Abstract
BACKGROUND
Anxiety produced by environmental threats can impair goal-directed processing and is associated with a range of psychiatric disorders, particularly when aversive events occur unpredictably. The prefrontal cortex (PFC) is thought to implement controls that minimize performance disruptions from threat-induced anxiety and goal distraction by modulating activity in regions involved in threat detection, such as the amygdala. The inferior frontal gyrus (IFG), orbitofrontal cortex (OFC), and ventromedial PFC (vmPFC) have been linked to the regulation of anxiety during threat exposure. We developed a paradigm to determine if threat-induced anxiety would enhance functional connectivity between the amygdala and IFG, OFC, and vmPFC.
METHODS
Healthy adults performed a computer-gaming style task involving capturing prey and evading predators to optimize monetary rewards while exposed to the threat of unpredictable shock. Psychophysiological recording (n = 26) and functional magnetic resonance imaging scanning (n = 17) were collected during the task in separate cohorts. Task-specific changes in functional connectivity with the amygdala were examined using psychophysiological interaction analysis.
RESULTS
Threat exposure resulted in greater arousal measured by increased skin conductance but did not influence performance (i.e., monetary losses or rewards). Greater functional connectivity between the right amygdala and bilateral IFG, OFC, vmPFC, anterior cingulate cortex, and frontopolar cortex was associated with threat exposure.
CONCLUSIONS
Exposure to unpredictable threat modulates amygdala-PFC functional connectivity that may help maintain performance when experiencing anxiety induced by threat. Our paradigm is well-suited to explore the neural underpinnings of the anxiety response to unpredictable threat in patients with various anxiety disorders.
Keywords: Amygdala, Functional connectivity, Inferior frontal gyrus, Orbitofrontal cortex, Psychophysiological interaction, Ventromedial prefrontal cortex
Unpredictable environmental threats can induce a subjective state of anxiety that is linked to impaired goal-directed processing and anxiety disorders (1–3). Researchers have proposed that excessive anxiety results from diminished top-down control in response to threat-related distractors, which has been associated with decreased prefrontal cortex (PFC) activation in highly anxious individuals (4). Prefrontal cortex dysfunction and altered connectivity with the amygdala have been demonstrated during threat processing in anxiety disorders (5–7). Prior studies have largely assessed threat processing during exposure to aversive stimuli (e.g., fearful/ angry faces, images of spiders, aversive sounds). Recent neurobiological frameworks have emphasized uncertainty and anticipatory processing in anxiety (8), which parallel clinical observations that anxiety symptoms (e.g., worry, intrusive thoughts, avoidance behaviors) often persist in the absence of the precipitating stimulus. Experimental paradigms that probe the processing of uncertain or ambiguous threat during concurrent goal-directed tasks are therefore relevant to elucidate etiological factors of clinical anxiety and inform potential treatment approaches. Understanding amygdala-PFC functional connectivity during threat-induced anxiety and goal distraction in healthy populations provides a foundation for how these functional connections may be compromised in anxiety disorders.
The regulation of threat-elicited anxiety is important for maintaining performance in a range of interpersonal and occupational activities (e.g., patrol/guard jobs, first responders) that require continuous goal-directed attention and contingent planning. Mobbs et al. (9,10) examined threat anticipation using an active avoidance paradigm that required navigating through a virtual maze where the threat of shock was contingent upon performance. Activation in the ventro-medial PFC (vmPFC) was observed when threat was present but spatially distant. However, threat of unpredictable compared with predictable aversive events is more strongly linked to anxiety and depressive states/disorders (11–13). Consequently, we examined amygdala-PFC functional connectivity during anxiety created by threat of unpredictable aversive stimuli.
Based on prior studies (14–18), we posit that effective regulation of the amygdala’s response to threat is critical to maintaining goal-directed behavior. Exposure to threat activates the amygdala, while cognitive processing in the presence of emotional stimuli engages ventral PFC, including the inferior frontal gyrus (IFG), vmPFC, and orbitofrontal cortex (OFC) (19–22). These PFC subregions have been previously implicated in the control of emotional distraction (19,23). The IFG is involved in inhibitory control and coping with elevated task demands posed by emotional distractors (15,24,25). Our prior research demonstrated visual threat stimuli presented as emotional distractors on a delayed-response working memory task activate the amygdala and IFG (19). Inferior frontal gyrus activation has been associated with better working memory performance during emotional distraction (22). Cognitive control of anxiety states from threat-related distractors and reappraisal of threat stimuli were associated with lateral PFC (IFG) and medial PFC (vmPFC, OFC) activation and simultaneously decreased amygdala activation (17,26).
