Abstract
Hepatocyte growth factor (HGF), through activation of the c-MET receptor, mediates biological processes critical for tissue regeneration; however, its clinical application is limited by protein instability and poor recombinant expression. We previously engineered a HGF fragment (eNK1) that possesses increased stability and expression yield, and developed a c-MET agonist by coupling eNK1 through an introduced cysteine residue. Here, we further characterize this eNK1 dimer, and show it elicits significantly greater c-MET activation, cell migration, and proliferation than the eNK1 monomer. The efficacy of the eNK1 dimer was similar to HGF, suggesting its promise as a c-MET agonist.
Keywords: Hepatocyte growth factor, protein engineering, c-MET receptor, tissue regeneration, ligand/receptor interaction, receptor agonist
1. Introduction
The c-MET tyrosine kinase receptor has been implicated in the processes of organogenesis and wound healing, and is dysregulated in numerous cancer malignancies [1–3]. Given its overexpression in both developing tissue and tumors, much effort has gone into developing both agonists and antagonists of c-MET for diseases such as ischemia and cancer, respectively [3–15]. In the case of c-MET agonists, there is a need for molecules that effectively stimulate the canonical c-MET pathway leading to proliferation, migration, and survival. Hepatocyte growth factor (HGF) is an 80 kDa, multi-domain protein that is the cognate ligand and activator of c-MET [1, 16]. Despite its biological potency, the clinical adoption of HGF as a therapeutic for tissue regeneration has been limited by its inherent instability. Moreover, there are significant challenges in manufacturing such a complex growth factor. HGF can only be produced in insect or mammalian cells in low quantities; one study reported a yield of 1.2 mg/L from Chinese hamster ovary cell culture [17]. Thus, there is great interest in approaches such as gene delivery for production of HGF [6, 18], development of HGF activators [4, 15], or development of alternative c-MET agonists [5, 19–22].
Many efforts to develop c-MET agonists have focused on a fragment of HGF, termed NK1, which encompasses the N-terminal domain, a linker region, and the first Kringle domain of HGF. The NK1 fragment contains high-affinity c-MET and heparin binding sites necessary for functional activity [11, 21, 23–27]; however, it is a weak c-MET agonist compared to full-length HGF [23]. NK1 can be recombinantly produced in both bacteria and yeast [26, 28], but like HGF suffers from poor stability and low expression yields. Previously, we used combinatorial protein engineering to identify NK1 variants with improved thermostability and up to 40-fold increase in recombinant expression yield compared to wild-type NK1 [29]. These NK1 variants functioned as weak agonists or antagonists, depending on retention or mutation of Asn127 in the linker region, which mediates NK1 homodimerization [11, 26, 29, 30]. In addition, we created a covalent dimer through introduction of an N-terminal cysteine residue into an engineered NK1 agonist (eNK1), which allowed specific crosslinking through a disulfide bond [29]. This eNK1 dimer functioned as a c-MET agonist, as measured through cell scatter and expression of urokinase-type plasminogen activator.
Here, we determined that the covalent eNK1 dimer can act as a surrogate for HGF by testing its ability to activate c-MET receptor phosphorylation and downstream signaling pathways, cell migration, and cell proliferation compared to HGF and the eNK1 monomer. The eNK1 dimer offers the combined properties of high thermostability, ease of recombinant production in yeast, and comparable biological activity to HGF, suggesting promise for further therapeutic development.
2. Experimental Methods
2.1. Cells and Reagents
The term “eNK1 monomer” refers to the engineered NK1 variant M2.2 D127N, and “eNK1 dimer” refers to the disulfide-linked dimer cd D127N from our previous work [29]. HGF was from R&D Systems. FGFb was from GIBCO. BaF3-MET cells were provided by Patrick Ma, Case Western Reserve University. BaF3-MET growth media was RPMI 1640 medium with Glutamax (Invitrogen) with 10% fetal bovine serum (FBS) (American Type Culture Collection), 1% penicillin-streptomycin, 0.5 ng/mL mouse interleukin-3 (R&D Systems), and 1 mg/mL Geneticin (GIBCO). Human umbilical vein endothelial cells (HUVECs) and the EGM-2 BulletKit were purchased from Lonza Walkersville Inc. A549 cells were grown in Dulbecco’s Modified Eagle Media (Invitrogen) with 10% FBS and 1% penicillin-streptomycin. The PE-conjugated anti-FLAG antibody for binding assays was from Prozyme. Primary antibodies for Western blot analysis were all from Cell Signaling except for β-tubulin (Covance) and c-MET (Santa Cruz Biotechnology). All secondary antibodies were purchased from Jackson ImmunoResearch, and chemoluminescent substrate was from ThermoScientific. NP-40 buffer was composed of 20 mM Tris pH 8.0, 137 mM NaCl, 10% glycerol, and 1% IGEPAL/NP40. All cells and reagents for the PathHunter Assay were from DiscoveRx Corporation.
