Abstract
Speaking, which naturally occurs in different modes or “tasks” such as conversation and repetition, relies on intact basal ganglia nuclei. Recent studies suggest that voice and fluency parameters are differentially affected by speech task. This study examines the effects of subcortical functionality on voice and fluency, comparing measures obtained from spontaneous and matched repeated speech samples. Parkinson subjects who are being treated with bilateral deep brain stimulation (DBS) of the subthalamic nuclei (STN) were tested with stimulators ON and OFF. Results indicated that a voice measure, harmonic to noise ratio, is improved in repetition and in DBS-ON, and that dysfluencies are more plentiful in conversation with little or variable influence of DBS condition. These findings suggest that voice and fluency are differentially affected by DBS treatment and that task conditions, interacting with subcortical functionality, influence motor speech performance.
The ability to speak clearly involves a complex brain system that is not fully understood. Parkinson’s Disease (PD), a disorder primarily affecting the basal ganglia, interferes with this ability. In earlier times, subcortical nuclei were regarded as subordinately relaying commands from motor and supplementary motor cortex to lower motor neurons. Intensive studies in the past several decades (De Long & Georgopoulous, 1981; DeLong, Georgopoulos & Crutcher, 1983) and the influential lecture by Marsden (1982) have led to a more complex view. The intricacies of motor control by the human basal ganglia have been revealed using several approaches to the question (Utter & Basso, 2008), including neurophysiological (Parent & Hazrati, 1995a), computational (Gurney, Prescott, & Redgrave, 2001a, b), and functional neuroimaging methods (Eidelberg, 2007).
Several kinds of control and management are now recognized as inherent to basal ganglia processing, including planning and execution (Watson & Montgomery, 2006; Beneke, Rothwell, Dick, Day, & Marsden, 1987; Brooks, 1996), initiation (Atchison, Thompson, Frackowiak, & Marsden, 1993; Burleigh, Norak, Nutt & Obeso, 1997; Gracco and Abbs, 1987), and monitoring of movements (Cummings, 1993; Gassler, 1978; Taylor & Saint-Cyr, 1992). Recent approaches to the basal ganglia describe a central role in behavioral “action selection” (Gurney, Prescott, & Redgrave, 2001a, p. 401). In Baev’s (1995) model of basal ganglia function, monitoring is achieved in the dopaminergic system through matching of the predicted gesture with the actual afferent flow coming from the executed motor gesture. Thus, far from constituting an indirect pathway, circuitry in the basal ganglia forms an “additional integrative station” enabling motor gestures in concert with widespread projections to other subcortical and to cortical neurons (Parent & Hazrati, 1995b, p. 128). In a similar view, Graybiel (1998) proposes that the basal ganglia formulate representations of motor and cognitive action sequences in order to implement them as performance units.
These interpretations of basal ganglia function drawn from motor control studies have important implications for our understanding of speech motor control. Poor coordination of speech gestures in hypokinetic dysarthria may be attributable to impaired motor planning as well as defective ongoing monitoring (Connor, Abbs, Cole, & Gracco, 1989; Gracco & Abbs, 1987). Speech disorders in basal ganglia disease may arise in part from deficient execution and maintenance of an appropriate internal model of the action plan (Georgiou, Bradshaw, Iansek, Phillips, Mattingley, & Bradshaw, 1993; Gurney, Prescott, & Redgrave, 2001a). The dysarthria associated with basal ganglia disease is characterized by imprecise articulation, changes in rate and rhythm, and soft, breathy vocal quality, which often seriously interferes with intelligibility (Canter, 1963, 1965a, b; Darley, Aronson, & Brown, 1975; Forrest, Weismer, & Turner, 1989). These characteristics are found in varying combinations and to varying degrees in PD, a disease in which inadequate production of dopamine negatively affects the complex network of inhibition and excitation relations among subcortical nuclei.
Parkinsonian speech disorder, called hypokinetic dysarthria, arises as a combination of deficient respiration, phonation, articulation, resonance, and prosody (Goberman & Coelho, 2002). Hypokinetic dysarthria was commonly held to manifest consistently across talking of any kind (Yorkston, Beukelman, & Bell, 1988, Shames and Wiig, 1990; Duffy, 1995, p. 62), not influenced by linguistic variables, such as complexity or lexical frequency, or task effects, such as reading versus spontaneous speech. However, contemporary models of the basal ganglia are consistent with the notion that specific vocal tasks might be expected to place different demands on processing, and therefore be differentially affected by disease.
As an instructive example of task effects, it is well known that singing, a vocal mode similar to speech, is associated with reduction of dysfluencies in chronic stuttering (Ludlow & Loucks, 2003). In cases of severe nonfluent aphasia, singing of a familiar melody (Yamadori, Osumi, Masuhara, & Okubo, 1977) with and without familiar lyrics (Hébert, Racette, Gagnon, & Peretz, 2003) may be preserved and singing improves intelligibility in some of the dysarthrias (Hughlings Jackson, 1874; Kempler & Van Lancker, 2002). Similarly, specific task demands have been used to induce fluency in stutterers (Andrews et al., 1982). External cues, such as a metronome or choral speech support, often provide a dramatic benefit to dysfluent speech (Alm, 2004). Task effects on vocal disability in spasmodic dysphonia have also been reported (Roy, Gouse, Mauszycki, Merrill, & Smith, 2005).
The study reported here arises from clinical observations, as well as previous reports, that compromise to basal ganglia competence affect articulatory and phonatory success for spontaneous and repeated speech quite differently. One of the important differences between these two speech modes may well be that while spontaneous speech requires the generation of an internal motor plan, followed by initiation, execution, and monitoring, an external template is provided for repeated speech, reducing the burden on motor speech control throughout the process.
The use of DBS of the subthalamic nucleus (STN) to treat PD provides a reversible means of modifying the activity of the basal ganglia. While effective in reducing non-speech motor symptoms, this form of therapy has no effect or even a negative effect on speech (Tripoli, Zrinzo, & Martinez-Torres, 2008; Wang, Verhagen Metman, Bakay, Arzbaecker, & Bernard, 2008). Some parallels exist with levadopa therapy, which has a variable effect on motor activation (Feigin, Ghilardi, Fukuda, Mentis, Dhawan, Barnes, Ghez, & Eidelberg, 2002), and gains from the pharmacological intervention are not as significant for speech as for limb function (Dromey, Kumar, Lang, & Lozano, 2000). These facts raise questions about the relationship between speech and non-speech motor control in PD, and more generally, about the nature of cortical-subcortical interactions during speech.
