Abstract
The dose-dependent effect of isoflurane on regional CBF of cortical and subcortical structures in anesthetized macaque monkeys was investigated with the Continuous ASL MRI technique. High concentration of isoflurane resulted in global CBF increase and blood pressure decrease. Evident CBF change was observed in the subcortical structures. Specifically, CBF in thalamus and cerebellum was increased about 39% and 55% when isoflurane concentration was changed from 0.75% to 1.5%, respectively. Also, those regional CBF changes correlated linearly with isoflurane inspiratory concentrations, indicating impaired CBF autoregulation in these structures. In contrast, no obvious CBF changes were observed in anterior cingulated cortex, motor cortex, medial prefrontal cortex, and caudate. The results demonstrate that, under the 0.75–1.5% isoflurane maintenance doses, the CBF auto-regulation is well preserved in the cerebral cortical regions and caudate, but impaired in thalamus and cerebellum, indicating disturbed CBF-metabolism coupling and functional response in specific subcortical regions of anesthetized macaque monkeys.
Keywords: Dose-dependent effect, Isoflurane, Cerebral blood flow (CBF), auto-regulation, non-human primate, arterial spin labeling(ASL)
Introduction
Isoflurane is a volatile inhalation anesthetic agent and commonly used in surgical procedures and in vivo neuroimaging examinations of animals and humans. Previous human and animal studies indicate that the induction of isoflurane results in cerebral vasodilatation and has evident effects on cerebral blood flow (CBF), cerebral blood volume (CBV), permeability, neurovascular coupling, neuron functionality [1, 5, 7, 8, 12, 13, 16]. Increased CBF and reduced rate of cerebral oxygen consumption (CMRO2) have been observed in the animal and human studies [1, 12]. Also, previous canine, baboon, and human studies have shown that the CBF autoregulation mechanism was disturbed under high dose isoflurane[10, 12, 21].
Non-human primates (NHP) resemble humans more than any other animals and are widely used in preclinical studies and various neuroscience researches. As isoflurane produces rapid induction and recovery from anesthesia, most NHP examinations and neuroimaging experiments (such as MRI and PET) are performed under isoflurane anesthesia. The 0.75%–1.5% isoflurane, mixed with 100% O2 or ambient air, is normally used as the maintenance dose for sedation purpose during general structural MRI, functional MRI or PET scanning. In each scanning session, the isoflurane concentration may be adjusted to obtain light or deep depth of anesthesia for specific study purpose or to accommodate to each subject’s physiological or pathological condition. Evident effects of large isoflurane dose variation on CBF have been reported in animal and human studies [9, 12, 21]. BOLD functional MRI responses in the default-mode network of the anaesthetized monkey brain (under 0.8%–1.5% isoflurane) have demonstrated evident reduction of neural activation under deep anesthesia (light vs deep anesthesia) [22]. A prior Xenon-133 study of anesthetized baboons has showed that isoflurane produces vasoconstriction in cerebral vessels in the low doses (~0.5% or less)) or vasodilation in the high doses (~0.95% or more), indicating there exist the transition doses of the biphasic CBF responses between the low and high doses [21]. The 0.75%–1.5% maintenance doses are within the range of the transition doses of the biphasic characteristic. However, due to the technique limitation in high-resolution CBF measurements in prior studies, the relevant dose-dependent effect of isoflurane on regional CBF in different cortical and subcortical structures is poorly understood.
The arterial-spin-labeling (ASL) MRI perfusion technique provides a unique means to quantitatively measure high-resolution CBF of humans and animals non-invasively [2, 17, 19]. In comparison with other perfusion techniques, ASL offers higher spatial resolution and temporal resolution to measure the brain hemodynamic response and has been used widely to access the neuronal activation and acquire basal CBF maps under various circumstances. As the continuous ASL(CASL) technique with a separate labeling coil provides improved signal-to-noise ratio(SNR) and reduced RF exposure, a specific setting with the CASL technique has been successfully developed to measure whole brain CBF of macaque monkeys at high-resolution and SNR [23]. In this study, the specific CASL technique was employed to investigate the dose-dependent effect of isoflurane on regional CBF in the cortical and subcortical structures of anesthetized NHPs.