2.2. Yeast production of NK1 variants
Growth and induction media for recombinant protein expression in Pichia pastoris and detailed protein purification methods were as previously described [29]. The eNK1 dimer was produced by expression of a modified variant of eNK1 containing an N-terminal cysteine residue. Protein purity was analyzed using SDS-PAGE and quantified using a Nanodrop 2000 (Thermo Scientific) with the extinction coefficients 25662 M−1cm−1 for the eNK1 monomer and 51324 M−1cm−1 for the eNK1 dimer. Protein was flash-frozen in 0.1% Tween20 in 1x phosphate buffered saline containing an additional 500 mM NaCl (PBS500) and stored at −80 °C. Thawed protein was kept at 4 °C and used within three weeks. In Figure 1A, purified proteins were analyzed on a 4–12% Bis-Tris gel (Invitrogen).
Figure 1. Biochemical and biophysical characterization of eNK1 monomer and dimer.
A) Non-reduced SDS-PAGE of purified eNK1 monomer and eNK1 dimer. B) Representative competition binding curves of eNK1 monomer and dimer on HUVECs, measured by flow cytometry and normalized to maximal binding levels. C) Circular dichroism spectra of eNK1 monomer and dimer acquired at 20 °C. D) Thermal melting curves of eNK1 monomer and dimer as measured by circular dichroism spectroscopy. In all panels, eNK1 monomer is indicated by filled circles, and eNK1 dimer by open circles.
2.3. Circular dichroism
Circular dichroism (CD) spectroscopy was performed on a J-815 CD Spectropolarimeter (Jasco Corporation). eNK1 monomer and eNK1 dimer were diluted in 1x PBS and spectra were collected at 20 °C from λ = 180–260 nm. Thermal melts were performed over a temperature range of 30–90°C. The Tm,eff was fit to a two-state unfolding curve using GraphPad Prism 6. Error bars represent the standard deviation of triplicate experiments.
2.4. Cell binding assays
For competition binding assays, a monomeric NK1 variant containing a FLAG epitope tag (FLAG-M2.2 D127A) [29] was used as a competitor. Competitor concentrations of FLAG-M2.2 D127A were 12 nM and 15 nM for HUVECs and BaF3-MET, respectively. HUVECs were incubated with 600 ng/mL of FGFb for 2 h at 4 °C to completely block heparan sulfate on the cell surface [29], followed by washing with BPBS (1x PBS + 0.1% bovine serum albumin (BSA)). After incubation with NK1 variants and competitor for 5 h, cells were washed with BPBS and incubated with PE-conjugated anti-FLAG antibody for 20 min at 4 °C, then washed and analyzed by flow cytometry using a Guava EasyCyte (Millipore). All data was analyzed using Flow-Jo software and IC50 values were determined by fitting to a four-parameter sigmoidal curve using GraphPad Prism 6. Binding reactions were performed at 4 °C to preclude receptor internalization. Values in Table 1 represent the standard deviation of triplicate experiments.
Table 1.
Compilation of melting temperature (Tmeff) and relative binding affinity (IC50) for two cell types.
| NK1 mutant | Tmeff (°C) | IC50 (nM)
|
|
|---|---|---|---|
| HUVEC | BaF3-MET | ||
| eNK1 | 65.0 ± 0.2 | 1.8 ± 0.7 | 2.2 ± 0.4 |
| eNK1 dimer | 61.9 ± 0.7 | 0.023 ± 0.02 | 0.014 ± 0.004 |
2.5. Phosphorylation assays
HUVECs or A549 cells were grown until 50% confluence. Cell quiescence was induced by incubation for 12 h in basal media + 0.1% BSA, followed by addition of either 1 nM or 10 pM of HGF or NK1 variants for time points indicated. Cells were incubated for the indicated times at 37 °C/5% CO2, then lysed with NP-40 buffer containing protease and phosphatase inhibitors (Thermo Scientific). Equal amounts of lysate were loaded on Tris-Glycine gels (Invitrogen) and transferred onto nitrocellulose membranes. Western Blot analysis was performed with the reagents listed above. Chemiluminescence was detected using the ChemiDoc XRS System (Bio-Rad).
2.6. DiscoveRx PathHunter® Platform
This assay, which was performed according to the manufacturer’s instructions, uses enzyme fragment complementation technology, where c-MET dimerization and trans-phosphorylation induces a downstream reaction, with chemiluminescence as the output. Relative chemiluminescence therefore corresponds to the level of c-MET phosphorylation and activation. Briefly, PathHunter® proprietary U2OS cells were seeded in CP16 media and grown overnight at 37 °C. HGF or NK1 variants were added to the cells and incubated for 3 h at room temperature. PathHunter® Detection Reagent was added to each well, and incubated for 1 h at room temperature in the dark. Chemiluminescence was measured using a microtiter plate reader (BioTek).
2.7. Cell migration assay
1.3×104 HUVECs were plated and grown to confluency. After inducing quiescence by incubating for 12 h in basal media + 0.1% BSA, a horizontal “wound” was created in cell monolayers using a scratching device. HGF or NK1 variants were added to the wells, along with 10 pM FGFb, and plates were incubated at 37 °C/5% CO2. Images were taken at 0 and 24 h using an ImageXpress 5000A Scanner (Molecular Devices). Controls of HGF or NK1 variants only without FGFb, FGFb only (negative control) or HUVEC complete growth media (positive control) were included in each assay. ImageJ was used to measure the wound area at both time points, and the average fraction wound closure was calculated for each condition over triplicate samples. The experiment was performed three times.