Our general aim is to better understand cortical-subcortical interactions in normal and dysarthric speech, and the role of such interactions in specific dimensions of speech production. To study the effects of DBS on cerebral control of speech, this portion of the larger project focused on voice quality and articulatory fluency, using acoustic measures obtained in two types of motor speech task, conversation and repetition. We tested repetition in two contexts. The first was conversation-repetition, which is defined as the repetition of phrases excerpted from the subject’s spontaneous conversation. This enabled a direct comparison of spontaneous and repetition modes using the same phrases. The second was the repetition of specific statements and questions presented by the examiner and performed twice, mirroring the conversation, conversation-repetition conditions. The first and second sentence repetitions provided an estimate of a practice effect that might occur in the conversation-repetition condition.
Methods
Subjects
Seven right-handed, male speakers of American English with Parkinson’s disease, ages 49–62 (mean = 58 years) and education 14–18 years (mean = 16.1 years), volunteered for the study. They had received the diagnosis of idiopathic Parkinson’s disease between 9 and 16 years (mean 11.9 years) before inclusion in this study, and were between two and 56 months (mean of 21 months) post DBS programming (Table 1). Subjects were otherwise healthy and had no significant psychiatric or medical disorders. For treatment of tremor and rigidity, they received bilateral electrodes surgically implanted in the subthalamic nuclei. Motor function improved with DBS therapy (Table 1). Pre and post-surgical speech testing was not performed, but there were no indications of micro-lesion effects in these subjects prior to DBS programming. Patient provided self-evaluations about their speech during the course of their time with PD including any changes following DBS surgery. During the presurgical period, three patients reported no speech problems, while four indicated that their speech became softer and two experienced dysfluencies. Following initiation of DBS therapy, three subjects reported that their speech became worse, one reported an improvement, and three experienced no change in speech. Informal clinical evaluation of conversational speech by a speech pathologist at time of testing prior to turning off DBS indicated that five of the seven subjects had evidence of dysfluencies or hesitations, six had impaired voice quality (soft, breathy or strangled), and three showed articulatory imprecision (Table 2).
Table 1.
Demographic information for the subjects in study. SID refers to subject identifier. Age, education, and PD duration (time since diagnosis) are in years. DBS duration is in the number of months since the stimulators were activated. Levodopa refers to the daily dose in mg. The Unified Parkinson’s Disease Rating Scale motor score (UPDRS III) (Fahn et al., 1987) and the Hoehn and Yahr (H & Y) disease staging scores (Hoehn & Yahr, 1967) are presented for the DBS-ON and DBS-OFF examinations. Both the UPDRS III [t(6) = 2.74; p = 0.034] and the Hoehn and Yahr [t(6) = 2.52; p = 0.045] scores demonstrate significant improvements with DBS-ON. The bottom row provides group means and standard deviations (S.D.).
| SID | AGE | ED | PD Duration | DBS Duration | Levodopa | UPDRS III OFF | UPDRS III ON | H & Y OFF | H & Y ON |
|---|---|---|---|---|---|---|---|---|---|
| 104 | 57 | 16 | 16 | 27 | 400 | 56.5 | 55.5 | 5.0 | 4.0 |
| 106 | 59 | 15 | 10 | 9 | 600 | 27.5 | 19.0 | 2.5 | 2.5 |
| 107 | 62 | 18 | 15 | 2 | 600 | 26.0 | 24.0 | 2.5 | 2.0 |
| 108 | 61 | 14 | 11 | 12 | 600 | 27.0 | 9.0 | 2.0 | 2.0 |
| 109 | 49 | 16 | 9 | 4 | 300 | 23.5 | 21.5 | 2.5 | 2.0 |
| 110 | 62 | 16 | 11 | 56 | 600 | 52.5 | 31.0 | 4.0 | 3.0 |
| 111 | 56 | 18 | 11 | 37 | 400 | 10.5 | 4.0 | 2.0 | 2.0 |
| Means (S.D.) | 58.0 (4.6) | 16.1 (1.5) | 11.9 (2.6) | 21.0 (20) | 500 (129) | 31.9 (16.5) | 23.4 (16.8) | 2.9 (1.1) | 2.5 (0.8) |
Table 2.
Subjects’ self report of speech changes associated with DBS therapy and the clinical impression at study intake.
| SID | Speech History | Pre-DBS Speech | Post-DBS Speech | Clinical Impression |
|---|---|---|---|---|
| 104 | None | Clear, fluent | Difficulty with word formation, speech initiation, low voice, mumbling, stuttering, lower pitch, dysfluent, spells out loud when speech freezes | Short rushes, freezing, dysfluent, strangled voice, aphonic |
| 106 | None | Soft voice, difficulty with word formation not readily noticable | Mumbling for 4 – 5 months, stuttering for 8 months | Slurring, soft voice variable rate, dysfluent |
| 107 | Mild stuttering until age 21 | Stammering, soft voice, poor intelligibility | Improved | Some imprecision, uneven loudness, fast rate, dysfluencies, breathy voice |
| 108 | None | Fine | No change | Somewhat soft voice |
| 109 | Articulation therapy, Elementary through HS | Soft speech | Mild dysfluency, pronouncing words not quite so fluid | Mild slurring, mild hesitation |
| 110 | None | Soft, hesitant speech | Good initially, later soft and hesitant | Soft voice, slowed, hesitation, word finding difficulties, flat prosody |
| 111 | None | Soft speech | Speech stronger | Some slurring, soft voice |
All subjects were free of Parkinsonian medication for at least 12 hrs when they provided speech samples in both the DBS-ON and the DBS-OFF conditions, which were conducted at least a week apart. Testing in the DBS-OFF condition occurred at least two hours after turning off the stimulators. This protocol was approved by the Institutional Review Board of the Nathan Kline Institute and all subjects provided written informed consent.