Methods and Materials
Animal preparation
Adult healthy female rhesus monkeys (n=4, 6–10 years old) were used in this study. The animals were initially anesthetized with ketamine (5–10 mg/kg, IM), then orally intubated. An IV catheter was placed for delivering lactated ringers solution (3.5–10 ml/kg/hr) in the scanner. The anesthetized and spontaneously breathing animals were immobilized with a custom-made head holder and placed in the “supine” position during MRI scanning. Isoflurane was administrated at three inspiratory concentrations with random order: 0.75%, 1.0%, and 1.5 % (0.6, 0.8. 1.2 MAC (minimum alveolar concentration) (end-tidal), respectively), mixed with ambient air. Et-CO2, inhaled CO2, O2 saturation, blood pressure, the mean arterial pressure (MAP), heart rate, respiration rate, and body temperature were monitored continuously in addition to visual inspection of animals every 30 minutes. These physiological parameters were recorded and maintained in normal ranges (PCO2:38–42 mmHg; PO2:25–35 mmHg; O2 saturation: 95%–100%; MAP: 60–100mmHg; heart rate: 120–150 beats/min) in each study session. The body temperature was maintained at 37.5°C by a feedback-regulated circulating warm-water blanket. All procedures followed the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University in accordance with the NIH Guide for Care and Use of Laboratory Animals.
MRI examination
MRI scans were performed on a Siemens 3T Trio whole body scanner (Siemens Medical, PA, USA) with an 8-channel high resolution knee coil (In vivo, Inc) and a home-made butterfly neck coil (ID = 2.9 cm, each loop) with active decoupling [23]. Isofluance concentrations were monitored and measured continuously with an anesthesia monitor (GE Datex - ohmeda Cardiocap/5). A cross-sectional image of the neck was taken to verify that the neck labeling coil was properly positioned. The single-shot, gradient-echo planar imaging (EPI) was applied for the CBF measurement. The MRI parameters were: TR/TE= 4000/25 ms, FOV= 96 mm × 96 mm, data matrix = 64 × 64, 16 slice with slice thickness = 1.5 mm, post-labeling-delay = 0.8 s, Labeling duration= 2 s. Seventy pairs of control and labeling axial images were acquired and the acquisition was repeated 3 times at each dose. Corresponding T2 weighted images also were acquired by using fast spin-echo sequences with TR/TE= 5900/125ms, FOV = 96 mm × 96 mm, matrix = 128 × 128, 2 averages. The CBF measurement was started at least 30 minutes later after the animal was moved into the scanner. The 15-minute minimum equilibrium time was applied after isoflurane dose was changed each time.
Data analysis
Data analyses were performed using Matlab (MathWorks, MA) and Stimulate software (http://www.cmrr.umn.edu/stimulate). CBF maps were calculated with the formula and parameters in our previous paper [23] except that a constant T1 (1.0 s) of brain tissue (grey matter and white matter) was applied instead of a measured T1 map. Also, the equilibrium value M0 was approximated with the amplitude of the correspondent non-tagged image, as the relative CBF changes were required in the present study and evaluated in data analysis. The simplified formula is:
where λ is the water brain–blood partition coefficient, α is the arterial spin-labeling efficiency, Snon-labeled and Slabeled are signal intensities of the non-labeled and labeled images, respectively, ATT is the arterial transit time, T1A is the longitudinal relaxation time (T1) of the arterial blood at 3T, PLD is the post-labeling delay and LD is the labeling duration. All the experiment parameters were as same as described in our previous paper. Briefly, α = 0.92, λ of 0.98 ml/g for gray matter and 0.82 ml/g for white matter, ATT = 0.8 s, T1A = 1.66 s. PLD for different imaging slices were adjusted in the CBF calculation based on known acquisition time per slice.