2.8. Cell proliferation assay
4×103 HUVECs were seeded and grown for 24 h, and quiescence was induced by incubating for 12 h in basal media + 0.1% BSA. HGF or NK1 variants were then added, along with 10 pM FGFb, and incubated for 24 h at 37 °C/5% CO2. Next, 2 μCi tritiated thymidine (MP Biomedicals) was added to each well and incubated for an additional 24 h at 37 °C/5% CO2, after which the supernatant was removed and the cells lysed via freeze-thaw. The amount of tritiated thymidine incorporated into newly synthesized DNA was measured using a scintillation counter (PerkinElmer). Error bars represent the standard deviation of triplicate wells. Data was measured against negative control with only FGFb added.
3. Results
3.1 eNK1 dimer retains biophysical characteristics of the eNK1 monomer, while exhibiting increased c-MET binding affinity
The eNK1 monomer and disulfide-linked eNK1 dimer were solubly expressed in Pichia pastoris, followed by purification using metal chelating chromatography and size exclusion chromatography. Protein purity was assessed by SDS-PAGE (Figure 1A). Competition binding assays were used to determine the apparent affinities of eNK1 monomer or eNK1 dimer to c-MET expressed on the surface of BaF3-MET, a murine cell line transfected with human c-MET receptor, [31] and HUVECs, a primary cell line used extensively in angiogenesis and tissue regeneration studies [32–34]. eNK1 retains strong binding to heparin/heparan sulfate proteoglycans (HSPG) [29]. Thus, direct measurement of eNK1/c-MET interactions was facilitated by BaF3-MET cells, which lack heparin/HSPG on their surface [35]. Since HUVECs have heparan sulfate on their surface, cells were preincubated with basic fibroblast growth factor (FGFb) prior to binding assays, as we previously showed this strategy could effectively block heparin binding sites on the cell surface [29]. For both cell lines, the eNK1 dimer had a significantly lower half-maximal inhibitory concentration value (IC50) in the picomolar range compared to the eNK1 monomer, indicating that dimerization increases the apparent binding affinity by over an order of magnitude through avidity effects (Figure 1B, Table 1). The equilibrium binding constant (KD) of HGF to c-MET has been measured to be in the picomolar range [36, 37].
A main advantage of the eNK1 monomer over wild-type NK1 is its increase in thermal stability [29]. We used circular dichroism spectroscopy to determine if structural elements or thermal stability were affected by creating the dimeric version of the protein. The CD spectra of the eNK1 monomer and eNK1 dimer were comparable (Figure 1C), indicating that covalent dimerization does not perturb secondary structure. The apparent thermal melting temperature (Tm,eff) of both proteins was similar: 65.0 ± 0.2 °C and 61.9 ± 0.7 °C for the eNK1 monomer and eNK1 dimer, respectively (Figure 1D, Table 1), indicating that the dimer maintains the enhanced thermal stability of its monovalent counterpart compared to wild-type NK1 (Tm,eff = 50.7 ± 0.2 °C) [29].
3.2 eNK1 dimer potently activates the c-MET receptor and downstream signaling pathways
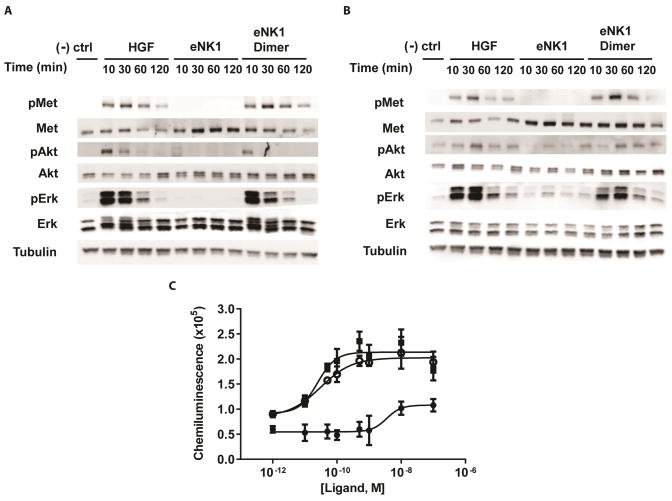
An ideal c-MET agonist is one that would have comparable biological potency to HGF. We measured the ability of the eNK1 dimer to activate the c-MET receptor and downstream cell signaling pathways in HUVECs compared to the eNK1 monomer and HGF (Figure 2). Phosphorylation of c-MET and downstream targets Erk and Akt lead to cellular responses of survival, migration, and proliferation [2]. The eNK1 monomer was unable to activate c-MET, Erk, or Akt to an appreciable degree, although weak activation levels were seen at longer exposure times. In contrast, the eNK1 dimer stimulated phosphorylation of c-MET, Erk, and Akt with similar potency to full-length HGF at both 1 nM (Figure 2A) and 10 pM (Figure 2B). Similar results were obtained in the human lung carcinoma cell line A549 (Supplementary Figure 1). HGF and the eNK1 dimer also activated c-MET phosphorylation to equivalent levels using the DiscoveRx PathHunter system (Figure 2C). In this assay, the eNK1 monomer had an EC50 for c-MET phosphorylation of more than an order of magnitude higher compared to the eNK1 dimer and HGF, and exhibited a lower maximal level of c-MET phosphorylation.