Speech samples
Speech samples were recorded using a head-worn microphone (Shure) and a Marantz digital recorder. Tasks chosen for this study were conversation, “conversation-repetition,” and sentence repetition (performed twice to mirror the conversation/conversation-repetition tasks). These measures were obtained by first eliciting a 5 minute sample of conversational speech, during which the subject discussed a hobby, family vacation, or other topic of his choice at the beginning of the speech examination. From this audio-recorded corpus, 30 phrases and sentences were excerpted from the subject’s conversation for a repetition task. These excerpted speech samples were complete linguistic units of 3–7 words, free of slang, specialty and low-frequency vocabulary or proper nouns. Approximately 30 minutes later, the subject was instructed to repeat the excerpted phrases and sentences, taken from his original spontaneous conversation. This enabled the analysis and comparison of identical forms of speech sample under two different task conditions. In order to estimate the extent to which a practice effect might contribute to differences between conversation and conversation-repetition, a sentence repetition task was performed twice during a comparable time interval. In this task, subjects repeated four statements and four questions at the behest of the examiner. Any differences in acoustic measures taken from these first and second sets of eight sentence repetitions could then be compared with differences obtained from the conversation and conversation-repetition tasks.
Speech Data
For this study, speech was characterized in terms of voice quality and fluency. To examine voice quality, harmonic-to-noise ratios (HNR) were obtained for vowels in the conversation, conversation-repetition, and the first and second sentence repetition samples using Praat (Boersma & Weenink, 2008). The HNR values were normalized (nHNR) using the durations of the measured vowel portions. The normalization for each segment was a multiplier calculated as a ratio obtained by dividing the longest duration across segments in all conditions by the duration of each segment. This normalized HNR values across short and long segments in the different conditions.
Dysfluencies were narrowly defined as the difference between the number of target syllables in an utterance and the number of syllables actually produced. Dysfluencies were quantified from the wave form by determining the difference between the number of target syllables and the number of syllables actually produced, and expressing the difference as a percentage of the number of target syllables.
Results
Subject Characteristics
Subject characteristics examined in relation to the acoustic measures were age, education, duration of DBS, and duration of disease (time since PD diagnosis). Neither age nor education values were correlated with any of the production measures. However, significant negative correlations were found between nHNR and the duration of DBS in the DBS-ON condition for the first [r = −0.95; p = 0.001] and second [r = −0.89; p = 0.007] sentence repetitions, such that nHNR decreased for the sentence repetition task as the duration of DBS therapy increased over months. Disease duration was positively correlated with dysfluencies during conversation, in both the DBS-ON [r = 0.85; p = 0.016] and DBS-OFF [r = 0.88; p = 0.009] conditions, such that the proportion of dysfluencies during conversation increased with disease duration. A similar relationship was observed for conversation repetition only in the DBS-OFF condition [r = 0.80; p = 0.03].
Voice Quality
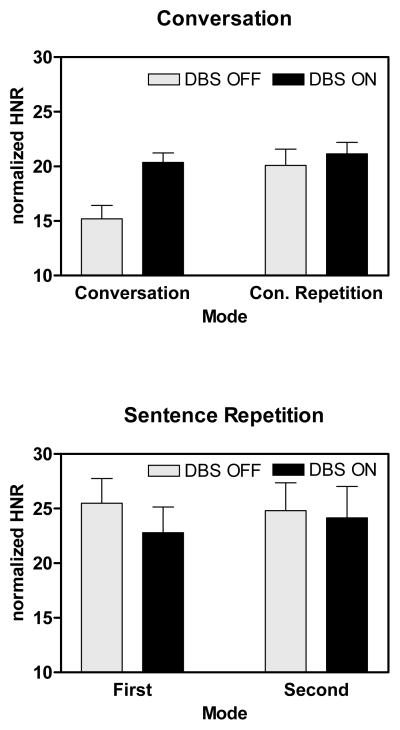
The nHNR values were analyzed using repeated measures analysis of variance (ANOVA). The factors were task (conversation, sentence), DBS (OFF, ON), and repetition (conversation and conversation repetition, first and second sentence repetition). The results are depicted in Figure 1. There was a significant effect of task [F(1,6) = 8.05; p = 0.03], with a higher nHNR for sentence repetition than for conversation. There was also an interaction between task and repetition [F(1,6) = 10.05; p = 0.019], with a significant increase in nHNR between conversation and conversation repetition but not between the first and second sentence repetitions. Finally, there was a significant interaction between task, repetition and DBS [F(1,6) = 7.23; p = 0.036], with a significant increase in nHNR during conversation with DBS-ON, but no DBS effect during conversation repetition or the first or second sentence repetitions.
Figure 1.
Average HNR values for speech produced during spontaneous conversation and conversation-repetition, and during the first and second sentence repetitions in the DBS-OFF and DBS-ON conditions. The nHNR values were normalized for the duration of the measured segment. Higher values represent higher nHNR.
An examination of the pair-wise differences in conditions provides an estimate of the relative effects of DBS, repetition, and task on nHNR. The difference in nHNR during conversation in the DBS-OFF and DBS-ON conditions revealed a 34% increase with DBS-ON [t(6) = −2.97; p = 0.025]. The effects of repetition were evaluated in the DBS-OFF conditions. The improvement in nHNR between conversation and conversation-repetition was 32.1% [t(6) = −2.79; p = 0.032]. In contrast, the improvement in nHNR between conversation and first sentence repetition was 67.8% [t(6) = −3.59; p = 0.009].
Fluency
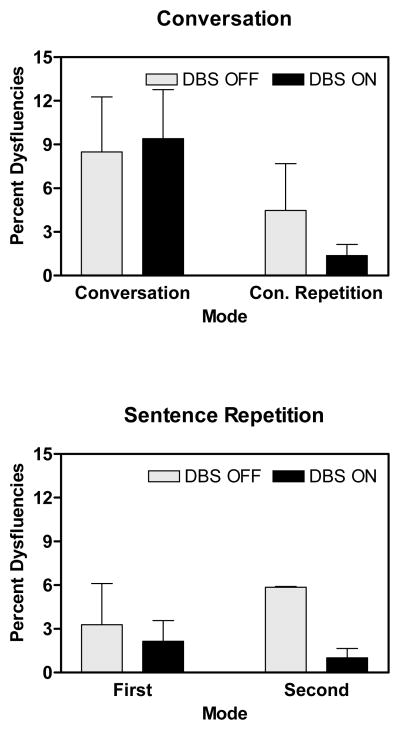
Because of the differences in the occurrences of dysfluencies in the conversation and sentence repetition tasks, these tasks were analyzed separately. For conversation, there was a significant reduction in the percent of dysfluencies during conversation- repetition compared to conversation [F(1,6) = 7.86; p = 0.031]. There was no significant effect of DBS. For sentence repetition, there were no significant effects of first and second repetition, or of DBS.
