Abstract
Stem cells are controlled, in part, by genetic pathways frequently dysregulated during human tumorigenesis. Either stimulation of Wnt/β-catenin signalling or overexpression of telomerase is sufficient to activate quiescent epidermal stem cells in vivo, although the mechanisms by which telomerase exerts these effects are not understood. Here we show that telomerase directly modulates Wnt/β-catenin signalling by serving as a cofactor in a β-catenin transcriptional complex. The telomerase protein component TERT (telomerase reverse transcriptase) interacts with BRG1 (also called SMARCA4), a SWI/SNF-related chromatin remodelling protein, and activates Wnt-dependent reporters in cultured cells and in vivo. TERT serves an essential role in formation of the anterior–posterior axis in Xenopus laevis embryos, and this defect in Wnt signalling manifests as homeotic transformations in the vertebrae of Tert−/− mice. Chromatin immunoprecipitation of the endogenous TERT protein from mouse gastrointestinal tract shows that TERT physically occupies gene promoters of Wnt-dependent genes. These data reveal an unanticipated role for telomerase as a transcriptional modulator of the Wnt/ β-catenin signalling pathway.
Mammalian tissues require strict regulation of progenitor cell proliferation and differentiation to facilitate both tissue maintenance with normal homeostasis and efficient repair in settings of organ damage. This exquisite control of tissue progenitor cells is executed by both secreted and cell-intrinsic factors. Dysregulation of pathways controlling normal progenitor cell function is a common event in human cancer, suggesting that altered progenitor cell regulation is an important step in carcinogenesis. One such pathway is controlled by Wnt proteins, secreted glycoproteins crucial for development, stem-cell maintenance and stem-cell activation1. Overexpression of β-catenin, a critical mediator of Wnt signalling, causes activation of epidermal stem cells and induces anagen in mouse skin2–4. A similar phenotype is elicited by overexpression of telomerase, the enzyme that adds DNA repeats to chromosome ends, although the mechanisms by which telomerase activates tissue progenitor cells remain poorly understood5,6. The central role of Wnt/β-catenin signalling in stem-cell biology and in differentiation explains how alterations in this pathway are frequently initiating lesions in human tumorigenesis7.
Another pathway essential for maintaining stem cells involves telomere repeat addition by telomerase. In settings of insufficient telomerase, telomeres shorten with each cell division, eventually compromising end protection at a subset of chromosome ends, which results in a local DNA-damage response that impairs stem-cell self renewal and progenitor cell survival8,9. In addition to this role for telomerase in supporting tissue progenitor cells by maintaining telomeres, gain-of-function experiments revealed that telomerase serves a more direct function in tissue progenitor cells. Conditional overexpression of TERT in mouse skin activated quiescent bulge stem cells and caused a rapid developmental transition in the hair follicle from telogen, the resting phase of the hair follicle cycle, to anagen, the active phase5, which strikingly phenocopies the effects of β-catenin overexpression in mouse skin2–4. The ability of TERT to activate tissue stem cells required neither the telomerase RNA TERC nor the reverse transcriptase activity of TERT and is therefore independent of its catalytic role in adding telomere repeats5,10. Gene expression studies on inducible TERT mouse skin together with pattern-matching algorithms suggested that the TERT-controlled gene expression pattern significantly resembled the transcriptional programs regulated by Wnt and Myc10.
Other experiments have suggested the existence of telomere length-independent activities for telomerase in cellular transformation11–13, proliferation14, stem-cell biology15–17, cell survival18 and chromatin regulation19. However, the mechanisms by which TERT exerts these effects in progenitor cells and cancers remain unknown. Here we report the unexpected finding that telomerase is a direct modulator of the Wnt/β-catenin pathway. TERT interacts with BRG1, occupies specific chromatin sites of Wnt/β-catenin target genes, activates Wnt reporters in culture and in vivo, and is important for the Wnt program in mouse embryonic stem (ES) cells and in Xenopus embryos. These data reveal a significant level of integration between two pathways required for stem-cell proliferation and self-renewal with profound implications for understanding stem-cell regulation, development and carcinogenesis.
TERT interacts with BRG1 and activates Wnt reporters
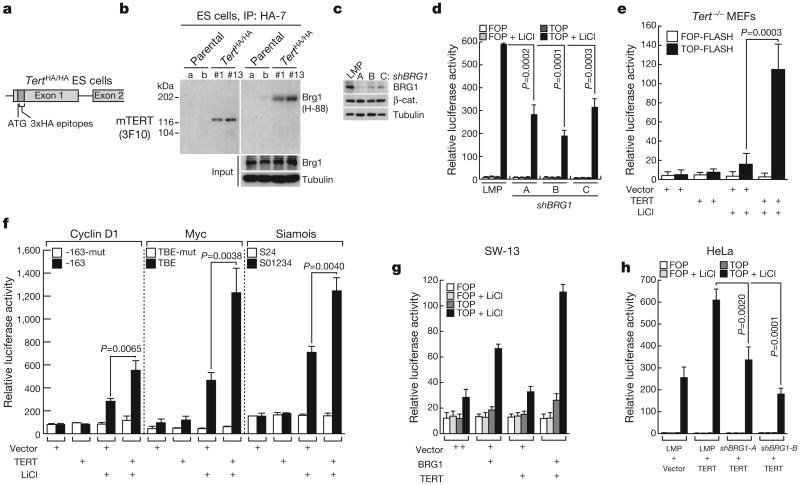
To understand telomerase-mediated signalling, we purified TERT protein complexes from HeLa cells using tandem affinity chromatography coupled with nano liquid chromatography tandem mass spectrometry20. In replicate purifications, we identified numerous peptides derived from BRG1, a SWI/SNF-related ATP-dependent chromatin-remodelling factor that acts to modulate chromatin conformation21 (Supplementary Fig. 1). To circumvent significant challenges in studying endogenous TERT protein22, we introduced epitope tags into the Tert locus in knock-in mouse ES cells (Fig. 1a and Supplementary Fig. 2) and in knock-in mice (see below). Endogenous TERT was readily detected by immunoprecipitation and western blot analysis from ES cells in which a haemagglutinin (HA) epitope tag was inserted at the start codon of the Tert gene through homologous recombination (Fig. 1a, b). Immunoprecipitation of endogenous TERT revealed the presence of BRG1 in TERT complexes (Fig. 1b and Supplementary Figs 3–5). Domain mapping experiments showed that TERT interacts with the bromodomain of BRG1 in glutathione S-transferase pull-down experiments (Supplementary Fig. 6).