Figure 2. Phosphorylation of c-MET and downstream targets.
HGF, eNK1 monomer, or eNK1 dimer was added to HUVEC cells at concentrations of A) 1 nM, or B) 10 pM for the indicated timepoints. Negative control indicates basal media alone without added c-MET agonist. Western blots of cell lysates analyzed using a phospho-specific antibody (‘p’) indicate activated c-MET, Akt and Erk. Total protein levels of c-MET, Akt and Erk are shown to relate the fraction of phosphorylated protein to total protein between treatment conditions. Tubulin is shown as a control to indicate total protein loaded per gel lane prior to Western blotting. C) HGF, eNK1 monomer, or eNK1 dimer were added to c-MET expressing cells, and activation was quantified using the DiscoveRx PathHunter system, which measures chemiluminescence as a readout. HGF is depicted as filled squares, eNK1 monomer as filled circles and eNK1 dimer by open circles.
3.3 eNK1 dimer stimulates increased cell migration compared to eNK1 monomer
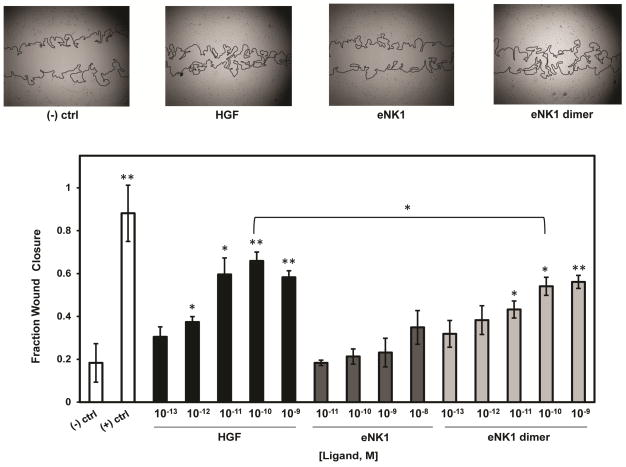
Tissue regeneration requires a host of biological processes including cell migration into the wound area and cell proliferation [38]. A directional migration assay was used to investigate cell migration in response to HGF, eNK1 dimer, or eNK1 monomer. In this assay, a scratch is created in a monolayer of HUVECs as an in vitro proxy for wound healing, and cell migration into this void is measured over time. Preliminary experiments showed only marginal increases in cell migration upon the addition of any of the three ligands alone. However, enhanced cell migration was observed when HGF was combined with FGFb (Supplementary Fig. 2), a growth factor whose receptor is known to crosstalk with c-MET [39, 40]. HGF or the eNK1 dimer, when combined with 10 pM FGFb, showed significant dose-dependent increases in cell migration into the wound area over 24 h compared to the FGFb-only negative control, with the eNK1 dimer showing levels of activity approaching that of HGF (Figure 3). In contrast, the eNK1 monomer stimulated minimal amounts of cell migration only at the highest concentration tested.
Figure 3. Cell migration assay.
Top images show representative HUVEC wounds 24 h following incubation with 10 pM FGFb and 1 nM HGF, eNK1 monomer, or eNK1 dimer. Bar graph shows the fraction of wound closure with increasing concentrations of HGF or NK1 variant. Negative control represents media containing 10 pM FGFb alone. Positive control represents incubation of cells in complete growth media. Significance was measured against the negative control except where indicated. *p<0.05, **p<0.005. p<0.005 for all corresponding concentrations between HGF/eNK1 dimer and eNK1 monomer.
3.4 eNK1 dimer stimulates increased cell proliferation compared to eNK1 monomer
Finally, we tested the ability of the eNK1 dimer to stimulate HUVEC proliferation compared to the eNK1 monomer and HGF. Cell proliferation was measured by incorporation of tritiated thymidine into newly synthesized DNA (Figure 4). For this assay, the addition of 10 pM FGFb was again required to amplify the biological effects of ligand stimulation. Both HGF and the eNK1 dimer showed positive dose response curves, with the eNK1 dimer stimulating slightly lower levels of cell proliferation. In contrast, the eNK1 monomer stimulated proliferation appreciably over background levels only at the highest concentration tested.
Figure 4. Cell proliferation assay.
HUVECs were incubated with varying concentrations of HGF, eNK1 monomer, or eNK1 dimer with 10 pM FGFb for 24 h. Cell proliferation was measured by uptake of tritiated thymidine into newly synthesized DNA. Significance was measured against the negative control (FGFb only) unless otherwise indicated. *p<0.05, **p<0.005.
4. Discussion
c-MET activators are currently under clinical development for a variety of biomedical applications. In particular, recombinant HGF is being tested in human clinical trials for treatment of spinal cord injury (Phase I/II trial), amyotrophic lateral sclerosis (Phase I trial) [41, 42], hepatitis (Phase I/II trial) [43], and venous leg ulcers (Phase I/II trial) [44]. In parallel with these efforts, challenges with recombinant expression and poor stability of HGF have motivated gene therapy approaches for protein production, which is being tested in clinical trials for peripheral arterial disease [45], critical limb ischemia [46], and amyotrophic lateral sclerosis [47]. In addition, a small molecule HGF mimetic is in Phase II trials for myocardial infarction [48]. Work from academic research groups has explored the use of HGF fragments as alternative c-MET agonists [5, 19–22]; however, these proteins possess similar limitations as HGF.