As with nHNR, an examination of pair-wise differences in the DBS OFF condition provided an estimate of the effects of repetition and task on dysfluencies. There were 90.2% more dysfluencies in conversation compared to conversation repetition [t(6) = 4.03; p = 0.007]. There were 158% more dysfluencies in conversation compared to the first sentence repetition [t(6) = 3.07; p = 0.022].
Discussion
The present results demonstrate that the effects of task, DBS, and subject characteristics (duration of DBS and disease) interact in their effects on speech. For voice quality, nHNR was 32% higher when conversational material was repeated compared to when it was originally spoken. There was a comparable 34% improvement in nHNR during conversation when DBS-ON was compared to DBS-OFF. There were no comparable nHNR effects when the first and second repetitions of sentences were compared. However, there was an influence of DBS on nHNR across subjects during sentence repetition in that nHNR decreased with duration of DBS therapy over months in the DBS-ON condition. With the relatively high nHNR during sentence repetition, this appears to be a subtle effect during the natural history or course of DBS therapy and PD.
Just as a subject effect on nHNR for DBS-ON was observed in the condition with the highest nHNR, a subject effect for dysfluency was observed in the condition with the highest percentage of dysfluency. In the conversation condition, the percent of dysfluencies increased with disease duration in both the DBS-ON and DBS-OFF conditions. For conversation repetition, this relationship was observed only in the DBS-OFF condition, suggesting that DBS does have an effect on dysfluencies under some task conditions. Task was the major factor in the fluency measure, with a reduction in dysfluencies when conversational material was repeated compared to when it was originally produced. In the DBS-OFF condition, there was a 90.2% reduction for repetition in the percentage of dysfluencies. There were no significant main effects or interactions with DBS in the fluency measure in group performance, although dysfluencies were more than doubled in DBS ON in two of the subjects.
Finally, as the effect of task was a major focus of this study, performances during conversation and the first sentence repetition were compared in the DBS-OFF condition. For voice quality, nHNR was 67.8% higher during sentence repetition than conversation. The task difference in percent dysfluencies was greater, with 158% greater percentage of dysfluencies during conversation.
Our results show that fluency and phonation were not uniformly affected by task or by DBS state. In the case of voice quality, the effect of task appears to be comparable to the effect of DBS in improving HNR. An improvement in acoustic measures for voice using a repetition task during DBS ON has been reported elsewhere (Gentil, Chauvin, Pinto, Pollak, & Benabid, 2001). Worsened speech following DBS was reported particularly for the spontaneous mode of speaking (Rousseax, Krystkowiak, Kozlowski, 2004). In our results, fluency was less affected by DBS state than was phonation on these tasks. Speech was less fluent in conversation than in either repetition condition (conversation-repetition or sentence repetition). With respect to fluency, the effect of task is much larger than the effect of DBS. However, it should be noted that dysfluency was narrowly defined in this study. Examinations of rate and pausing are in progress.
Our findings of less than dramatic changes in speech in association with DBS ON and OFF find correspondence in the current literature. Reports of speech changes are inconsistent and variable and appear to involve small differences. The differential effects of DBS on the elements of speech becomes apparent in recent summary reports (Tripoli, Zrinzo, Martines-Torres, et al., 2008; Krack, Batir, Van Blercom, et al., 2003). Dromey, Kumar, Lang & Lozano (2000) found only small changes in vocal fundamental frequency (F0) and amplitude in seven subjects who had undergone DBS treatment. The Dromey et al. paper is relevant in several respects. They found a task effect with increased F0 during conversation but not sustained phonation. Further, this increase was found only when subjects were receiving both DBS and levodopa. D’Alatri et al. (2008) reported that STN DBS produced more stable glottal vibration with reduced frequency and amplitude tremor, which was not associated with an improvement in speech performance.
Role of task factors
Certain effects of task conditions on speech effectiveness have previously been reported. Using intelligibility measures, differences have been seen for spontaneous speech versus reading or repetition. In a study of accelerated speech following bilateral thalamic surgery in a patient with PD, Canter and Van Lancker (1985) observed a task effect in the patient’s intelligibility. Speech samples read aloud by this patient were more intelligible than samples of the patient’s spontaneous speech. Similarly, a comparison of sentences from the Assessment of Intelligibility of Dysarthric Speech (Yorkston & Beukelman, 1981) with spontaneous speech obtained from dysarthric subjects revealed, again, that dysarthric speakers were more intelligible when reading aloud (Frearson,1985). In both of these studies, different speech samples were used for the comparison tasks. A later study of a single Parkinson’s patient with severe dysarthria revealed a 50% decrease in intelligibility for spontaneous speech exemplars, when compared with the same speech examplars in reading and repetition (Kempler & Van Lancker, 2002).
Acoustic differences as a function of speech task also appear in the literature as early as 1943, when differences in pitch and duration were observed for reading and “impromptu speaking” (Snidecor, 1943, p. 50). Schulz, Greer & Friedman (2004) studied task effects on speech production in Parkinson’s patients who had undergone pallidotomy surgery. Measures of sentence duration, pause duration, and frequency of pauses differed for reading, picture description, and conversation. These authors suggested that picture description and conversation, as spontaneous tasks, may place a greater burden on the vocal system than reading. Brown and Docherty (1995) compared several acoustic parameters in dysarthric speakers of variable etiology while they read a paragraph versus spoke spontaneously. The reading condition was associated with longer vowel durations in some patients, but not in those with PD. The PD patients showed a different speech task effect—longer voice onset times in reading. Kent et al. (1997) compared repetition with conversational speech in patients with cerebellar ataxia. The dysarthric speakers produced longer syllable durations than the control group in sentence repetition, but not in conversation. Acoustic differences were also found in the single case of dysarthria reported by Kempler and Van Lancker (2002) using blind rating of spectrograms. Formant structures on spectrograms were noisier and more incoherent on speech examplars spontaneously produced than on the same exemplars in read and repeated task modes, and this measure—a visual representation of signal-to-noise ratio--correlated with intelligibility ratings.
Kent and Kent (2000) developed profiles of the different type of dysarthrias comparing sustained vowel phonation, diadochokinesis and conversational speech tasks. In hypokinetic dysarthria in PD, vowel prolongation revealed greater fundamental frequency and first formant variability than in normals. Prosodic irregularities that are usually present in dysarthria were highlighted in conversation, but less so in the rote tasks. Other researchers have noted that the relative rankings of deviant perceptual characteristics are not the same in syllable repetition as in reading for patients with dysarthria (Zeplin & Kent, 1996). These reports, although somewhat fragmentary, suggest that motor speech characteristics vary, in some cases consistently, with task.