The PFC regulates emotional distraction and maintains ongoing performance via its modulatory interactions with the amygdala [and regions that lie downstream from the amygdala (14,27,28)]. To minimize performance disruptions from threat-induced anxiety, compensatory neural processes may be engaged to modulate the resulting neural response (3). It is therefore important to test task-dependent functional connectivity rather than testing local mean changes in activity. Functional connectivity between the amygdala and the IFG and frontopolar cortex is increased as a function of emotional distraction (e.g., visual threat) during working memory tasks (22,29) and as a function of motor inhibition during threat exposure (e.g., fearful/angry faces) (30). Increased functional connectivity during emotion regulation has been demonstrated between the amygdala and the IFG, vmPFC, and OFC, although there is variability in the specific PFC regions across studies (17,26,31,32). These findings informed our hypothesis that the regulation of threat-induced anxiety will be manifest as increased functional connectivity of the amygdala with ventral PFC.
We adapted an arcade style game in which participants faced the threat of unpredictable shocks while navigating through a virtual maze to flee from a predator and pursue prey. Escape from the predator and capture of prey were motivated by monetary gains or losses unrelated to shock delivery. Our goal of studying threat modulation during these dual tasks was to create a symmetric design with the same tracking behaviors across threat and nonthreat conditions. This is in contrast to the control condition in prior studies (9,10) where participants mimicked the avatar’s movements that did not probe active avoidance during safety from shock. Moreover, the source of threat was unpredictable, unlike the Mobbs et al. (9,10) paradigm where escape from the predator was motivated by shock upon capture.
To address our goal of eliciting psychological state changes linked to anxiety, we used threat of shock to induce anxiety (33,34) and increase psychophysiological arousal (35–38). Activation in the thalamus, striatum, insula, and lateral and medial PFC (IFG and anterior cingulate cortex [ACC]) but inconsistent findings of amygdala activation have been demonstrated in prior threat-of-shock studies (35,37,39–42). We predicted increased skin conductance response (SCR) and decreased heart rate variability (HRV), reflecting greater psychophysiological arousal, and increased functional connectivity between the amygdala and the IFG, OFC, and vmPFC for threat versus nonthreat conditions.
METHODS AND MATERIALS
Forty-five participants completed two experiments: 28 participated in psychophysiological recording (16 female participants; mean age = 25.50, SD = 5.49) and 17 underwent functional magnetic resonance imaging (fMRI) scanning (8 female participants; mean age = 25.29, SD = 5.89). Two psychophysiology participants were excluded for failure to show shock-evoked SCR during pretask calibration (final sample: n = 26; 15 female participants; mean age = 24.81, SD = 4.95). Participants were free of past psychiatric illness, neurological illness/injury, current psychotropic medications or substance abuse, and magnetic resonance imaging contra-indications. The Structured Clinical Interview for DSM-IV (43) was administered to the fMRI sample to rule out current Axis I disorders (44), history of bipolar disorder, psychotic disorder, and substance dependence. Trauma exposure defined by DSM-IV was ruled out in fMRI participants.
The Yale University Human Investigations Committee approved this study and participants provided written informed consent. Participants were compensated $20 per hour plus the opportunity to win up to $20 based upon their task performance. Shock calibration and psychophysiology procedures, including SCR and HRV methods, are reported in Supplement 1.
Experimental Paradigm
Participants performed a computer gaming style task adapted from Mobbs et al. (9,10) that engaged goal-directed attention and planning while maximizing monetary reward and avoiding loss (Figure 1). Participants manipulated a joystick to navigate an avatar through a two-dimensional maze with no dead ends to capture prey in return for monetary reward. A predator was programmed to follow the minimum path through the maze to pursue the avatar. Participants were instructed to evade predator capture to minimize monetary loss.