Development of NK1-based c-MET effectors have been informed by insights into the HGF/NK1 mechanism of action [11, 21, 26, 30, 49, 50]. NK1 is the minimal fragment of HGF that has been shown to activate c-MET; however, it functions as a weak agonist [23]. Compared to wild-type NK1, the engineered NK1 variant identified in our previous work (eNK1; also termed M2.2 D127N) has identical functional activity, a 15 °C increase in thermal stability, and up to ~40-fold increased recombinant expression yield from yeast cultures, but is still limited by low biological potency compared to HGF. In the present study we showed that a covalently-crosslinked dimer created from eNK1 retains this high stability and binds to c-MET with a relative affinity that is over an order of magnitude stronger than the eNK1 monomer due to avidity effects. Because of the tight affinity of this eNK1 dimer, avoiding ligand depletion conditions that affect Langmuir binding isotherms would have required reaction volumes of over 1 liter and several days to reach equilibrium, which is not compatible with cell surface binding assays. Thus, our binding measurements may underestimate c-MET affinity, but the picomolar IC50 values we observed for the eNK1 dimer on two different cell lines lie within a similar range of affinities previously measured for HGF [36, 37].
In addition to stimulating c-MET phosphorylation, the eNK1 dimer activated similar cell signaling pathways as HGF, including downstream targets Akt and Erk, with comparable potency and temporal effects. The eNK1 monomer induced cell signaling only at high concentration, highlighting the importance of ligand-mediated receptor dimerization for activation. The DiscoveRx PathHunter assay offers a more quantitative measure of c-MET activation, and the results roughly mirror the Western blot phosphorylation experiments where the eNK1 dimer activates c-MET to a similar degree compared to HGF, while the eNK1 monomer activates a lower level of c-MET phosphorylation at the highest concentration tested. In functional assays, both the eNK1 dimer and HGF stimulated HUVEC migration and proliferation at low nanomolar concentrations, but required the addition of FGFb. In these experiments, we observed trends of dose response-mediated activity from protein ligands alone, but these results were not statistically significant. The dependence of c-MET mediated cell migration and proliferation on FGFb suggests that synergistic effects of signaling through multiple receptor tyrosine kinase receptors is necessary for these biological outputs.
HGF is a unique growth factor, encompassing 6 domains including an N-terminal domain, four Kringle domains (K1, K2, K3, and K4), and a serine protease homologous (SPH) domain [1]. While the NK1 and the SPH domains have been shown to bind both c-MET and heparin [25, 51], the role of the other Kringle domains is unclear due to the lack of high resolution structural information available for HGF and c-MET. One likely hypothesis is that the K2-K4 domains serve to orient HGF in a way that facilitates an optimal NK1 conformation for dimerization and receptor activation [49, 50]. In our study, dimerization of eNK1 through a covalent disulfide bond obviates the need for these additional HGF domains, at least for the biological functions tested here, positioning the c-MET receptor in a conformation that drives activation and cell signaling.
Comparable agonistic activity observed with the eNK1 dimer suggests that it could be a suitable replacement for HGF, with the additional benefits of increased stability and ability to be produced in a microbial expression system. Future directions of this work include testing the therapeutic efficacy of the eNK1 dimer in animal models of disease such as ischemia, multiple sclerosis, and diabetes [52–54]. As the eNK1 dimer retains binding to heparin [29], it may also find utility in combination with naturally-derived or synthetic biomaterial scaffolds that contain heparin or heparin-binding epitopes [55–57] for localized, sustained delivery in vivo for tissue regeneration applications.
Supplementary Material
1 nM of HGF, eNK1 monomer, or eNK1 dimer was added to A549 cells for the indicated time points. Negative control indicates basal media alone without added c-MET agonist. Western blots of cell lysates analyzed using a phospho-specific antibody (‘p’) indicate activated c-MET, Akt and Erk. Total protein levels of c-MET, Akt and Erk are shown to relate the fraction of phosphorylated protein to total protein between treatment conditions. Tubulin is shown as a control to indicate total protein loaded per gel lane prior to Western blotting.
A) Scratched HUVEC monolayers were incubated with 1 nM each of VEGF121, EGF, or FGFb, either individually or in combinations with 1 nM HGF. Wound closure of HUVECs was compared between conditions. B) Either 0.01 or 0.1 nM of VEGF121 or FGFb was added, in addition to varying concentrations of HGF, to identify the optimal concentration of VEGF121 or FGFb that resulted in synergistic effects on wound closure. Negative control indicates media alone without growth factor added. Positive control indicates the addition of complete growth media. Wound area was measured immediately after scratching and at 24 h.
Highlights.
HGF is difficult to produce and unstable, highlighting a need for c-MET agonists.
A disulfide-linked dimer (eNK1 dimer) was created from an engineered HGF fragment.