Figure 1. TERT activates Wnt reporter plasmids in a BRG1-dependent manner.
a, Diagram of HA–TERT knock-in ES cells. b, Interaction of endogenous TERT with BRG1 in TertHA/HA ES cells by immunoprecipitation (IP) and immunoblot (IB). HA-7 and 3F10, anti-HA antibodies. c, Depletion of BRG1 protein by shRNAs in HeLa cells by immunoblot. d, TOP-FLASH reporter activity in HeLa cells transduced with empty vector (LMP) or shRNA BRG1 retroviruses, then transfected with TOP-FLASH plasmid (wild-type TCF sites) and treated with LiCl (n = 3) (FOP-FLASH, mutant TCF sites). e, TOP-FLASH activity in Tert−/− MEFs co-transfected with empty vector or mouse TERT expression plasmid and treated with or without LiCl (n = 4). f, Luciferase activity after transient co-transfection of reporter plasmids comprising cyclin D1, Myc, or siamois promoters driving luciferase with TERT plasmid or empty vector in HeLa cells, followed by LiCl treatment (n = 3). Filled bar, wild-type TCF binding elements (TBE); open bar, mutant TBEs. g, Effect on TOP-FLASH activity of transient co-transfection of BRG1, TERT or BRG1 combined with TERT in SW-13 cells lacking BRG1 (n = 2). h, Effect of BRG1 depletion with shRNA on TERT-mediated activation of TOP-FLASH activity in HeLa cells (n = 3). Error bars indicate standard deviation; P values produced by Student's t-test.
Because BRG1 had been implicated as a β-catenin-interacting protein in the Wnt pathway23,24, we tested whether BRG1 was required for transactivation of Wnt reporter plasmids. Stabilization of β-catenin caused by LiCl-mediated inhibition of GSK3-β led to strong upregulation of luciferase from a transfected reporter plasmid driven by multimerized TCF-binding sites (TOP-FLASH). Depletion of BRG1 with retroviral-encoded short hairpin RNAs (shRNAs) reduced luciferase expression by more than 50%, consistent with a role for BRG1 in controlling β-catenin-mediated transcription at TCF-dependent promoters (Fig. 1c, d). These data showing that TERT interacts with BRG1, and that BRG1 is involved in the Wnt pathway, together with the similarities between the effects of Wnt proteins and those of TERT in hair follicle stem-cell activation prompted us to investigate a role for TERT in the Wnt/β-catenin signalling pathway.
We asked whether TERT could activate the Wnt pathway by co-transfecting a TERT expression plasmid together with TOP-FLASH. Remarkably, overexpression of TERT was sufficient to hyperactivate the TOP-FLASH reporter in cells treated with LiCl, (Fig. 1e) without altering β-catenin protein level or subcellular localization (Supplementary Fig. 7). Similarly, reporter plasmids driven by promoters of other β-catenin target genes, including cyclin D1, c-myc (hereafter referred to as Myc) and siamois25, were also hyperactivated by TERT upon LiCl treatment (Fig. 1f). Overexpression of TERT in SW-13 cells, which do not express BRG1, failed to enhance β-catenin reporter activity upon lithium treatment, and this defect in TERT signalling was restored through BRG1 overexpression (Fig. 1g). Furthermore, BRG1 depletion using RNA interference abrogated the ability of TERT to hyperactivate TOP-FLASH reporter plasmids in HeLa cells (Fig. 1h). TERT stimulated TOP-FLASH plasmids in Terc−/− mouse embryonic fibroblasts (MEFs) and a catalytically inactive TERT mutant (TERTci) stimulated Wnt reporters, indicating that the catalytic function of telomerase is not required for transcriptional co-activation (Supplementary Fig. 8). These results suggest that TERT protein interacts with BRG1 to regulate Wnt-dependent promoters.
TERT activates the Wnt axis in vivo and in ES cells
To determine if the ability of TERT to activate the Wnt pathway extends to an in vivo context, we investigated the stem-cell niche of the gastrointestinal tract, where Wnt signalling through β-catenin and TCF proteins is required for maintenance of stem cells and progenitor cells26. Wnt signalling in the gastrointestinal tract was monitored using Axin2lacZ/+ reporter mice, a knock-in strain in which β-galactosidase is expressed from the Axin2 promoter27. Axin2lacZ/+ mice were intercrossed with inducible TERT (i-TERTci) mice, which allow tetracycline-regulated expression of catalytically inactive TERT5,10. β-galactosidase activity in crypts in the small intestine and colon was significantly enhanced by induction of TERTci in i-Tertci Axin2lacZ/+ mice by both whole mount and histology (Fig. 2a, b). In addition, CD44, a transcriptional target of β-catenin in crypts, was upregulated by TERTci overexpression (Fig. 2c). These data show that TERT overexpression is sufficient to enhance significantly the transcriptional output of the Wnt/β-catenin pathway in progenitor cells of the small intestine.
Figure 2. TERT activates the Wnt pathway in vivo and is required for efficient target gene activation by WNT3A ligand in mouse ES cells.