The influence of task on speech observed in this study may be attributable to the lack of an external model in conversation versus the presence of an external model in repetition. These findings for speech are in agreement with numerous observations of other motor behaviors. Parkinson subjects have been described as “enormously disadvantaged” by lack of internal cues (Georgiou et al., 1993, p. 1575). “Gait ignition failure” is commonly seen clinically in Parkinson’s disease; taking a step is aided by an external stimulus (Atchison, Thompson, Frackowiak, & Marsden, 1993; Burleigh, Norak, Nutt, & Obeso, 1997), such as a hockey stick or a line on the floor. The subcortical systems performance circuit proposed by Baev (1995, p. 38) contains a “model of the controlled object,” implying that a deficient subcortical system will falter when an internal model is required, as in conversational speech. Studies have demonstrated that motor deficits in Parkinson’s disease are more severe in “internally guided” than in externally guided motor tasks using reaching gestures (Lewis et al., 2007; Schenk et al., 2003).
Research in the field of stuttering also suggests a task-dependent motor speech system (Alm, 2004), which may involve basal ganglia mechanisms. The speech of people who stutter has been known to improve when provided with an external cue in the form of rhythmic support. The insufficiently organized system supporting spontaneous speech can be in part bypassed in a condition where external support is provided.
DBS and levadopa effects
Comparisons can reasonably be made with studies of levodopa, as neurologists anticipate that the effects of DBS in the ON state on motor function will be comparable to an optimal dose of levodopa (Marks, 2009; Okun, 2009). As mentioned previously, the effects of levodopa on speech are variable. De Letter, Santens & Van Borsel (2005) observed improved intelligibility scores using the Yorkston & Beukelman Intelligibility Test (1980) (which utilizes reading and repetition). In numerous studies, various speech parameters show no effect or a negative effect of the medication. A study measuring dysfluencies in PD subjects ON and OFF levodopa in reading and producing a monologue, a significant group effect was not found, but increases in dysfluencies in individual speakers suggested a role of increased dopamine levels in the brain (Goberman & Blomgren, 2003). Similarly, Rousseax, Krystkowiak, Kozlowski et al. (2004) reported articulation difficulties in two of seven patients in DBS-ON. We found a two-fold elevated proportion of dysfluencies in two subjects (29%) in DBS-ON state (in conversation). de Letter, Santens, de Bodt et al. (2006) reported increased rate variability in 25 PD patients in a reading task after levodopa administration, which may have been related to more dysfluencies. In another DBS subject, DBS treatment improved oral control over what had occurred during levodopa treatment (Gentil, Tournier, Pollak, & Benabid, 1999) and greater lip mobility following DBS surgery was observed (Rousseaux, Krystkowiak, Kozlowski, et al.,2004).
Several factors may account for the higher harmonic to noise ratios, reflecting a stronger acoustic signal arising from improved voice quality, in DBS-ON. These factors include increased respiratory function, higher subglottal air pressure and increased air flow over the glottis, firmer and more stable vocal folds, and improved and more consistent control of muscles of the larynx and vocal tract. De Letter, Santens, De Bodt et al (2007) reported improved respiratory measures following levodopa administration in all of 25 PD subjects tested. A single case study reported improved respiration and phonation time in DBS on (Hoffman-Ruddy et al., 2001) and longer phonation time was seen in a group of seven DBS patients (Rousseaux, Krystkowiak, Kozlowski, et al., 2004). Levodopa was observed to upscale the overall levels of vocal amplitude and tempo (Ho et al., 2008), presumably benefiting from enhanced respiratory function. Pitch and loudness variability improved with medication in 10 patients (De Letter, Santens, Estercam, et al., 2007). Recent studies of coordination of breath and phonation using intra-oral pressure measures revealed improved function for both levodopa and DBS conditions (Sarr, Pinto, Ludovic, Purson, Ghio, Epresser, Teston, & Viallet, 2009). Several voice measures in 20 Parkinson’s subjects improved with levodopa, and the tremor intensity measure decreased (Sanabria, Ruiz, Gutierrez, Marquez, Escobar, Gentil, & Cenjor, 2001). Positive effects of DBS and of levodopa on phonatory capacity in seven subjects were reported by Sung, Kim, Kim, et al. (2004) and in 20 subjects evaluated for pitch (Gentil et al., 2001). In a study of 19 DBS-treated subjects, speech was rated by the treating physicians, speech pathologists, and subjects themselves as worse during DBS-ON, but glottal tremor was reduced and phonation time increased (Klostermann, 2008), again suggesting a selective improvement in phonation. As noted previously, D’Alatri et al. (2008) reported reductions in both frequency and amplitude tremor with DBS.
Summary
The significant influence of task on motor speech measures in the present study has implications for the study of motor speech. It is clear from results such as these and others reviewed above that task must be taken into account to describe motor speech processes and to understand the effects of brain dysfunction on articulation and voice. It follows that efforts to understand the effects of DBS on motor speech competence must carefully consider task demands when evaluation and treatment is undertaken. These findings further suggest that DBS may affect different components of motor speech processes in different or even opposite ways. It appeared from this study that in the DBS-ON state, voice characteristics were improved yielding higher HNR values. This occurred during conversation, the condition in which nHNR was lowest in the untreated state. The effect on dysfluencies is less straightforward due to the strong influence of task. It is likely that the nonuniform effects of DBS on elements of motor speech, compounded by the fact that speech performance varies with task, may account in part for the variable reports by patients of the impact of DBS on their speech.
These results may lead to more specific and empirical questions about the causal relationships between subcortical nuclei and separable components of the motor speech process. Components of speech production are widely understood to be differentially controlled (Murdoch, 2001). The distribution of management of these elements across cortical and subcortical systems is a topic of active research for articulation (Sidtis, Strother & Rottenberg, 2003; Sidtis, Gomez, Naoum, Strother, & Rottenberg, 2006; Ackermann & Riecker, 2004; Hillis, Work, Barker, Jacobs, Breese and Maurer, 2004) and voice (Simonyan & Jürgens, 2003; Ludlow, 2005; Loucks, Poletto, Simonyan, Reynolds, & Ludlow, 2007). Further, reflecting the differences in task demands, patterns of brain activity likely differ during external versus internally guided tasks (Lewis, Slagle, & Smith, et al., 2007; Sidtis, Tagliati, Sidtis, Dhawan, & Eidelberg, 2009). Compounding the difficulties in understanding the breakdown of a complex control system in PD and its alteration with DBS is the evidence that the progression of PD is the result of neuropathology that progresses by encroaching on a series of brain structures based on their neurobiological properties rather than simply increased destruction in a restricted neuroanatomical region (Braak et al., 2006). This raises the possibility that different aspects of motor speech control in PD are associated with changes in different neurotransmitter systems (Goberman, 2005).