The eNK1 monomer is a weak agonist and only activates c-MET at high concentrations.
The eNK1 dimer is a potent activator of c-MET, cell migration, and proliferation.
The eNK1 dimer elicits similar biological activity compared to HGF.
Acknowledgments
This work was funded in part by NIH R01 CA151706 (J.R.C.), an NSF Graduate Research Fellowship (C.J.L.), NIH training grants 5T32 GM008412 (D.S.J.) and 5T32 CA09302 (P.C.T.), a Siebel Graduate Fellowship (D.S.J.), and the Stanford Bioengineering REU program (A.V.). We acknowledge Dr. Arnold Hayer and Dr. Feng-Chiao Tsai for their technical support with the cell migration assay, and thank Patrick Ma (Case Western Reserve) for BaF3-MET cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakamura T, Mizuno S. The discovery of Hepatocyte Growth Factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad, Ser B. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 3.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 4.Landgraf KE, Santell L, Billeci KL, et al. Allosteric peptide activators of pro-hepatocyte growth factor stimulate Met signaling. J Biol Chem. 2010;285:40362–72. doi: 10.1074/jbc.M110.179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietronave S, Forte G, Locarno D, et al. Agonist monoclonal antibodies against HGF receptor protect cardiac muscle cells from apoptosis. Am J Physiol Heart Circ Physiol. 2010;298:H1155–65. doi: 10.1152/ajpheart.01323.2008. [DOI] [PubMed] [Google Scholar]
- 6.Pyun W-B, Hahn W, Kim D-S, et al. Naked DNA expressing two isoforms of hepatocyte growth factor induces collateral artery augmentation in a rabbit model of limb ischemia. Gene Ther. 2010;17:1442–52. doi: 10.1038/gt.2010.101. [DOI] [PubMed] [Google Scholar]
- 7.Rickert KW, Patel SB, Allison TJ, et al. Structural basis for selective small molecule kinase inhibition of activated c-Met. J Biol Chem. 2011;286:11218–25. doi: 10.1074/jbc.M110.204404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzone M, Basilico C, Cavassa S, et al. An uncleavable form of pro – scatter factor suppresses tumor growth and dissemination in mice. J Clin Invest. 2004;114:1418–1432. doi: 10.1172/JCI200422235.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenall Sa, Gherardi E, Liu Z, et al. Non-agonistic bivalent antibodies that promote c-MET degradation and inhibit tumor growth and others specific for tumor related c-MET. PLoS One. 2012;7:e34658. doi: 10.1371/journal.pone.0034658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchhofer D, Lipari MT, Santell L, et al. Utilizing the activation mechanism of serine proteases to engineer hepatocyte growth factor into a Met antagonist. Proc Natl Acad Sci U S A. 2007;104:5306–11. doi: 10.1073/pnas.0700184104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolbert WD, Daugherty J, Gao C, et al. A mechanistic basis for converting a receptor tyrosine kinase agonist to an antagonist. Proc Natl Acad Sci U S A. 2007;104:14592–7. doi: 10.1073/pnas.0704290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youles M, Holmes O, Petoukhov MV, et al. Engineering the NK1 fragment of hepatocyte growth factor/scatter factor as a MET receptor antagonist. J Mol Biol. 2008;377:616–22. doi: 10.1016/j.jmb.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Merchant M, Ma X, Maun HR, et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci U S A. 2013;110:E2987–96. doi: 10.1073/pnas.1302725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon Y, Smith BD, Zhou Y, et al. Effective inhibition of c-MET-mediated signaling, growth and migration of ovarian cancer cells is influenced by the ovarian tissue microenvironment. Oncogene. 2013:1–10. doi: 10.1038/onc.2013.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landgraf KE, Steffek M, Quan C, et al. An allosteric switch for pro-HGF/Met signaling using zymogen activator peptides. Nat Chem Biol. 2014;10:567–73. doi: 10.1038/nchembio.1533. [DOI] [PubMed] [Google Scholar]
- 16.Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 17.Wilke S, Krausze J, Gossen M, et al. Glycoprotein production for structure analysis with stable, glycosylation mutant CHO cell lines established by fluorescence-activated cell sorting. Protein Sci. 2010;19:1264–71. doi: 10.1002/pro.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirakawa Y, Sawa Y, Takewa Y, et al. Gene transfection with human hepatocyte growth factor complementary DNA plasmids attenuates cardiac remodeling after acute myocardial infarction in goat hearts implanted with ventricular assist devices. J Thorac Cardiovasc Surg. 2005;130:624–32. doi: 10.1016/j.jtcvs.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann G, Prospero T, Brinkmann V, et al. Engineered mutants of HGF/SF with reduced binding to heparan sulphate proteoglycans, decreased clearance and enhanced activity in vivo. Curr Biol. 1998;8:125–34. doi: 10.1016/s0960-9822(98)70059-4. [DOI] [PubMed] [Google Scholar]