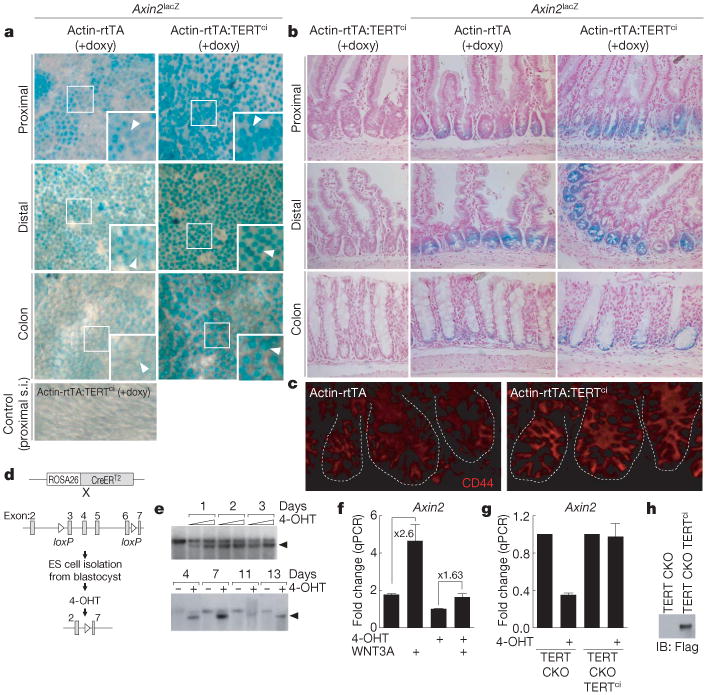
a, b, X-Gal staining for β-galactosidase activity in small intestine and colon of Axin2lacZ/+ reporter mice, i-Tertci Axin2lacZ/+ mice or controls. Whole mounts seen from abluminal side (a); histology, nuclear fast red (b). Arrowheads indicate crypts. Doxy, doxycycline; s.i., small intestine. c, CD44 protein in small intestine crypts by immunofluorescence. d, Schematic for deletion of TERT by 4-OHT treatment of conditional TERT knockout (CKO) ES cells. e, Cre-mediated recombination in ES cells by Southern blot. Arrowhead, recombined Tert allele. f, Induction of Axin2 by WNT3A ligand in TERT conditional knockout (CKO) mouse ES cells treated with vehicle or with 250nM 4-OHT for 3 days, exposed to WNT3A (100 ng ml−1) for 24 h and analysed by qPCR (n = 3). g, h, Basal expression of Axin2 mRNA by qPCR in TERT conditional knockout mouse ES cells treated with vehicle or 4-OHT, and Axin2 mRNA levels in TERT conditional knockout cells with stable overexpression of mouse TERTci (n = 3), shown in h by immunoprecipitation and western blot analysis. Error bars indicate s.d. Original magnification: a, ×4 (insets ×8); b, ×20; c, ×40.
To understand if TERT is required for Wnt signalling, we generated TERT conditional knockout mouse ES cells, incorporating a ROSA26-CreER allele, which enabled efficient deletion of TERT with tamoxifen treatment (Fig. 2d, e and Supplementary Fig. 9)28. WNT3A ligand efficiently induced Axin2 messenger RNA in TERT conditional knockout ES cells that retained TERT sequences. However, deletion of TERT in TERT conditional knockout ES cells with tamoxifen significantly diminished induction of Axin2 by WNT3A treatment (Fig. 2f). Furthermore, deletion of TERT reduced basal expression of Axin2, which was rescued by overexpression of mouse TERTci (Fig. 2g, h). Thus, TERT is required for efficient induction of Wnt target genes in mouse ES cells treated with WNT3A ligand.
TERT is required for Xenopus anterior–posterior axis formation
Activation of Wnt/β-catenin signalling in the ventral vegetal region of Xenopus embryos causes duplication of the anterior–posterior axis29. Injecting increasing amounts of Xenopus Tert mRNA together with a low amount of Xenopus β-catenin mRNA promoted formation of a duplicate anterior–posterior axis in a dose-dependent manner (Fig. 3a, b). Similarly, injection of Xenopus (x)TERTci (D770A) mRNA in conjunction with Xenopus β-catenin mRNA also promoted secondary axis formation, indicating that this activity does not require reverse transcriptase catalytic function (Fig. 3c).
Figure 3. TERT promotes anterior–posterior axis duplication and is required for efficient anterior–posterior axis in Xenopus.
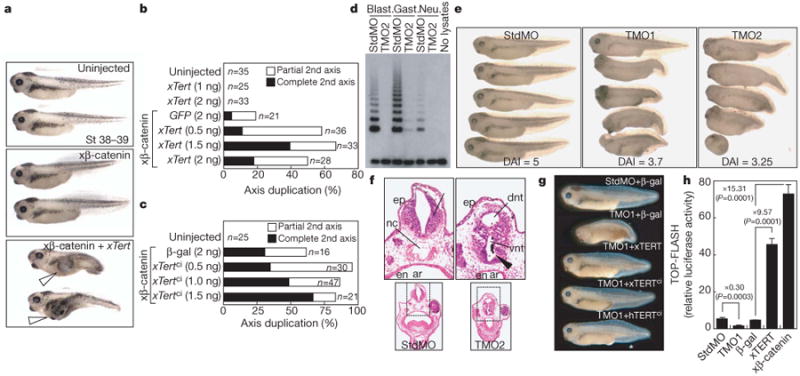
a, b, Duplicate anterior–posterior axis formation in Xenopus embryos co-injected with Xenopus(x)β-catenin mRNA (0.2 ng) and increasing amounts of xTert mRNA. Open arrowheads, extra axes. c, Duplicate axis with co-injection of xTertci and xβ-catenin mRNA (0.4 ng). d, TRAP activity at blastula, gastrula and neurula stages in embryos injected with TERT morpholino (TMO2) or control (StdMO). e, Defects in anterior–posterior axis development in embryos injected with TERT morpholinos (TMO1 or TMO2), but not StdMO, scored using dorso-anterior index (DAI). f, Ectopic neural tube formation (arrowhead) in TMO2-injected embryos. ar, archenteron; dnt, dorsal neural tube; en, endoderm; ep, epidermis; nc, notochord; vnt, ventral neural tube. Transverse sections; haematoxylin and eosin staining. g, Rescue of developmental phenotypes with xTERT, xTERTci and human (h)TERTci, stages 37–38. h, TOP-FLASH analysis by co-injecting reporter plasmid at the 2-cell stage with either StdMO or TMO1, or co-injecting reporter plasmid with β-gal, xTERT or β-catenin (n = 3, P values produced by Student's t-test, error bars indicate s.d.).