The motor cortex, medial frontal cortex (mPFC), anterior cingulated cortex (ACC), caudate, thalamus, cerebellum, were selected for region of interest (ROI) analysis (Fig. 1). Each ROI was outlined based upon the corresponding non-tagged high resolution EPI images and T2-weighted images with the Stimulate software (www.cmrr.umn.edu/stimulate/). For each animal, CBF of each ROI was normalized to the mean CBF value of the three dose levels to reduce the inter-subject variations of CBF measurements in the data analysis. Repeated ANOVA was performed to analyze the CBF differences statistically across the different doses. Correlation analysis was used to study the dose-dependence effects on CBF. SPSS 17.0 was used for statistical analysis. P-values less than 0.05 were considered statistically significant.
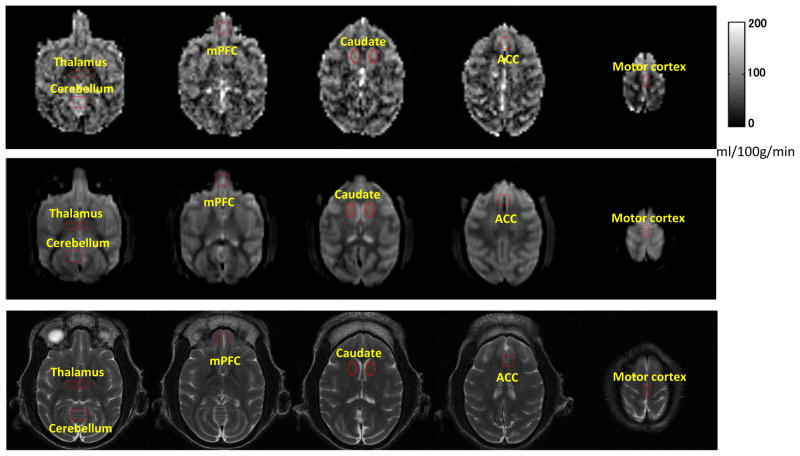
Fig 1.
Selected cerebral blood flow (CBF) maps of an adult rhesus monkey acquired with the Continuous ASL technique (top); Non-tagged EPI images (middle) and T2 weighted images (bottom). Spatial Resolution: 1.5 mm ×1.5 mm ×1.5 mm.
ROIs are illustrated on CBF maps and corresponding EPI and T2 weighted images.
mPFC: medial frontal cortex; ACC: anterior cingulated cortex.
Results
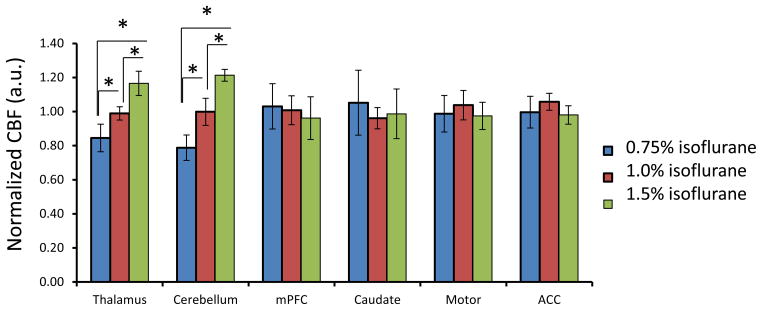
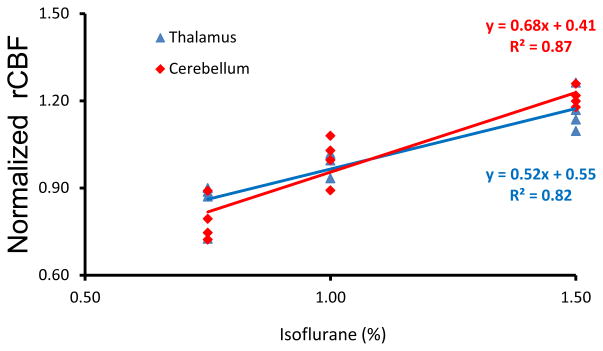
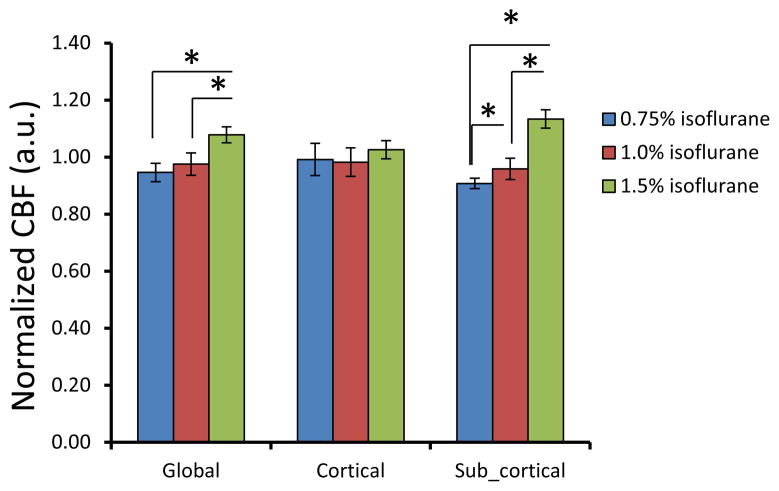
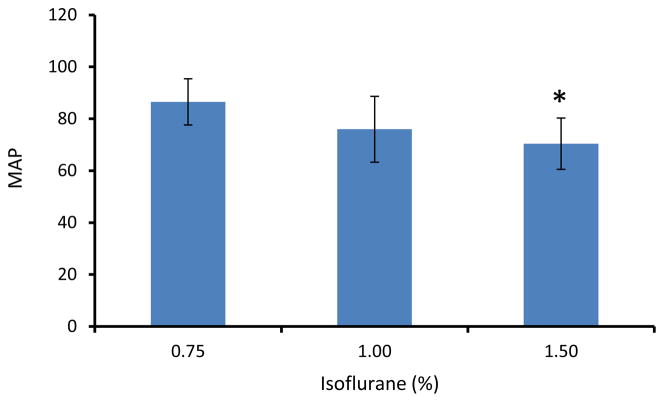