Figure 1.
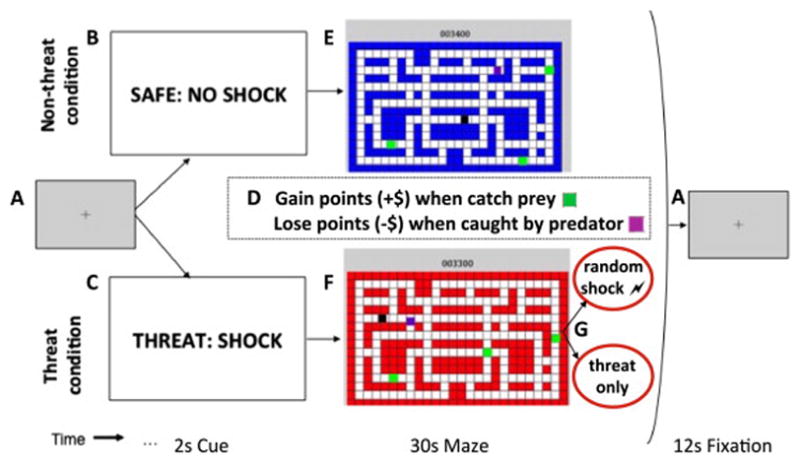
Schematic of experimental trial for predator-prey task. (A) The nonthreat and threat conditions were separated by 12-second rest periods. (B, C) The nonthreat and threat conditions were explicitly conveyed via 2-second visual cues before each 30-second maze period. (D) The dual task was identical in both conditions: to maximize points by manipulating the movement of the avatar to capture prey (green squares), resulting in point gains, and to avoid capture by the predator (purple square), resulting in point losses. (E, F) Participants used a joystick to navigate an avatar (black square) through a two-dimensional maze. In the nonthreat condition, the blue background indicated no shocks would be administered at any time. In the threat condition, the red background indicated participants may receive a shock at any time. (G) Shock was randomly delivered on a subset of threat trials. To eliminate the confound of shock effects, only threat trials with no shock delivery were included in the threat versus nonthreat comparisons.
Before the main task, participants completed an instructional run that demonstrated the loss and reward contingencies, followed by an adaptive practice run to measure the participant’s skill in navigating the maze and to set the difficulty level of the task that remained constant throughout the experiment. The task difficulty was adjusted by setting the predator’s speed from level 5 (most difficult: predator speed = avatar speed) to level 1 (least difficult). In the fMRI sample, 16 participants were assigned level 5 and 1 participant was assigned level 4. In the psychophysiology sample, 5 participants were assigned level 5, 10 were assigned level 4, and 11 were assigned level 3.
Participants performed the five runs under the presence/ absence of threat of mild electrical shock. Participants were instructed they would not be shocked in the nonthreat condition but might be shocked unconditionally at random times in the threat condition. Each run consisted of four threat and four nonthreat trials lasting 32 seconds presented in alternating order and interspersed with 12-second rest periods. The order of trial types was reversed across consecutive runs. A 2-second cue at the start of each trial signaled threat or nonthreat trial types. Shock was randomly delivered on a subset of threat trials, which included at least one shock per run. The potential confound of shock on the neural response to threat was eliminated by including only threat trials with no shock in the threat condition for all fMRI and behavioral analyses. The threat trials with shock (shock condition) were modeled as a nuisance regressor in the fMRI analyses. The shock condition included 49.63% and 51.92% of threat trials for the fMRI and psychophysiology samples, respectively. Immediately following the fMRI scan (posttest), a subset of participants (n = 10) rated their subjective experience of anxiety during the task (Supplement 1).
Behavioral Data Analysis
Avatar captures by the predator and prey captures by the participant were recorded every 500 milliseconds. Paired t tests were conducted to compare the average rate of captures across threat versus nonthreat conditions.