- 20.Roy RS, Soni S, Harfouche R, et al. Coupling growth-factor engineering with nanotechnology for therapeutic angiogenesis. PNAS. 2010;107:13608–13613. doi: 10.1073/pnas.1006007107/-/DCSupplemental. www.pnas.org/cgi/doi/10.1073/pnas.1006007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lietha D, Chirgadze DY, Mulloy B, et al. Crystal structures of NK1-heparin complexes reveal the basis for NK1 activity and enable engineering of potent agonists of the MET receptor. EMBO J. 2001;20:5543–55. doi: 10.1093/emboj/20.20.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross J, Gherardi E, Mallorqui-Fernandez N, et al. Protein engineered variants of hepatocyte growth factor/scatter factor promote proliferation of primary human hepatocytes and in rodent liver. Gastroenterology. 2012;142:897–906. doi: 10.1053/j.gastro.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Jakubczak JL, Larochelle WJ, Merlino G. NK1, a Natural Splice Variant of Hepatocyte Growth Factor/Scatter Factor, Is a Partial Agonist In Vivo. Mol Cell Biol. 1998;18:1275–1283. doi: 10.1128/mcb.18.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwall RH, Chang LY, Godowski PJ, et al. Heparin induces dimerization and confers proliferative activity onto the hepatocyte growth factor antagonists NK1 and NK2. J Cell Biol. 1996;133:709–18. doi: 10.1083/jcb.133.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes O, Pillozzi S, Deakin Ja, et al. Insights into the structure/function of hepatocyte growth factor/scatter factor from studies with individual domains. J Mol Biol. 2007;367:395–408. doi: 10.1016/j.jmb.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 26.Chirgadze DY, Hepple JP, Zhou H, et al. Crystal structure of the NK1 fragment of HGF/SF suggests a novel mode for growth factor dimerization and receptor binding. Nat Struct Biol. 1999;6:72–9. doi: 10.1038/4947. [DOI] [PubMed] [Google Scholar]
- 27.Sakata H, Stahl SJ, Taylor WG, et al. Heparin Binding and Oligomerization of Hepatocyte Growth Factor/Scatter Factor Isoforms: Heparan Sulfate Glycosaminoglycan Requirement for MET Binding and Signaling. J Biol Chem. 1997;272:9457–9463. doi: 10.1074/jbc.272.14.9457. [DOI] [PubMed] [Google Scholar]
- 28.Stahl SJ, Wingfield PT, Kaufman JD, et al. Functional and biophysical characterization of recombinant human hepatocyte growth factor isoforms produced in Escherichia coli. Biochem J. 1997;326:763–772. doi: 10.1042/bj3260763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones DS, Tsai P-C, Cochran JR. Engineering hepatocyte growth factor fragments with high stability and activity as Met receptor agonists and antagonists. Proc Natl Acad Sci U S A. 2011;108:13035–40. doi: 10.1073/pnas.1102561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K, Chirgadze DY, Lietha D, et al. A new crystal form of the NK1 splice variant of HGF/SF demonstrates extensive hinge movement and suggests that the NK1 dimer originates by domain swapping. J Mol Biol. 2002;319:283–8. doi: 10.1016/S0022-2836(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 31.Sattler M, Pride YB, Ma P, et al. A Novel Small Molecule Met Inhibitor Induces Apoptosis in Cells Transformed by the Oncogenic TPR-MET Tyrosine Kinase. Cancer Res. 2003;63:5462–5469. [PubMed] [Google Scholar]
- 32.Park H-J, Zhang Y, Georgescu SP, et al. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006;2:93–102. doi: 10.1007/s12015-006-0015-x. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, von Ballmoos MW, Faessler D, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211:103–9. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Miya M, Maeshima A, Mishima K, et al. Enhancement of in vitro human tubulogenesis by endothelial cell-derived factors : implications for in vivo tubular regeneration after injury. Am J Physiol Ren Physiol. 2011;301:387–395. doi: 10.1152/ajprenal.00619.2010. [DOI] [PubMed] [Google Scholar]
- 35.Ornitz DM, Yayon a, Flanagan JG, et al. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240–7. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–41. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higuchi O, Mizuno K, Vande Woude GF, Nakamura T. Expression of c-met proto-oncogene in COS cells induces the signal transducing high-affinity receptor for hepatocyte growth factor. FEBS Lett. 1992;301:282–6. doi: 10.1016/0014-5793(92)80257-h. [DOI] [PubMed] [Google Scholar]
- 38.Chen J-A, Shi M, Li J-Q, Qian C-N. Angiogenesis: multiple masks in hepatocellular carcinoma and liver regeneration. Hepatol Int. 2010;4:537–47. doi: 10.1007/s12072-010-9192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavallaro UGO, Wu Z, Palo ADI, et al. FGF-2 stimulates migration of Kaposi’s sarcoma-like vascular cells by HGF-dependent relocalization of the urokinase receptor. FASEB J. 1998;12:1027–1034. doi: 10.1096/fasebj.12.11.1027. [DOI] [PubMed] [Google Scholar]
- 40.Marui A, Kanematsu A, Yamahara K, et al. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J Vasc Surg. 2005;41:82–90. doi: 10.1016/j.jvs.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Kringle Pharma, Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. A Phase I/II Study to Evaluate the Safety and Efficacy of Intrathecal Injection of KP-100IT in Subjects With Acute Spinal Cord Injury. [cited 2014 August 26]. Available from: http://clinicaltrials.gov/show/NCT02193334 NLM Identifier: NCT02193334. [Google Scholar]
- 42.Kringle Pharma, Inc.; Keio University School of Medicine. A Phase I, Dose-Escalating Study to Evaluate the Safety and Pharmacokinetics of Single and Multiple Intrathecal Infusion of KP-100IT through SM-1500 in Subjects with Amyotrophic Lateral Sclerosis (ALS) World Health Organization; 2014. [Internet] [cited 2014 August 26]. Available from: http://apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN-UMIN000007062 NLM Identifier: JPRN-UMIN000007062. [Google Scholar]
- 43.Ido A, Moriuchi A, Numata M, et al. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: a phase I/II clinical trial, following preclinical studies to ensure safety. J Transl Med. 2011;9:55. doi: 10.1186/1479-5876-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kringle Pharma Europe AB. . A Phase I/II Double-Blind, Dose Ranging, Vehicle Controlled, Randomized, Parallel Groups, Safety, Tolerability and Efficacy Study of ChronSeal® (5-amino-acid deleted recombinant human Hepatocyte Growth Factor (KP-dHGF)), in Subjects with Venous Leg Ulcers. EU Clinical Trials Register [Internet] 2012 [cited 2014 August 26]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2007-002695-34 Identifier: 2007-002695-34.