CBF in each monkey was obtained under 0.75%, 1.0%, and 1.5% isoflurane. The isoflurane dose-dependent effects on global, cortical and subcortical mean CBF are shown in Fig 2. CBF in global and subcortical regions were increased 14% (± 5%) and 25% (± 4%) (1.5% vs 0.75% isoflurane), respectively. The corresponding CBF changes in different brain structures are illustrated in Fig 3. Similarly, CBF in frontal cortex, ACC and motor cortex (Fig 3) increased 4.5% (±1.9%), and 10% (±5%) in caudate. There was no significant difference observed between the high dose groups and the low dose base line. However, CBF in thalamus and cerebellum were increased 39% (±23%) and 55% (±15%) (1.5% vs 0.75% isoflurane), respectively. In addition, the changes were linearly and significantly correlated with the three isoflurane dose levels (Fig 4). During each scanning session, the mean arterial pressure (MAP) of animals decreased at high dose levels but was still maintained in the normal range (Fig 5), although significant MAP decrease was observed at 1.5% isoflurane.
Fig 2.
Dose-dependence effect of isoflurane on mean CBF in global, cortical and subcortical regions of anesthetized macaque monkeys (mean±SD). CBF in each ROI is normalized to the mean CBF value of three dose levels: 0.75%, 1.0%, 1.5%.*: p<0.05
Fig 3.
Dose-dependence effect of isoflurane on regional CBF in different brain regions of anesthetized macaque monkeys (mean±SD). CBF in each ROI is normalized to the mean CBF value of three dose levels: 0.75%, 1.0%, 1.5%(end -tidal), respectively.
*: p<0.05, in comparison with CBF under 0.75%isoflurane anesthesia.
Fig 4.
The correlation of isoflurane dose levels with regional CBF changes in thalamus (blue) and cerebellum (red)of macaque monkeys.
Fig 5.
