did not examine the relationship between breast cancer subtype and margins since hormone receptor status and HER2 status were not reported in a majority of the included studies. Although it may seem counterintuitive that more widely clear margins do not reduce LR, an emerging body of literature supports the idea that biology and the use of effective systemic therapy are the major determinants of local control. Rates of both local and distant recurrence vary by breast cancer subtype.8,11-13 A meta-analysis by Lowery et al8 examined LRR rates by subtype in 7174 patients undergoing BCT. At a median follow-up of 57 months, patients with a non-TNBC (ER, PR positive or HER2/neu overexpressing) had lower rates of LRR than the triple negative subtype (relative risk [RR], 0.49; 95% CI, 0.33-0.73). A similar pattern was seen in 5416 women treated with mastectomy where patients with non-TNBC were significantly less likely to develop an LRR following mastectomy then the triple negative cohort (RR, 0.66; 95% CI, 0.53-0.83). In a more recent study, Russo et al14 examined the impact on LR of margin widths of 2 mm or greater to margins less than 2 mm in 906 women undergoing BCT between 1998 and 2006, controlling for tumor subtype. The 5-year rate of LR was 0% for margins less than 2 mm and 2.3% for ≥ 2 mm margins. On multivariate analysis, margin width was not associated with LR, but triple negative subtype was a highly significant predictor of LR with an adjusted hazard ratio of 3.7 (95% CI, 1.6-8.8; p = 0.003).
The impact of biology on LR is mitigated by effective systemic therapy. In the National Adjuvant Breast and Bowel Project (NSABP) B14 trial of tamoxifen versus placebo in ER positive women, the use of tamoxifen reduced LR to 4.3% compared to 14.7% in the placebo group.15 A similar reduction in LR was seen with the addition of chemotherapy in ER negative women from 13.4% to 2.6% in NSABP B13.16 However, the experience with HER2 overexpressing patients indicates that even greater reductions in LR can be obtained with the addition of targeted therapy to chemotherapy.13,17,18 A meta-analysis of randomized trials comparing adjuvant chemotherapy with or without trastuzumab for HER2/neu positive breast cancer showed significant reductions in LR in the trastuzumab treated patients (RR, 0.58; 95% CI, 0.43-0.77, p = 0.0002).18 At this time, there is no targeted therapy for TNBC, so it could be postulated that bigger surgery might improve LR for the triple negative subtype. A mastectomy represents the widest margin that can be obtained in breast cancer surgery, and 3 studies have examined the effect of surgery type (BCT compared to mastectomy without RT) on LR in TNBCs. Patients treated with mastectomy had larger tumors and higher risk features; however, on multivariate analysis, type of surgery was not a predictor of LRR in this patient population.19-21 To the best of our knowledge, our study is the first to directly address the question of margin width in BCT in TNBC. In addition to finding no impact of margins > 2 mm on LR, it is noteworthy that rates of local control were high in this patient cohort, with only 5% of those with margins of 2 mm or less and 4% of those with margins greater than 2 mm experiencing LR. The non-significant reported hazard ratio for LR after controlling for chemotherapy and tumor size, coupled with the studies showing no difference in outcome for TNBC patients treated with BCT and mastectomy, strongly suggests that bad biology is unlikely to be overcome with larger surgical procedures. This finding has implications for clinical practice as significant controversy currently exists among surgeons and radiation oncologists regarding the acceptable margin width in BCT for invasive breast cancer, with only 11-40% of surveyed surgeons comfortable with a negative margin definition of no ink on tumor.4-6 However, acceptance of this negative margin definition has the potential to benefit a significant number of women, as recent data show that 25% of all women treated with BCT undergo a re-excision22,23, and approximately half of re-excisions are performed to achieve wider negative margins.23 Minimizing the need for re-excision decreases the risk of poorer cosmetic outcome associated with the resection of larger amounts of breast tissue24, delayed time to adjuvant therapy, additional operating room costs, and the possible psychological impact of reoperation on patients.
Strengths of this study include a large cohort of contemporarily treated TNBC patients at a single institution, with detailed information available on margin and treatment data. Furthermore, because TNBCs recur early, with peaks of LR seen in the first 3 years25,26, we have likely captured the majority of LR events with a median follow-up of 84 months. However, this study is a retrospective analysis with known potential limitations. There was a trend toward decreased chemotherapy utilization in the ≤ 2 mm margin group. During the time period of this study, there was no consensus on the use of chemotherapy for tumors < 1 cm in size, and this difference likely represents chance variation. On univariate analysis, factors such as age, lymphovascular invasion, use of chemotherapy, nodal status, tumor size, and use of a boost were not found to be significant predictors of LR, which may be secondary to insufficient power to detect a difference based on our population size. However, the numerically low rates of LR in both groups in our study suggest that even if a larger sample size resulted in a statistically significant difference, the magnitude of difference is unlikely to be clinically meaningful.