Endogenous Wnt/β-catenin signalling has an important role in axial development and patterning of the ventrolateral mesoderm30,31. We therefore asked if inhibiting TERT through injection of specific morpholinos altered Xenopus embryo development (Fig. 3d). Remarkably, xTERT depletion by TERT morpholinos (TMO) caused a pronounced defect in anterior–posterior axis formation32 (Fig. 3e and Supplementary Table 1; dorso-anterior index score: TMO1, 3.7; TMO2, 3.2; StdMO control, 5.0). TERT inhibition led to ectopic neural tube formation and loss of the notochord, phenotypes also seen in knockout mouse embryos defective in components of the Wnt pathway (Fig. 3f)33. These TERT phenotypes were efficiently rescued by co-injection with xTert, xTertci or human TERTci mRNAs resistant to TMO1, ruling out off-target effects of the TMOs and revealing that the developmental defects in TMO-treated embryos are due to impaired TERT-mediated signalling rather than a defect in catalytic function (Fig. 3g and Supplementary Fig. 10). Overexpression of xTert mRNA strongly activated co-injected TOP-FLASH reporter plasmids and xTERT depletion significantly reduced TOP-FLASH expression (Fig. 3h). These results show that TERT is required for proper Wnt signalling and for formation of the anterior–posterior axis during frog development.
Homeotic transformations in Tert−/− mice
In addition to its critical role in formation of the anterior–posterior axis, Wnt signalling is also required for formation of the paraxial mesoderm33 and subsequent generation of the somites from presomitic mesoderm34. We speculated that impaired Wnt signalling caused by TERT inhibition might therefore result in defects in somitogenesis. Somite-specific staining of Xenopus embryos revealed that TERT depletion disrupted somitogenesis, resulting in abnormal somite shape and impaired segment polarity (Fig. 4a). Notably, the effect of TERT morpholinos on caudal development was particularly pronounced, consistent with the established role of WNT3A in posterior development in vertebrates35. WNT3A regulates posterior development in part through transcriptional regulation of Cdx1, a homeobox gene that directly regulates posterior Hox gene expression36. WNT3A ligand induced expression of Cdx1 in mouse ES cells by 12 fold, whereas conditional deletion of TERT in TERT conditional knockout ES cells strongly suppressed induction of Cdx1 by WNT3A (Fig. 4b). Moreover, depletion of xTERT reduced levels of xCdx1, xCdx2 and xCdx3, as well as the Wnt target gene siamois, in TMO-treated Xenopus embryos at the late gastrulation stage (Fig. 4c). Cdx genes are also regulated by FGF, retinoic acid and BMP signalling, in addition to Wnts36–38; however, we detected no change in these pathways with TERT depletion, indicating that TERT contributes to Cdx gene regulation through its specific role in the Wnt/β-catenin pathway (Supplementary Fig. 11). These data show that the endogenous TERT protein is required for appropriate regulation of Cdx genes by the Wnt/β-catenin pathway in mouse ES cells and in Xenopus embryos.
Figure 4. Somite defects in Xenopus embryos treated with TERT morpholino and homeotic transformations in Tert−/− mice.
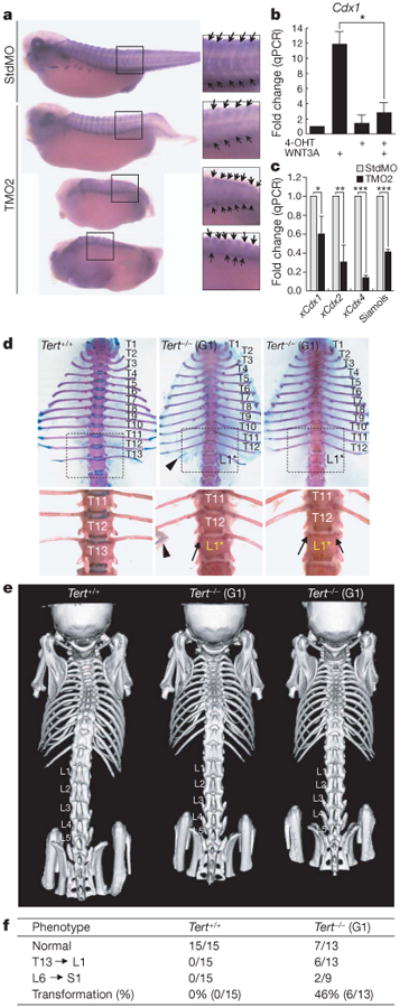
a, Somite staining using 12/101 antibody of Xenopus embryos injected with either StdMO or TMO2. Arrows show somite boundaries. b, WNT3A-mediated induction of Cdx1 in TERT conditional knockout ES cells treated with vehicle or 4-OHT for 3 days, followed by WNT3A for 24 h. * P = 0.002 by Student's t-test (n = 3). c, Levels of xCdx1, xCdx2and xCdx4measured by qPCR in Xenopus embryos injected with StdMO or TMO2 and collected at the late gastrulation stage. *P = 0.0066, **P = 0.0003, ***P < 0.0001 by Student's t-test (n = 4). d, e, T13 to L1 vertebral homeotic transformations in G1 Tert−/− mice. Unilateral loss of 13th rib (middle) or bilateral loss of 13th ribs (right). Arrowhead, 13th rib remnant; arrows, missing 13th ribs. Adult skeletons were stained with alcian blue and alizarin red (ventral; d). Three-dimensional reconstruction of microCT scan images of independent mice (dorsal; e).f, Summary of axial skeletal defects in G1 Tert−/− mice. P = 0.0046 by Fisher's exact test.