The mean arterial pressure (MAP) at different isoflurane dose levels. *: p<0.05, in comparison with 0.75% isoflurane.
Discussion and conclusion
The dose dependence effects of isoflurane on mean CBF have been investigated systemically at several dose levels ( 0, ~0.24, ~0.5, ~0.95, and ~1.4%) in previous study in baboons [21]. It has been demonstrated that isoflurane induces vasoconstriction in the low concentrations (~0.5% or less) and vasodilatation in the high concentration (~0.95% or more), indicating that there exist transition doses in the range of 0.5% to 0.95%. In addition, at the high isoflurane concentration (1.4%), MAP was decreased to 50% of the baseline (without isoflurane) and impaired CBF auto-regulation was observed. Murphy et al has reported that isoflurane did not cause CBF increase in normacapic volunteers at 0.6 to 1.1 MAC, but resulted in significant increase at 1.6 MAC [11]. Reinstrup et al observed that relative CBF was increased with 1.0 MAC isoflurane in subcortical regions of human by using SPECT imaging [14, 15]. Similarly, the present monkey results demonstrate that the global CBF increase is more prominent from 1.0% to 1.5% than from 0.75% to 1.0% (Fig 2). More specifically, there was no observable CBF change in the cortex regions, and the global CBF increase is majorly contributed by the CBF increase in the subcortical structures(Fig 2).
The previous studies in canines and baboons have shown that the CBF autoregulation was disturbed under high doses of isoflurane [8, 9]. Our study indicates that the CBF autoregulation in the subcortical regions is more susceptible than that in the cortical regions. The regional CBF changes in different brain structures were illustrated (Fig 3). It is demonstrated that regional CBF changes in the frontal cortex, ACC and motor cortex of monkeys were less than 7%, and not significant (Fig 3), indicating the preserved CBF autoregulation in these cortical regions. Also, no obvious CBF change was observed in caudate (Fig 3). However, CBF in thalamus and cerebellum increased about 39% and 55% (0.75% vs 1.5% isoflurane) and the CBF changes correlated linearly and significantly with the isoflurane dose levels (Fig 4), suggesting impaired CBF autoregulation in those subcortical structures.
Thalamus plays critical roles in relaying signals from the basal ganglia and cerebellum to the cerebral cortex. Prior rat results demonstrate that isoflurane has dose-dependent effects on neural functionality of thalamus [3]. Higher CBF changes in thalamus and cerebellum have been reported in previous SPECT study of human under 1 MAC isoflurane [14, 15]. Also, CBF increasing in the thalamic area (0.4 MAC isoflurane vs baseline) was observed in the human study by using contrast-enhanced MRI perfusion measurement [6]. Previous cat study has demonstrated that the blood-brain-barrier (BBB) in thalamus can be opened with 1% isoflurane while BBB in the cortex will be broken down at higher dose (3%) [20]. Those prior results indicate that thalamus is more susceptible to high-dose isoflurane than cortical structures, consistent with our finding. Also, it has been reported that CBF in young adult human thalamus was reduced at higher isoflurane dose level in previous PET study(1.0 vs 0.4 MAC isoflurane)[16]. The disagreement between the previous study and the present one may be due to the different isoflurane doses and experimental setting applied between the two studies and probably species difference as well.
CBF in cerebellum is more susceptible to isoflurane dose levels than in other ROIs (Fig 3). Previous study in human has showed that isoflurane caused CBF increase in cerebellum [15]. In this study, the ROI is placed in the upper part of the cerebellum structure and mainly covers the anterior lobe of cerebellum (Fig 1). Our results demonstrate that the regional CBF in cerebellum is associated significantly with the isoflurane dose levels, suggesting that the CBF auto-regulation in cerebellum is also impaired under the maintenance doses.