This is a preliminary study in which seven subjects have been carefully evaluated as part of a broader study that include other tasks, other measures, and functional neuroimaging aimed at better understanding the nature and consequences of cortical-subcortical interactions during speech. Better understanding of the sequelae of surgical treatment can lead to better informed explication of risks and benefits and to enlightened postsurgical counseling. Knowledge of which components of the motor speech function are more or less affected, negatively or positively, will assist in treatment planning for post surgical PD patients.
Figure 2.
Mean number of dysfluencies measured in speech produced during spontaneous conversation and the repetition of utterances produced during conversation (conversation-repetition) and the first and second repetitions. Both tasks were performed in the DBS-OFF and DBS-ON states. The number of dysfluencies represents the difference between the number of target syllables in the utterance and the actual number of syllables produced. This number is then expressed as a percentage of the total syllables produced. Higher values represent greater percentages of dysfluencies.
Acknowledgments
Kelly Bridges, Krista Cameron, Dora Katsnelson, Judy Yuen, Lisa Yeung, and Elizabeth Sweeting assisted in data acquisition, management and analysis. Ji Sook Ahn’s translation of the article by Sung et al. (2004) from the original Korean is greatly appreciated. This work was supported by NIDCD R01 DC007658 and the Parkinson’s Disease Foundation.
References
- Ackermann Hermann, Riecker Alex. The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain and Language. 2004;2004:320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Alm P. Stuttering and basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders. 2004;37:325–369. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Andrews G, Howie PM, Dozsa M, Guitar BE. Stuttering: speech pattern characteristics under fluency-inducing conditions. Journal of Speech and Hearing Research. 1982;25:208–216. [PubMed] [Google Scholar]
- Atchison PR, Thompson PD, Frackowiak RS, Marsden CD. The syndrome of gait ignition failure: a report of six cases. Movement Disorders. 1993;8:285–292. doi: 10.1002/mds.870080306. [DOI] [PubMed] [Google Scholar]
- Baev KV. Disturbances of learning processes in the basal ganglia in the pathogenesis of Parkinson’s disease: a novel theory. Neurol Res. 1995;17:38–48. doi: 10.1080/01616412.1995.11740285. [DOI] [PubMed] [Google Scholar]
- Belin P, Van Eeckhout P, Zilbovicius M, Remy P, Francois C, Guillaume S. Recovery from nonfluent aphasia after melodic intonation therapy: A PET study. Neurology. 1996;47:1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- Beneke R, Rothwell J, Dick J, Day B, Marsden C. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat: doing phonetics by computer (Version 5.1.11) [Computer program] 2009 Retrieved July 19, 2009, from http://www.praat.org/
- Braak H, Müller CM, Bohl JR, Rüb U, de Vos RAI, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson disease reconsidered. Movement Disorders. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Brooks D. Basal ganglia function during normal and parkinsonian movement; PET activation studies. In: Battistin L, Scarlatto G, Caraceni T, Ruggieri S, editors. Advances in Neurology. Vol. 69. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 433–441. [PubMed] [Google Scholar]
- Brown A, Docherty GJ. Phonetic variation in dysarthric speech as a function of sampling task. European Journal of Disorders of Communication. 1995;30:17–35. doi: 10.3109/13682829509031320. [DOI] [PubMed] [Google Scholar]
- Burleigh JA, Norak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Movement disorders. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- Canter GJ. Speech characteristics of patients with Parkinson’s disease. I. Intensity, pitch and duration. Journal of Speech and Hearing Disorders. 1963;28:221–229. doi: 10.1044/jshd.2803.221. [DOI] [PubMed] [Google Scholar]
- Canter GJ. Speech characteristics of patients with Parkinson’s disease. II. Physiological support for speech. Journal of Speech and Hearing Disorders. 1965a;30:44–49. doi: 10.1044/jshd.3001.44. [DOI] [PubMed] [Google Scholar]
- Canter GJ. Speech characteristics of patients with Parkinson’s disease: III. Articulation, diadochokinesis, & overall speech adequacy. Journal of Speech & Hearing Disorders. 1965b;30:217–224. doi: 10.1044/jshd.3003.217. [DOI] [PubMed] [Google Scholar]
- Canter GJ, Van Lancker D. Disturbances of the temporal organization of speech following bilateral thalamic surgery in a patient with Parkinson’s disease. Journal of Communication Disorders. 1985;18:329–349. doi: 10.1016/0021-9924(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Connor N, Abbs J, Cole K, Gracco V. Parkinsonian deficits in serial multiarticulate movements for speech. Brain. 1989;112:997–1009. doi: 10.1093/brain/112.4.997. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Neurol Rev. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- D’Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on Parkinsonian speech impairment. J of Voice. 2008;22(3):365–372. doi: 10.1016/j.jvoice.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Motor Speech Disorders. Philadelphia: W. B. Saunders; 1975. [Google Scholar]
- De Letter M, Santens P, De Bodt M, Boon P, Van Borsel J. Levodopa-induced alterations in speech rate in advanced Parkinson’s disease. Acta Neurol Belg. 2006;106(1):19–22. [PubMed] [Google Scholar]
- De Letter M, Santens P, Estercam I, Van Maele G, De Bodt M, Boon P, Van Borsel J. Levodopa-induced modifications of prosody and comprehensibility in advanced Parkinson’s disease as perceived by professional listeners. Clin Linguist Phon. 2007;21(10):783–91. doi: 10.1080/02699200701538181. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, De Bodt M, Van Maele G, Van Borsel J, Boon P. The effect of levodopa on respiration and word intelligibility in people with advanced Parkinson’s disease. Clin Neurol Neurosurg. 2007;109(6):495–500. doi: 10.1016/j.clineuro.2007.04.003. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, Van Borsel J. The effects of levodopa on word intelligibility in Parkinson’s disease. J Commun Disord. 2005;38(3):187–96. doi: 10.1016/j.jcomdis.2004.09.001. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Georgopoulos AP, Crutcher MD. Cortico-basal ganglia relations and coding of motor performance. Expl Brain Res Suppl. 1983;7:30–40. [Google Scholar]