Analysis of telomerase knockout mice, including germline deletion of either Terc or Tert, led to the paradigm that first generation (G1) telomerase knockout mice are normal, but that continued breeding of telomerase-deficient strains leads to defects in renewing tissues caused by progressive telomere shortening and eventual loss of telomere protection39,40. On the basis of our data showing clear involvement of TERT in Wnt/β-catenin signalling in diverse contexts, we analysed Tert−/− mice, focusing on the vertebrae of the spinal column, which derives from somites and depends on proper expression of Cdx and Hox genes41. G1 Tert−/− mice lacked telomerase activity but retained functional telomeres and showed none of the apoptotic defects seen in late generation (G5–G6) Tert−/− mice (Supplementary Fig. 12)42,43. Detailed analyses using skeletal preparations and high-resolution CT scanning revealed that G1 Tert−/− mice have homeotic transformations of the vertebrae (T13 to L1), characterized by loss of the thirteenth rib on one or both sides (6 out of 13 G1 Tert−/− mice with T13 to L1 transformation versus 0 out of 15 Tert+/+ controls, P = 0.0046; Fig. 4d–f and Supplementary Table 2). In addition, some G1 Tert−/− mice showed an L6 to S1 transformation (Supplementary Fig. 13). These homeotic defects in G1 Tert−/− mice strikingly resemble those in vestigial tail mice, which harbour a hypomorphic mutation inWNT3A and have homeotic transformations with similar penetrance (Supplementary Fig. 14)36.
TERT occupies Wnt target gene promoters
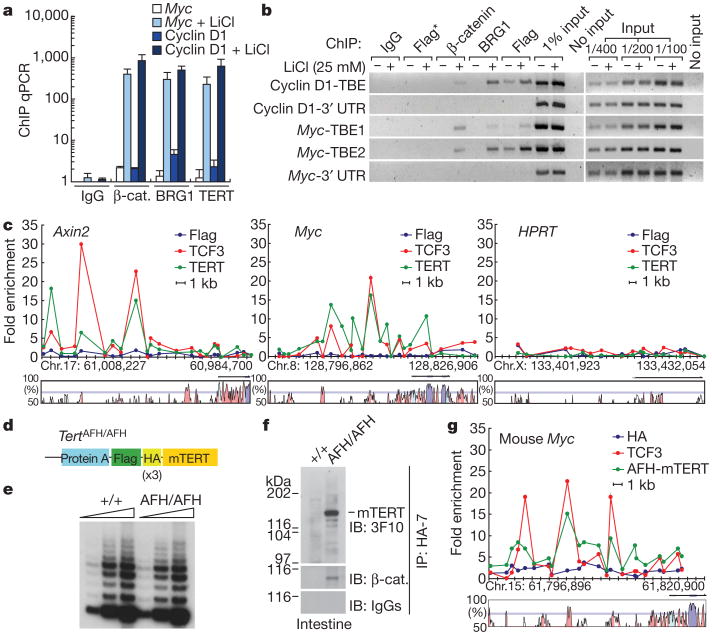
To address the mechanism by which TERT regulates transcription of Wnt/β-catenin target genes, we investigated occupancy of β-catenin-dependent gene promoters by chromatin immunoprecipitation (ChIP). Upon lithium treatment of HeLa cells, β-catenin and BRG1 became associated with promoter fragments containing known TCF binding elements (TBEs), including those within cyclinD1 and Myc, two genes directly regulated by β-catenin. ChIP revealed that stably expressed Flag–TERT was also associated with the TBE-containing promoter fragments of the cyclin D1 and Myc promoters in a lithium-dependent manner (Fig. 5a, b). Furthermore, sequential ChIP revealed that Flag–TERT was bound to the same promoter elements as BRG1 and β-catenin (Supplementary Fig. 15). TERT binding to TCF elements was analysed further through quantitative ChIP experiments using primer pairs spanning 20 kb upstream of the Axin2 and Myc genes at average intervals of 1 kb. The profile of Flag–TERT binding closely resembled that of TCF3 at both the Axin2 and Myc genes in HeLa cells stimulated with lithium (Fig. 5c). Notably, the Flag antibody ChIP performed on parental HeLa cells lacking expression of Flag–TERT did not detect these promoter fragments, indicating that the Flag–TERT ChIP signals depended on the presence of stably overexpressed TERT protein. These data reveal that Flag–TERT physically associates with TBE-containing promoter fragments, along with BRG1 and β-catenin.
Figure 5. TERT occupies Wnt target gene promoters in HeLa cells and in mouse small intestine.
a, b, Association of Flag–TERT, BRG1 or β-catenin with TBE-containing fragments of the cyclin D1 and Myc promoters in Flag–TERT HeLa cells with or without LiCl treatment by ChIP qPCR (a) and semi-qPCR (b). Error bars indicate s.d. 3′ UTR sequences lacking TBEs, negative controls. Flag*, Flag ChIP from HeLa parental cells. c, Scanning ChIP across 20 kb of Axin2, Myc and HPRT promoters in lithium-treated HeLa parental and Flag–TERT HeLa cells by qPCR. Non-coding sequences conserved between human and mouse genomes are shown (VISTA graphs: exon, purple; 5′ UTR, blue; conserved non-coding sequence, pink). d, AFH-TERT knock-in mouse allele. e, Telomerase activity in small intestine from wild-type and TertAFH/AFH mice (TRAP). f, Association of AFH-TERT and β-catenin at the endogenous level in extracts from small intestine of TertAFH/AFH mice. Anti-HA antibody immunoprecipitation, followed by immunoblot with anti-HA antibody or anti-b-catenin antibody. IgGs, negative control for immunoblot. g, In vivo ChIP from Tert+/+ and TertAFH/AFH mouse small intestine shows co-occupancy across 20 kb of the Myc promoter for TCF3 (red) and AFH-TERT (green). ChIP from Tert+/+ mouse with anti-HA-7 antibody, negative control (blue).