In the present study, MAP was showing a decreasing trend while isoflurane concentration was increasing (Fig 5). However, it was still maintained in the normal range (between 60 to 100 mmHg) during each MRI scanning session [4, 21]. The CBF autoregulation and BBB can be disrupted when MAP is beyond the normal range between 60 to 150 mmHg in human. Doses of less than 1.5 MAC have been suggested in neuroanesthesia of human in order to maintain the cerebral autoregulatory function [18]. The present findings in monkeys demonstrated that the CBF auto-regulation was disrupted in thalamus and cerebellum under routine maintenance isoflurane. Further experiments are needed to evaluate the BBB integration in these subcortical regions.
Neuroimaging examination of NHPs is usually conducted under anesthesia with the routine maintenance doses. The administered isoflurane concentration may be adjusted for different study purpose or to accommodate to each subject’s physiological or pathological condition. It is known that the BOLD function response is reduced under isoflorane anesthesia. Our finding demonstrates that, under the 0.75–1.5% maintenance doses, the CBF auto-regulation is well preserved in the cortical structures and caudate, but impaired in thalamus and cerebellum, indicating disturbed CBF-metabolism coupling in specific subcortical regions. The finding suggests that the isoflurane dose effect on regional CBF needs be taken into account in the relevant neuroimaging examinations of NHPs or human patients.
In addition, the blood flow velocity could be affected due to the isoflurane dose changes in each scanning session, resulting in potential variations in labeling efficiency. Accordingly, the CBF quantification in the CASL perfusion measurement might be affected. In this study, the labeling process at different isoflurane dose levels still meets the conditions for adiabatic inversion, as the blood water is labeled with the flow induced adiabatic inversion in CASL with a separate labeling coil. Therefore, the influence of the labeling efficiency variation to CBF quantification due to the isoflurane dosage related flow velocity changes is very minor in this study.
In conclusion, within 0.75–1.5% isoflurane for maintenance of anesthesia, subcortical structures are susceptible to the isoflurane concentration. The regional CBF changes correlate linearly with the isofluane dose levels in thalamus and cerebellum and the CBF autoregulation is impaired. In contrast, the CBF autoregulation in the cortical structures and caudate is preserved and no obvious CBF changes are observed, indicating disturbed CBF-metabolism coupling and functional response in specific subcortical regions of anesthetized macaque monkeys.
Acknowledgments
The authors are grateful to Wendy Williamson Coyne and Ruth Connelly for animal handling and the anonymous reviewers for their valuable suggestions. The project was funded by the National Center for Research Resources P51RR000165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD011132, and by PHS GrantUL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
Footnotes
Conflict of interest statement
The authors have no conflict of interest in this work.
References
- 1.Cucchiara RF, Theye RA, Michenfelder JD. The effects of isoflurane on canine cerebral metabolism and blood flow. Anesthesiology. 1974;40:571–574. doi: 10.1097/00000542-197406000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- 3.Detsch O, Vahle-Hinz C, Kochs E, Siemers M, Bromm B. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res. 1999;829:77–89. doi: 10.1016/s0006-8993(99)01341-4. [DOI] [PubMed] [Google Scholar]
- 4.Hom GJ, Bach TJ, Carroll D, Forrest MJ, Mariano MA, Trainor CE, Wang PR, MacIntyre DE. Comparison of Cardiovascular Parameters and/or Serum Chemistry and Hematology Profiles in Conscious and Anesthetized Rhesus Monkeys (Macaca mulatta) Contemp Top Lab Anim Sci. 1999;38:60–64. [PubMed] [Google Scholar]