In conclusion, while patients with TNBCs have higher reported rates of LR than other breast cancer subtypes, it does not appear that bigger surgery improves outcome. Our current data continue to support the definition of a negative margin as no ink on tumor, even in this high-risk breast cancer subset.
Synopsis.
Local recurrence (LR) rates in women with triple negative breast cancer treated with breast conserving therapy were compared by margin width: 0.1-2.0 mm and >2 mm. No significant difference in LR rates was observed between the two margin groups.
Acknowledgments
This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The findings presented in this manuscript were presented in part in a poster discussion session at the 2013 American Society of Clinical Oncology Annual Meeting, May 31-June 4, 2013. This study was also the recipient of the 2013 Conquer Cancer Foundation of ASCO Merit Award.
References
- 1.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Bouganim N, Tsvetkova E, Clemons M, et al. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat. 2013;139(2):603–6. doi: 10.1007/s10549-013-2561-7. [DOI] [PubMed] [Google Scholar]
- 3.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184(5):383–93. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 4.Azu M, Abrahamse P, Katz SJ, et al. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol. 2010;17(2):558–63. doi: 10.1245/s10434-009-0765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovrics PJ, Gordon M, Cornacchi SD, et al. Practice patterns and perceptions of margin status for breast conserving surgery for breast carcinoma: National Survey of Canadian General Surgeons. Breast. 2012;21(6):730–4. doi: 10.1016/j.breast.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Hassani A, Griffith C, Harvey J. Size does matter: High volume breast surgeons accept smaller excision margins for wide local excision - A national survey of the surgical management of wide local excision margins in UK breast cancer patients. Breast. 2013 doi: 10.1016/j.breast.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46(18):3219–32. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Lowery AJ, Kell MR, Glynn RW, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–41. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 9.Groot G, Rees H, Pahwa P, et al. Predicting local recurrence following breast-conserving therapy for early stage breast cancer: the significance of a narrow (</= 2 mm) surgical resection margin. J Surg Oncol. 2011;103(3):212–6. doi: 10.1002/jso.21826. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Stroom J, Sonke JJ, et al. Impact of negative margin width on local recurrence in breast conserving therapy. Radiother Oncol. 2012;104(2):148–54. doi: 10.1016/j.radonc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Hattangadi-Gluth JA, Wo JY, Nguyen PL, et al. Basal subtype of invasive breast cancer is associated with a higher risk of true recurrence after conventional breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2012;82(3):1185–91. doi: 10.1016/j.ijrobp.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panoff JE, Hurley J, Takita C, et al. Risk of locoregional recurrence by receptor status in breast cancer patients receiving modern systemic therapy and post-mastectomy radiation. Breast Cancer Res Treat. 2011;128(3):899–906. doi: 10.1007/s10549-011-1495-1. [DOI] [PubMed] [Google Scholar]
- 13.Kim MM, Dawood S, Allen P, et al. Hormone receptor status influences the locoregional benefit of trastuzumab in patients with nonmetastatic breast cancer. Cancer. 2012;118(20):4936–43. doi: 10.1002/cncr.27502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo AL, Arvold ND, Niemierko A, et al. Margin status and the risk of local recurrence in patients with early-stage breast cancer treated with breast-conserving therapy. Breast Cancer Res Treat. 2013;140(2):353–61. doi: 10.1007/s10549-013-2627-6. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88(21):1529–42. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Dignam J, Mamounas EP, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J Clin Oncol. 1996;14(7):1982–92. doi: 10.1200/JCO.1996.14.7.1982. [DOI] [PubMed] [Google Scholar]
- 17.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 18.Dahabreh IJ, Linardou H, Siannis F, et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008;13(6):620–30. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 19.Adkins FC, Gonzalez-Angulo AM, Lei X, et al. Triple-negative breast cancer is not a contraindication for breast conservation. Ann Surg Oncol. 2011;18(11):3164–73. doi: 10.1245/s10434-011-1920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker CC, Ampil F, Burton G, et al. Is breast conservation therapy a viable option for patients with triple-receptor negative breast cancer? Surgery. 2010;148(2):386–91. doi: 10.1016/j.surg.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Zumsteg ZS, Morrow M, Arnold B, et al. Breast-Conserving Therapy Achieves Locoregional Outcomes Comparable to Mastectomy in Women with T1-2N0 Triple-Negative Breast Cancer. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-3011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–6. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467–75. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 24.Al-Ghazal SK, Blamey RW, Stewart J, et al. The cosmetic outcome in early breast cancer treated with breast conservation. Eur J Surg Oncol. 1999;25(6):566–70. doi: 10.1053/ejso.1999.0707. [DOI] [PubMed] [Google Scholar]
- 25.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 26.Pogoda K, Niwinska A, Murawska M, et al. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388. doi: 10.1007/s12032-012-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]