To understand if endogenous TERT protein participates in regulatory complexes at β-catenin-dependent genes, we engineered knock-in mice in which a multiple epitope tag was inserted in frame at the beginning of the Tert gene (AFH–TERT) (Fig. 5d and Supplementary Fig. 16). TertAFH/AFH mice were viable and telomerase activity from small intestine extracts measured by the telomere repeat amplification protocol (TRAP assay) was equivalent in TertAFH/AFH mice and in wild-type littermate controls (Fig. 5e). Immunoprecipitation and western blot analysis from protein extracts of the small intestine using anti-HA antibodies revealed an AFH–TERT band of the expected size specifically in TertAFH/AFH mice (Fig. 5f). Chromatin association of endogenous TERT with TCF sites was assessed by performing in vivo ChIP on small intestine using anti-HA antibodies and TCF3 antibodies. Primer pairs spanning 20 kb of the mouse Myc promoter at an average of 1-kb intervals were used in quantitative PCR to detect chromatin fragments associated with AFH–TERT and TCF3. AFH–TERT and TCF3 were bound to several chromatin regions spanning the Myc genomic sequences in a remarkably similar pattern (Fig. 5g). Importantly, ChIP experiments performed in parallel using anti-HA antibodies and wild-type small intestine samples did not pull down Myc promoter chromatin, indicating that the chromatin fragments in the AFH–TERT samples were dependent on the presence of AFH–TERT protein. Consistent with these findings, HA–TERT was associated with chromatin from the Axin2 promoter in a pattern that overlapped with β-catenin in TertHA/+ mouse ES cells (Supplementary Fig. 17). Furthermore, β-catenin was detected in AFH–TERT complexes purified from small intestine extracts of TertAFH/AFH mice, but not in Tert+/+ controls, indicating that endogenous TERT and β-catenin exist in a common complex (Fig. 5f and Supplementary Fig. 18). These data show that endogenous TERT and TCF3 occupy similar chromatin sites in the Myc gene in small intestine, consistent with a role for TERT in β-catenin-mediated transcriptional responses in vivo.
Conclusions
Wnt signalling serves a critical role in development, in proliferating tissue progenitor cells and in human cancers1. In the absence of a Wnt signal, TCF/LEF transcription factors occupy their cognate sites in Wnt responsive gene promoters, where they recruit repressor proteins such as CtBP and Groucho/TLE44. In the setting of Wnt ligand, β-catenin displaces these repressors and recruits proteins that help to confer transcriptional activation at TCF/LEF binding sites, although the mechanisms governing this activation are not fully understood45. Proteins recruited by β-catenin to promoters include several complexes that modify or remodel histones, such as BRG1, CBP/p300, TRAAP, Mll1/Mll2 and SWR1, as well as proteins coupled to initiation and elongation by RNA polymerase II, such as MED12, hyrax, pygopus and CDK8 (refs 44, 46–48). Thus, many modulators may be required for efficient tuning of the Wnt response and it is likely that some cofactors may be context-specific to explain how Wnt signalling leads to alternative cell fates, such as proliferation or differentiation. Our data indicate that telomerase represents one such cofactor that probably acts in a progenitor cell context to facilitate a Wnt-regulated program of self-renewal, proliferation or survival.
This unexpected role for TERT as a regulatory molecule modulating transcription complements the more widely appreciated function of telomerase in maintaining telomere repeats at chromosome ends. Dysfunctional telomeres that arise in settings of insufficient telomerase strongly inhibit stem-cell self-renewal and impair survival of tissue progenitor cells, resulting in defects in telomerase knockout mouse tissues with high renewal requirements, including blood, gastrointestinal tract and testis39,49. Similarly, mutations in telomerase components underlie the human stem-cell disorder dyskeratosis congenita, which is characterized by defects in blood, lung epithelium and epidermal structures50. Thus, it is likely that activation of the Wnt pathway to support progenitor cell proliferation also requires coordinated regulation of telomerase at telomeres to ensure that telomere dysfunction does not undermine the effects of Wnt proteins. This need for coordinated regulation of telomere maintenance and Wnt signalling in progenitor cells may have led to the direct incorporation of telomerase into the Wnt signalling pathway as a cofactor for β-catenin.
The contrast between the modest effects on Wnt signalling seen with germline deletion of Tert in mouse and the more severe consequences of inhibiting TERT in ES cells or in Xenopus embryos suggests the possibility that TERT function in the Wnt pathway is partially compensated in germline knockout mice. Such developmental compensation may occur through adaptation of the Wnt network and explain the mild phenotypes in mouse knockouts of other Wnt regulators, such asAxin2, Nkd1, Nkd2, Pygo1 and Pygo2. Our findings provide a mechanism to understand previous observations showing that TERT overexpression activates epidermal stem cells, as well as previous findings linking TERT to proliferation, survival and stem-cell biology in diverse contexts. Our data reveal an unanticipated level of convergence between the telomerase and Wnt/β-catenin signalling pathways with important implications for understanding development, stem-cell regulation and cancer.
Methods
TERT protein complex purification and mass spectrometry
Lysates from HeLa S3 cells expressing AH3-TERT were used for protein purification and mass spectrometry as described previously20. In brief, TERT was fused at its amino terminus to a Staph protein A domain and an HA-epitope tag (AH3), separated by a TEV protease cleavage site. Telomerase complexes from HeLa AH3-TERT extracts were purified on IgG-agarose, released by TEV protease, and captured again on anti-HA antibody resin. After final elution, TERT complexes were fractionated by SDS–PAGE and TERT-associated proteins were excised from the gel and analysed by LC MS/MS.