Imaging Acquisition/Preprocessing
Data were acquired using a 3.0T Siemens Trio scanner (Siemens, Erlangen, Germany) with a 12-channel head coil. Co-planar images were acquired using T1 flash sequence (repetition time [TR] = 300 msec, echo time [TE] = 2.47 msec, flip angle α = 60°, field of view [FOV] = 224 mm, matrix = 256 × 256, in-plane resolution = .875 mm ×.875 mm, slice thickness = 3.5 mm, 37 oblique axial slices). High-resolution images were acquired using a three-dimensional magnetization prepared rapid acquisition gradient-echo sequence (TR = 2530 msec, TE = 3.34 msec, flip angle α = 7°, FOV = 256 mm, matrix = 256 × 256, voxel size = 1 mm3, 160 slices, sagittal plane). Five runs of functional images were collected using standard echo-planar pulse sequence (TR = 2000 msec, TE = 25 msec, flip angle α = 90°, FOV = 224 mm, matrix = 64 × 64, voxel size = 3.5 mm3, 37 oblique axial slices, no interslice gap). Each functional run consisted of 186 volumes (182 volumes for four subjects for which there was 12s fixation, as opposed to 20s, at the end of each run) plus 3 discarded volumes to allow for magnetic resonance equilibration.
Magnetic resonance imaging analyses were conducted using the Functional MRI of the Brain (FMRIB) Software Library (FSL Version 4.1.6; FMRIB, Oxford, United Kingdom) (45,46). Nonbrain voxels were removed using the FSL brain extraction tool (47). Functional data were temporally realigned to correct for interleaved slice acquisition and motion corrected using the FSL MCFLIRT linear realignment tool (48). Images were spatially smoothed with an isotropic Gaussian kernel of 5 mm full width at half maximum. To eliminate low-frequency drift, time series were filtered below .011 Hz.
General Linear Model fMRI Data Analysis
All fMRI analyses were conducted using whole-brain voxel-wise regression with the FSL FMRI Expert Analysis Tool (FEAT). Using the FMRIB Linear Image Registration Tool, functional images were registered to co-planar images, which were registered to high-resolution structural images and normalized to the Montreal Neurological Institute 152 template.
We conducted general linear model (GLM) analysis of the main effect of shock and the threat versus nonthreat comparison on regional activation. First-level GLM analyses were computed for each participant including the shock explanatory variables (EVs) and the threat and nonthreat condition EVs, plus nuisance regressors for shock condition trials and six motion parameters. We included nuisance regressors for time points corresponding to motion outliers using the FSL motion outliers program (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers), which defined outlier time points using the upper threshold for creating box plots or the 75th percentile plus 1.5 times the interquartile range. The shock regressor was modeled with onset defined as the TR in which the shock was delivered. Shocks were modeled using finite impulse response, resulting in four EVs spanning 8 seconds. Contrasts were calculated to test the main effect of shock and its evolution over 8 seconds. The three condition regressors were modeled as box car functions for the trial duration (2-second cue plus 30-second maze = 32 seconds), convolved with a single-gamma hemodynamic response function.
Group-level FEAT analysis was performed using a mixed-effects model, with the random effects component estimated using the FMRIB Local Analysis of Mixed Effects 1 + 2 procedure (49). This model allows an unequal number of trials across conditions because it passes variance from first-level to higher-level analyses. Multiple comparison correction was performed on whole-brain tests with the FSL two-step cluster thresholding procedure to define clusters as contiguous sets of voxels with z > 1.96 and test the significance of resulting cluster(s) at a corrected p < .05 threshold using Gaussian random field theory (50). The main effect of shock analysis used an additional Bonferroni correction of p < .0125 for the four tests modeled by finite impulse response.
Psychophysiological Interaction Analysis
We used psychophysiological interaction (PPI) analysis (51) to measure changes in functional connectivity modulated by threat. We conducted whole-brain PPI tests, reflecting greater correlation with the seed time series (physiological regressor) for threat versus nonthreat conditions (psychological regressor). Separate analyses were conducted using right and left amygdala seeds as defined by automated anatomical labeling with the FMRIB Integrated Registration and Segmentation Tool (52) (Figure S1 in Supplement 1). First-level GLM analyses included four regressors: psychological, physiological, PPI, and nuisance. Threat and nonthreat trial durations comprised the psychological regressor, modeled as a box car function with values 1 and −1, respectively, convolved with a single-gamma hemodynamic response function. The PPI regressor was the product of the demeaned physiological regressor and the psychological regressor, which was zero-centered about the minimum and maximum values. The following nuisance regressors were modeled: shock condition trials, global mean time series of each preprocessed run, six motion parameters, and motion outliers. Mixed-effects group-level FEAT analysis was conducted using FSL cluster thresholding.