- DeLong MR, Georgopoulos AP. Motor functions of the basal ganglia. In: Brookhart JM, Mountcastle VB, Brooks VB, editors. Handbook of Physiology: Section 1. The Nervous System, Vol. 2, Motor Control, Part 2. Bethesda, MD: American Physiological Society; 1981. pp. 1017–1061. [Google Scholar]
- Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Movement Disorders. 2000;15(6):1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis and Management. St. Louis, MO: Mosby; 1995. [Google Scholar]
- Eidelberg D. The assessment of neurological systems with functional imaging. Brain & Language. 2007;102(2):192–199. doi: 10.1016/j.bandl.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton RL. UPDRS program members. United Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinsons disease. Vol. 2. Florham Park NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Feigin A, Ghilardi MF, Fukuda M, Mentis MJ, Dhawan V, Barnes A, Ghez CP, Eidelberg D. Effects of levodopa infusion on motor activation responses in Parkinson’s disease. Neurology. 2002;59(2):220–6. doi: 10.1212/wnl.59.2.220. [DOI] [PubMed] [Google Scholar]
- Forrest K, Weismer G, Turner GS. Kinematic, acoustic, and perceptual analyses of connected speech produced by Parkinsonian and normal geriatric adults. Journal of the Acoustic Society of America. 1989;85:2608–2622. doi: 10.1121/1.397755. [DOI] [PubMed] [Google Scholar]
- Frearson B. A comparison of the A.I.D.S. sentence list and spontaneous speech intelligibility scores for dysarthric speech. Australian Journal of Human Communication Disorders. 1985;13:5–21. [Google Scholar]
- Gassler R. Striatal control of locomotion, intentional actions and of integrating and perceptive activity. Journal of Neurological Science. 1978;36:187–224. doi: 10.1016/0022-510x(78)90082-5. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Iansek R, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain. 1993;116:1575–1587. doi: 10.1093/brain/116.6.1575. [DOI] [PubMed] [Google Scholar]
- Gentil M, Chauvin P, Pinto P, Pollak P, Benabid AL. Effect of bilateral stimulation of the subthalamic nucleus on Parkinsonian voice. Brain and Language. 2001;78:233–240. doi: 10.1006/brln.2001.2466. [DOI] [PubMed] [Google Scholar]
- Gentil M, Tournier CL, Pollak P, Benabid AL. Effect of bilateral subthalamic nucleus stimulation and dopatherapy on oral control in Parkinson’s disease. Eur Neurol. 1999;42(3):136–40. doi: 10.1159/000008087. [DOI] [PubMed] [Google Scholar]
- Goberman AM. Correlation between acoustic speech characteristics and non-speech motor performance in Parkinson disease. Med Sci Monit. 2005;11(3):CR109–116. [PubMed] [Google Scholar]
- Goberman AM, Coelho C. Acoustic analysis of Parkinsonian speech I: Speech characteristics and L-Dopa therapy. Neuro Rehabilitation. 2002;17(3):237–46. [PubMed] [Google Scholar]
- Goberman AM, Blomgren M. Parkinsonian speech disfluencies: Effects of L-dopa-related fluctuations. J Fluency Disord. 2003;28(1):55–70. doi: 10.1016/s0094-730x(03)00005-6. [DOI] [PubMed] [Google Scholar]
- Gracco VL, Abbs J. Programming and execution processes of speech movement control: potential neural correlates. In: Killer E, Gopnik M, editors. Motor and sensory processes of language. Hillsdale, NJ: Erlbaum; 1987. pp. 163–201. [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia I: A new functional anatomy. Biol Cybern. 2001a;84:401–410. doi: 10.1007/PL00007984. [DOI] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia II. Analysis and simulation of behavior. Biol Cybern. 2001b;84:411–423. doi: 10.1007/PL00007985. [DOI] [PubMed] [Google Scholar]
- Hébert S, Racette A, Gagnon L, Peretz I. Revisiting the dissociation between singing and speaking in expressive aphasia. Brain. 2003;126:1838–1850. doi: 10.1093/brain/awg186. [DOI] [PubMed] [Google Scholar]
- Hillis Argye E, Work Melissa, Barker Peter B, Jacobs Michael A, Breese Elisabeth L, Maurer Kristin. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127 (7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Ho AK, Bradshaw JL, Iansek R. For better or worse: The effect of levodopa on speech in Parkinson’s disease. Mov Disord 15. 2008;23(4):574–80. doi: 10.1002/mds.21899. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:152–157. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hoffman-Ruddy B, Schulz G, Vitek J, Evatt M. A preliminary study of the effects on the subthalamic nucleus (STN) deep brain stimulation (DBS) on voice and speech characteristics in Parkinson’s Disease. Clinical Linguistics & Phonetics. 2001;15(1–2):97–101. doi: 10.3109/02699200109167638. [DOI] [PubMed] [Google Scholar]
- Hughlings Jackson J. On the nature of the duality of the brain. In: Taylor J, editor. Selected Writings of John Hughlings Jackson. Vol. 2. London: Hodder & Stoughton; 1874. pp. 129–145. [Google Scholar]
- Kempler D, Van Lancker D. Effect of speech task on intelligibility in dysarthria: A case study of Parkinson’s Disease. Brain and Language. 2002;80:449–464. doi: 10.1006/brln.2001.2602. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF. Task-based profiles of the dysarthrias. Folia Phoniatrica et Logopaedica. 2000;52:48–53. doi: 10.1159/000021512. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Rosenbek JC, Vorperian HK, Weismer G. A speaking task analysis of the dysarthria in cerebellar disease. Folia Phoniatrica et Logopaedica. 1997;49:63–82. doi: 10.1159/000266440. [DOI] [PubMed] [Google Scholar]
- Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, Marzinzik F, Curio G, Sappok T. Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, McKeown MJ, Mailman RB, Belger A, Huang X. Task specific influences of Parkinson’s disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience. 2007;147(1):224–35. doi: 10.1016/j.neuroscience.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-Year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s Disease. New England Journal of Medicine. 2003;349:1925–1834. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Loucks TMJ, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL. Human brain activation during phonation and exhalation: Common volitional control for two upper airway functions. NeuroImage. 2007;36:131–143. doi: 10.1016/j.neuroimage.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL, Loucks T. Stuttering: a dynamic motor control disorder. Journal of Fluency Disorders. 2003;28:273–295. doi: 10.1016/j.jfludis.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Marks W., Jr Overview of DBS, indications, and patient selection. Workshop #8AC.005, Deep Brain Stimulation, presented at the American Academy of Neurology 61st Annual Meeting; Seattle, Washington. May 2.2009. [Google Scholar]