X-gal staining
Axin2lacZ/+ reporter mice were intercrossed with actin-rtTA+ tetop-TERTci+ double transgenic mice (i-TERTci) and TERT was induced by administration of doxycycline (2 mg ml−1, 5% sucrose) in drinking water beginning at age 21 days. After 14 days of doxycycline treatment, tissues were processed for X-gal staining. Tissues were fixed with 4% paraformaldehyde for 1 h at 4 °C, rinsed in PBS, washed 5 times for 30 min in wash buffer (2mM MgCl2, 0.01%deoxycholate, 0.02% NP-40 in PBS), then stained with X-gal solution (4mM K3Fe(CN)6, 4mM K4Fe(CN)6, 20mM Tris pH 7.4, 1mg ml−1 X-gal in wash buffer) overnight at room temperature. The stained samples were washed, post-fixed and analysed.
Immunoprecipitation and immunoblot
The following antibodies were used: anti-Flag (M2, Sigma), BRG1 (H-88, SantaCruz and J1 from G. Crabtree), β-catenin (Transduction) and tubulin (Sigma). For immunoblotting, whole-cell lysates were prepared using NP-40 lysis buffer (0.5% NP-40, 1.5mM MgCl2, 25 mM HEPES, 150 mM KCl, 10% glycerol, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF and1 μg ml−1 each of aprotinin, leupeptin and pepstatin) for 15 min at 4 °C, followed by centrifugation (13,200 r.p.m., 10 min). Supernatant fractions were denatured with 5× SDS sample buffer (200 mM Tris-Cl, pH 6.8, 40% glycerol, 8% SDS, 200 mM DTT, 0.08%bromophenol blue) at 95 °C for 5 min, fractionated by SDS–PAGE and transferred onto nitrocellulose membrane. For immunoblot blocking and antibody incubation, 1% non-fat dry milk in TBST (25 mM Tris-HCl, pH 8.0, 125 mM NaCl, 0.5% Tween-20) was used. SuperSignal WestPico (Pierce Biotechnology) reagents were used to detect HRP (horseradish peroxidase)-conjugated secondary antibodies. For immunoprecipitation, the cells on 10-cm plates were lysed with 1 ml NP-40 lysis buffer for 15 min at 4 °C, followed by spinning at 13,200 r.p.m. for 10 min. 100 μl extract (10% of input) was saved for later immunoblotting. The cell lysates were pre-cleared with either mouse or rabbit IgG (SantaCruz) for 30 min and 30 μl of protein A/G agarose PLUS (SantaCruz) beads for an additional 30 min. After spinning down (2,000 r.p.m., 2 min), the supernatant was transferred into fresh tubes and incubated with antibodies at 4 °C overnight. Subsequently, 20 μl of protein A/G agarose PLUS was added and incubated for 1 h. Immunoprecipitates were washed with NP-40 lysis buffer and PBS, then eluted by boiling in loading buffer and analysed by immunoblotting. For quantification of immunoblots, ImageJ (NIH) was used.
Xenopus embryo culture, microinjections and duplicate axis formation assay
Fertilization, embryo culture and microinjections were performed in accordance with standard methods. Embryos were microinjected with 5′-capped mRNAs synthesized in vitro (mMessage mMachine, Ambion), or with morpholinos(Gene Tools, TMO1, 5′-CATAGTGAGCACAATGGCAAAGTCC-3′; TMO2, 5′-TGGCTCCTCCTGTACGCAAAGGCAT-3′ and Std-MO). For duplicate axis formation assays, mRNAs were injected into ventral vegetal region of blastomere at the four-cell stage and ectopic axis formation was visually scored at embryonic stages 17 (late neurulation) through to 32 (tadpole). Partial axis duplication was scored based on incomplete formation of a duplicate head structure including eyes and cement gland pigmentation from the additional axis, whereas complete axis duplication required a complete head from the duplicated axis. Xenopus β-catenin mRNA was titrated to yield approximately 20% axis duplication and co-injected with increasing amounts of Xenopus Tert mRNA. For loss-of-function studies, morpholinos (40 ng) were injected at the one-cell stage. Average DAI (dorso-anterior index) score was calculated from more than 50 embryos of each condition. For rescue experiments, each mRNA (50 pg) was co-injected with TMO1 at the one-cell stage. Somites were analysed by 12/101 antibody staining of embryos fixed in MEMFA (0.1M MOPS (pH 7.4), 2mM EGTA, 1mM MgSO4 and 4% formaldehyde).
MicroCT and skeleton staining
ImTek microCT images (ImTek microCT scanner) were analysed by Microview 2.2 software (GE healthcare). Alcian blue/alizarin red staining was performed as previously described36.
Chromatin immunoprecipitation assays
ChIP assays were performed using Flag (M2, Sigma), β-catenin (Transduction), BRG1 (H-88, SantaCruz), TCF3 (M-20, SantaCruz) and HA (3F10, Roche) antibodies. HeLa cells were treated with 25 mM LiCl for 4 h before ChIP. For in vivo ChIP assays, mouse small intestine was dissected, opened longitudinally, washed twice in cold PBS and immediately cross-linked with 1% formaldehyde for 30 min at room temperature. Formaldehyde was quenched by adding glycine (final 0.125M concentration). Tissues were homogenized (maximum speed, 30 s), and processed in ChIP-RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.1% SDS, 0.5%deoxycholate, 1% NP40, 1 mM EDTA) containing proteinase inhibitors and further incubated on ice for 15 min. Tissue lysates were sonicated (70% output, 10 times, 30s) with glass beads (Sigma) and centrifugated (13,200 r.p.m., 30min). Supernatants were removed, diluted with ChIP-RIPA lysis buffer and precleared with protein A/G agarose PLUS (SantaCruz) overnight at 4 °C. Supernatant from precleared lysates was immunoprecipitated with antibodies overnight at 4°C and pulled down using protein A/G agarose PLUS by centrifugation (3,400 r.p.m., 2 min). Immunoprecipitates were further washed serially with ChIP-RIPA, high salt (50 mM Tris pH 8.0, 500 mM NaCl, 0.1% SDS, 0.5%deoxycholate, 1% NP40, 1 mM EDTA), LiCl wash buffer (50 mM Tris pH 8.0, 1 mM EDTA, 250 mM LiCl, 1% NP40, 0.5%deoxycholate) and TE buffer. Finally, immunoprecipitates cross-linked were reversed by incubation at 65 °C overnight and treated with RNaseA and proteinase K to extract DNA. The isolated DNAs were analysed by real-time PCR.