- 45.Makino H, Aoki M, Hashiya N, et al. Long-term follow-up evaluation of results from clinical trial using hepatocyte growth factor gene to treat severe peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2012;32:2503–9. doi: 10.1161/ATVBAHA.111.244632. [DOI] [PubMed] [Google Scholar]
- 46.Powell RJ, Goodney P, Mendelsohn FO, et al. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J Vasc Surg. 2010;52:1525–30. doi: 10.1016/j.jvs.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ViroMed Co., Ltd. dba VM BioPharma. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. Safety Study of VM202 to Treat Amyotrophic Lateral Sclerosis. [cited 2014 August 26]. Available from: http://clinicaltrials.gov/show/NCT02039401 NLM Identifier: NCT02039401. [Google Scholar]
- 48.Angion Biomedica Corp; National Heart, Lung and Blood Institute. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2012. Study to Evaluate the Safety and Activity of BB3 to Treat Heart Attack. [cited 2014 August 26]. Available from: http://clinicaltrials.gov/ct2/show/NCT01539590?term=NCT01539590&rank=1 NLM Identifier: NCT01539590. [Google Scholar]
- 49.Gherardi E, Sandin S, Petoukhov MV, et al. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. PNAS. 2006;103:4046–4051. doi: 10.1073/pnas.0509040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolbert WD, Daugherty-Holtrop J, Gherardi E, et al. Structural basis for agonism and antagonism of hepatocyte growth factor. Proc Natl Acad Sci U S A. 2010;107:13264–9. doi: 10.1073/pnas.1005183107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deakin Ja, Blaum BS, Gallagher JT, et al. The binding properties of minimal oligosaccharides reveal a common heparan sulfate/dermatan sulfate-binding site in hepatocyte growth factor/scatter factor that can accommodate a wide variety of sulfation patterns. J Biol Chem. 2009;284:6311–21. doi: 10.1074/jbc.M807671200. [DOI] [PubMed] [Google Scholar]
- 52.Bai L, Lennon DP, Caplan AI, et al. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–70. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez-Perez JC, Ernst S, Demirci C, et al. Hepatocyte Growth Factor/c-Met Signaling Is Required for β-Cell Regeneration. Diabetes. 2014;63:216–23. doi: 10.2337/db13-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKinnon H, Gherardi E, Reidy M, Bowyer D. Hepatocyte growth factor/scatter factor and MET are involved in arterial repair and atherogenesis. Am J Pathol. 2006;168:340–8. doi: 10.2353/ajpath.2006.050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hortensius Ra, Harley BaC. The use of bioinspired alterations in the glycosaminoglycan content of collagen-GAG scaffolds to regulate cell activity. Biomaterials. 2013;34:7645–52. doi: 10.1016/j.biomaterials.2013.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8:3695–703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martino MM, Briquez PS, Ranga A, et al. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–8. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1 nM of HGF, eNK1 monomer, or eNK1 dimer was added to A549 cells for the indicated time points. Negative control indicates basal media alone without added c-MET agonist. Western blots of cell lysates analyzed using a phospho-specific antibody (‘p’) indicate activated c-MET, Akt and Erk. Total protein levels of c-MET, Akt and Erk are shown to relate the fraction of phosphorylated protein to total protein between treatment conditions. Tubulin is shown as a control to indicate total protein loaded per gel lane prior to Western blotting.
A) Scratched HUVEC monolayers were incubated with 1 nM each of VEGF121, EGF, or FGFb, either individually or in combinations with 1 nM HGF. Wound closure of HUVECs was compared between conditions. B) Either 0.01 or 0.1 nM of VEGF121 or FGFb was added, in addition to varying concentrations of HGF, to identify the optimal concentration of VEGF121 or FGFb that resulted in synergistic effects on wound closure. Negative control indicates media alone without growth factor added. Positive control indicates the addition of complete growth media. Wound area was measured immediately after scratching and at 24 h.