- Marsden C. The mysterious motor function of the basal ganglia: The Robert Wartenberg Lecture. Neurology. 1982;32:514–539. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- Murdoch BE. Subcortical brain mechanisms in speech and language. Folia phoniatrica et Logopaedica. 2001;53(5):233–251. doi: 10.1159/000052679. [DOI] [PubMed] [Google Scholar]
- Okun MS. Long-term DBS patient management and troubleshooting. Workshop #8AC.005, Deep Brain Stimulation, presented at the American Academy of Neurology 61st Annual Meeting; Seattle, Washington. May 2.2009. [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Reviews. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Research Reviews. 1995b;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Rousseaux Marc, Krystkowiak Pierre, Kozlowski Odile, Özsancak Canan, Blond Serge, Destée Alain. Effects of subthalamic nucleus stimulation on parkinsonian dysarthria and speech intelligibility. Journal of Neurology. 2004;251:327–334. doi: 10.1007/s00415-004-0327-1. [DOI] [PubMed] [Google Scholar]
- Roy N, Gouse M, Mauszycki SC, Merrill RM, Smith ME. Task specificity in adductor spasmodic dysphonia versus muscle tension dysphonia. The Laryngoscope. 2005;115(2):311–316. doi: 10.1097/01.mlg.0000154739.48314.ee. [DOI] [PubMed] [Google Scholar]
- Sanabria J, Ruiz PG, Gutierrez R, Marquez F, Escobar P, Gentil M, Cenjor C. The effect of levodopa on vocal function in Parkinson’s disease. Clin Neuropharmacol. 2001;24(2):99–102. doi: 10.1097/00002826-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Sarr M, Pinto S, Jankowski L, Purson A, Ghio A, Espesser R, Teston B, Viallet F. L-dopa and STN stimulation effects on pneumophonic coordination in parkinsonian dysarthria: Intra-oral pressure measurements. Movement Disorders Society Conference; Paris, France. June; 2009. p. Abstract Th-240. [Google Scholar]
- Schenk T, Baur B, Steude U, Bötzel K. Effects of deep brain stimulation on prehensile movements in PD patients are less pronounced when external timing cues are provided. Neuropsychologia. 2003;41:783–794. doi: 10.1016/s0028-3932(02)00286-5. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Greer M, Friedman W. The effects of pallidotomy surgery on sentence measures across three tasks in Parkinson patients. Journal of Medical Speech-Language Pathology. 2004;12(4):195–205. [Google Scholar]
- Shames GH, Wiig EH. Human communication disorders. Columbus, OH: Merrill; 1990. [Google Scholar]
- Sidtis JJ, Strother SC, Rottenberg DA. Predicting performance from functional imaging data: Methods matter. Neuroimage. 2003;20:615–624. doi: 10.1016/S1053-8119(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Gomez C, Naoum A, Strother SC, Rottenberg DA. Mapping cerebral blood flow during speech production in hereditary ataxia. NeuroImage. 2006;31:246–254. doi: 10.1016/j.neuroimage.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Tagliati M, Sidtis D, Dhawan V, Eidelberg D. Globally increased peak cerebral blood flow (CBF) during high-frequency deep brain stimulation (STN-DBS) in Parkinson’s Disease (PD) Movement Disorders. 2009;24 (Supplement 1):S473–474. [Google Scholar]
- Snidecor J. A comparative study of the pitch and duration characteristics of impromptu speaking and oral reading. Speech Monographs. 1943;10:50–56. [Google Scholar]
- Sung JE, Kim H, Kim HS, et al. Effects of subthalamic nucleus deep brain stimulation on the phonation and articulation of the patients with Parkinson’s disease. J Korean Neurological Association. 2004;22:472–477. [Google Scholar]
- Taylor E, Saint-Cyr JA. Executive function. In: Cummings JL, Hubert SJ, editors. PD: behavioral and neuropsychological aspects. Oxford: Oxford University Press; 1992. pp. 74–85. [Google Scholar]
- Tripoli E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E, Hariz MI, Limousin P. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Movement Disorders. 2008;23(16):2377–2383. doi: 10.1002/mds.22296. [DOI] [PubMed] [Google Scholar]
- Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev. 2008;32:333–42. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Wang E, Verhagen, Metman L, Bakay R, Arzbaecher J, Bernard B. The effect of unilateral electrostimulation of the subthalamic nucleus on respiratory/phonatory subsystems of speech production in Parkinson’s disease: a preliminary report. Clinical Linguistics and Phonetics. 2003;17(4–5):283–289. doi: 10.1080/0269920031000080064. [DOI] [PubMed] [Google Scholar]
- Watson P, Montgomery EW., Jr The relationship of neuronal activity within the sensorimotor region of the STN to speech. Brain and Language. 2006;97:233–240. doi: 10.1016/j.bandl.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Weiss P, Stelmach GE, Hefter H. Programming of a movement sequence in Parkinson’s disease. Brain. 1997;120:91–102. doi: 10.1093/brain/120.1.91. [DOI] [PubMed] [Google Scholar]
- Wolfe VI, Garvin JS, Bacon M, Waldrop W. Speech changes in Parkinson’s disease during treatment with L-dopa. J Commun Disord. 1975;8(3):271–9. doi: 10.1016/0021-9924(75)90019-2. [DOI] [PubMed] [Google Scholar]
- Yamadori A, Osumi Y, Masuhara S, Okubo M. Preservation of singing in Broca’s aphasia. Journal of Neurology, Neurosurgery, and Psychiatry. 1977;40:221–224. doi: 10.1136/jnnp.40.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston KM, Beukelman DR. Assessment of intelligibility of dysarthric speech. Austin: C.C. Publications; 1981. [Google Scholar]
- Yorkston KM, Beukelman DR, Bell KR. Clinical management of dysarthric speakers. Boston: College Hill Press; 1988. [Google Scholar]
- Zeplin J, Kent RD. Reliability of auditory-perceptual scaling of dysarthria. In: Robin D, Yorkston K, Beukelman DR, editors. Disorders of motor speech: recent advantages in assessment, treatment and clinical characterization. Baltimore, MD: Baltimore Books; 1996. pp. 145–154. [Google Scholar]