Supplementary Material
Acknowledgments
We thank P. Chu of the Stanford Comparative Medicine Histology Research Core Laboratory for technical assistance. We thank F. Ishikawa for Xenopus TERT plasmid, G. Crabtree for antibodies and plasmids, T. Jacks for ROSACreERT2 mice, and K. Park for constructive comments. J.-I.P. was supported by a Stanford Comprehensive Cancer Center Fellowship. This work was supported by NCI grants CA111691 and CA125453 and by a grant from the California Breast Cancer Research Program to S.E.A.
Footnotes
Supplementary Information: is linked to the online version of the paper at www.nature.com/nature.
Author Contributions: J.-I.P., A.S.V., J.Y.H., J.C., M.S., T.D.V., R.N., P.D.M. and S.E.A. designed the experiments and analysed data; J.-I.P., A.S.V., J.Y.H., J.C., S.J., M.S., W.C., Z.M., P.C., H.J. and M.M. performed the experiments; and J.-I.P. and S.E.A. wrote the manuscript.
References
- 1.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 2.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 3.Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo Celso C, Prowse DM, Watt FM. Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 5.Sarin KY, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 7.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 9.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artandi SE, et al. Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc Natl Acad Sci USA. 2002;99:8191–8196. doi: 10.1073/pnas.112515399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Suarez E, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart SA, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci USA. 2002;99:12606–12611. doi: 10.1073/pnas.182407599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nature Cell Biol. 2003;5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong L, et al. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells. 2005;23:516–529. doi: 10.1634/stemcells.2004-0269. [DOI] [PubMed] [Google Scholar]
- 16.Imamura S, et al. A non-canonical function of zebrafish telomerase reverse transcriptase is required for developmental hematopoiesis. PLoS ONE. 2008;3:e3364. doi: 10.1371/journal.pone.0003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, et al. A key role for telomerase reverse transcriptase unit in modulating human embryonic stem cell proliferation, cell cycle dynamics, and in vitro differentiation. Stem Cells. 2008;26:850–863. doi: 10.1634/stemcells.2007-0677. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, et al. TERT promotes cellular and organismal survival independently of telomerase activity. Oncogene. 2008;27:3754–3760. doi: 10.1038/sj.onc.1211037. [DOI] [PubMed] [Google Scholar]
- 19.Masutomi K, et al. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc Natl Acad Sci USA. 2005;102:8222–8227. doi: 10.1073/pnas.0503095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132:945–957. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YL, et al. Immunodetection of human telomerase reverse-transcriptase (hTERT) re-appraised: nucleolin and telomerase cross paths. J Cell Sci. 2006;119:2797–2806. doi: 10.1242/jcs.03001. [DOI] [PubMed] [Google Scholar]
- 23.Barker N, et al. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBOJ. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Major MB, et al. New regulators of Wnt/β-catenin signaling revealed by integrative molecular screening. Sci Signal. 2008;1:ra12. doi: 10.1126/scisignal.2000037. [DOI] [PubMed] [Google Scholar]
- 25.Henriksson M, Luscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 26.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 27.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 29.McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- 30.Huelsken J, et al. Requirement for β-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heasman J, et al. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 32.Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann' organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- 34.Dubrulle J, Pourquie O. Coupling segmentation to axis formation. Development. 2004;131:5783–5793. doi: 10.1242/dev.01519. [DOI] [PubMed] [Google Scholar]
- 35.Greco TL, et al. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- 36.Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev. 2001;103:27–33. doi: 10.1016/s0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- 37.Pownall ME, Tucker AS, Slack JM, Isaacs HV. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development. 1996;122:3881–3892. doi: 10.1242/dev.122.12.3881. [DOI] [PubMed] [Google Scholar]
- 38.Houle M, Prinos P, Iulianella A, Bouchard N, Lohnes D. Retinoic acid regulation of Cdx1: an indirect mechanism for retinoids and vertebral specification. Mol Cell Biol. 2000;20:6579–6586. doi: 10.1128/mcb.20.17.6579-6586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 40.Erdmann N, Liu Y, Harrington L. Distinct dosage requirements for the maintenance of long and short telomeres in mTert heterozygous mice. Proc Natl Acad Sci USA. 2004;101:6080–6085. doi: 10.1073/pnas.0401580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohnes D. The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. Bioessays. 2003;25:971–980. doi: 10.1002/bies.10340. [DOI] [PubMed] [Google Scholar]
- 42.Farazi PA, Glickman J, Horner J, Depinho RA. Cooperative interactions of p53 mutation, telomere dysfunction, and chronic liver damage in hepatocellular carcinoma progression. Cancer Res. 2006;66:4766–4773. doi: 10.1158/0008-5472.CAN-05-4608. [DOI] [PubMed] [Google Scholar]
- 43.Rajaraman S, et al. Telomere uncapping in progenitor cells with critical telomere shortening is coupled to S-phase progression in vivo. Proc Natl Acad Sci USA. 2007;104:17747–17752. doi: 10.1073/pnas.0706485104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 45.Daniels DL, Weis WI. β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nature Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 46.Firestein R, et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature. 2008;455:547–551. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrera I, Janody F, Leeds N, Duveau F, Treisman JE. Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13. Proc Natl Acad Sci USA. 2008;105:6644–6649. doi: 10.1073/pnas.0709749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/β-catenin signaling. J Biol Chem. 2006;281:14066–14075. doi: 10.1074/jbc.M602696200. [DOI] [PubMed] [Google Scholar]
- 49.Wong KK, et al. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nature Genet. 2000;26:85–88. doi: 10.1038/79232. [DOI] [PubMed] [Google Scholar]
- 50.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009 doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.