- 5.Kimme P, Ledin T, Sjoberg F. Dose effect of sevoflurane and isoflurane anesthetics on cortical blood flow during controlled hypotension in the pig. Acta Anaesthesiol Scand. 2007;51:607–613. doi: 10.1111/j.1399-6576.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz IH, Kolbitsch C, Hormann C, Schocke M, Felber S, Zschiegner F, Hinteregger M, Kremser C, Pfeiffer KP, Benzer A. Subanesthetic concentration of sevoflurane increases regional cerebral blood flow more, but regional cerebral blood volume less, than subanesthetic concentration of isoflurane in human volunteers. J Neurosurg Anesthesiol. 2001;13:288–295. doi: 10.1097/00008506-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30:242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matta BF, Heath KJ, Tipping K, Summors AC. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology. 1999;91:677–680. doi: 10.1097/00000542-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Mcpherson RW, Traystman RJ. Effects of Isoflurane on Cerebral Auto-Regulation in Dogs. Anesthesiology. 1988;69:493–499. doi: 10.1097/00000542-198810000-00008. [DOI] [PubMed] [Google Scholar]
- 10.McPherson RW, Traystman RJ. Effects of isoflurane on cerebral autoregulation in dogs. Anesthesiology. 1988;69:493–499. doi: 10.1097/00000542-198810000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Murphy FL, Jr, KE, Johnstone RE. The effects of enflurane, isoflurane and halothane on cerebral blood flow and metablism in man. Abstracts of scientific papers, annual meeting of the American Society of Anesthesiologists. 1974:61–62. [Google Scholar]
- 12.Olsen KS, Henriksen L, Owen-Falkenberg A, Dige-Petersen H, Rosenorn J, Chraemmer-Jorgensen B. Effect of 1 or 2 MAC isoflurane with or without ketanserin on cerebral blood flow autoregulation in man. Br J Anaesth. 1994;72:66–71. doi: 10.1093/bja/72.1.66. [DOI] [PubMed] [Google Scholar]
- 13.Oshima T, Karasawa F, Okazaki Y, Wada H, Satoh T. Effects of sevoflurane on cerebral blood flow and cerebral metabolic rate of oxygen in human beings: a comparison with isoflurane. Eur J Anaesthesiol. 2003;20:543–547. doi: 10.1017/s0265021503000863. [DOI] [PubMed] [Google Scholar]
- 14.Reinstrup P, Ryding E, Algotsson L, Berntman L, Uski T. Regional cerebral blood flow (SPECT) during anaesthesia with isoflurane and nitrous oxide in humans. Br J Anaesth. 1997;78:407–411. doi: 10.1093/bja/78.4.407. [DOI] [PubMed] [Google Scholar]
- 15.Reinstrup P, Ryding E, Algotsson L, Messeter K, Asgeirsson B, Uski T. Distribution of cerebral blood flow during anesthesia with isoflurane or halothane in humans. Anesthesiology. 1995;82:359–366. doi: 10.1097/00000542-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Schlunzen L, Cold GE, Rasmussen M, Vafaee MS. Effects of dose-dependent levels of isoflurane on cerebral blood flow in healthy subjects studied using positron emission tomography. Acta Anaesthesiol Scand. 2006;50:306–312. doi: 10.1111/j.1399-6576.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- 17.Silva AC, Zhang W, Williams DS, Koretsky AP. Estimation of water extraction fractions in rat brain using magnetic resonance measurement of perfusion with arterial spin labeling. Magn Reson Med. 1997;37:58–68. doi: 10.1002/mrm.1910370110. [DOI] [PubMed] [Google Scholar]
- 18.Strebel S, Lam AM, Matta B, Mayberg TS, Aaslid R, Newell DW. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83:66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Talagala SL, Ye FQ, Ledden PJ, Chesnick S. Whole-brain 3D perfusion MRI at 3.0 T using CASL with a separate labeling coil. Magn Reson Med. 2004;52:131–140. doi: 10.1002/mrm.20124. [DOI] [PubMed] [Google Scholar]
- 20.Tetrault S, Chever O, Sik A, Amzica F. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur J Neurosci. 2008;28:1330–1341. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- 21.Van Aken H, Fitch W, Graham DI, Brussel T, Themann H. Cardiovascular and cerebrovascular effects of isoflurane-induced hypotension in the baboon. Anesth Analg. 1986;65:565–574. [PubMed] [Google Scholar]
- 22.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Nagaoka T, Auerbach EJ, Champion R, Zhou L, Hu X, Duong TQ. Quantitative basal CBF and CBF fMRI of rhesus monkeys using three-coil continuous arterial spin labeling. Neuroimage. 2007;34:1074–1083. doi: 10.1016/j.neuroimage.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]