Correlational Analyses
Correlational analyses tested the relationship between behavioral performance (prey captures and avatar captures) and the strength of right amygdala functional connectivity with left and right IFG, medial prefrontal cortex (mPFC), and vmPFC regions of interest. We compared whether correlations of performance with functional connectivity were significantly different during threat versus nonthreat using the modified Pearson-Filon (ZPF) statistic (Supplement 1).
RESULTS
Subjective Ratings
Posttest responses collected from 10 of the 17 participants from the fMRI sample confirmed that threat induction increased subjective anxiety. Anxiety was rated as higher at trial onset (t8 = −3.85, p = .005) and trial duration (t9 = −3.16, p = .012) for the combined threat/shock condition compared with the nonthreat condition (Supplement 1).
Behavioral Performance
For the fMRI sample, threat and nonthreat conditions did not differ in the average rate of avatar captures by the predator, t16 = −.55, p = .59, or average rate of prey captures, t16 = .24, p = .82. No significant effects of threat on monetary losses or rewards were observed for the psychophysiology sample (p values > .18).
Psychophysiology Results
In the psychophysiology sample, we confirmed increased nonspecific SCR rate during the shock versus nonthreat conditions, t25 = 2.97, p = .007 (Figure S2 in Supplement 1). There was no significant difference in mean SCR rate for the shock versus threat conditions, t25 = −.10, p = .92. As a test of the efficacy of our threat manipulation, mean SCR rate was significantly greater during threat versus nonthreat, t25 = 2.46, p = .02. The predicted decrease in HRV (root mean square successive difference) for threat versus nonthreat was marginally significant, t25 = −1.96, p = .06. There was no significant difference in heart rate between threat and nonthreat conditions.
Shock-Related Activation
Activation from shock occurred in expected regions, including bilateral insula, thalamus, cingulate cortex, amygdala, IFG, and somatosensory cortex (Figure 2). Activation in the insula, parietal lobe, and temporal lobe was sustained for all four time points, whereas activation in the thalamus was limited to the second time point. There was activation in the amygdala and postcentral gyrus during the second and third time points. Activation in IFG and OFC was observed for the second through fourth time points. Cingulate cortex activation, spanning the ACC, midcingulate cortex, and posterior cingulate cortex, was observed in the third and fourth time points.
Figure 2.
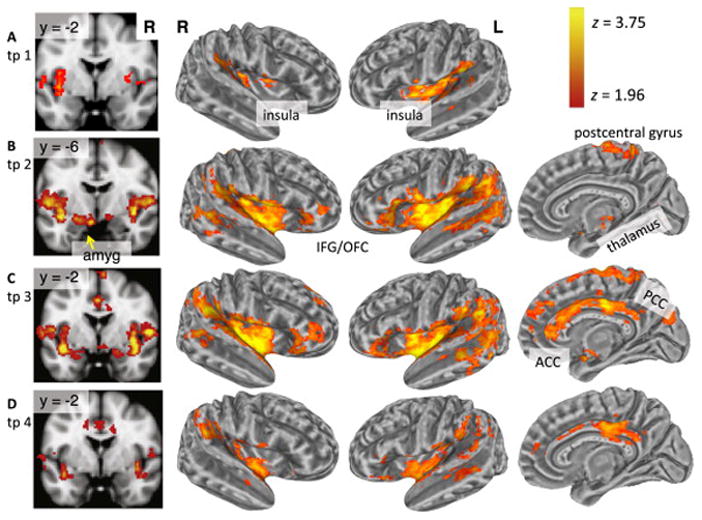
Voxel-wise results: Main effect of shock occurrences. Shock activation map (z > 1.96, p < .0125 cluster corrected) on the volume rendering (left column) and inflated cortical surface for the following post-stimulus onset time points (tp): (A) tp 1: onset to 2 seconds; (B) tp 2: 2 to 4 seconds; (C) tp 3: 4 to 6 seconds; and (D) tp 4: 6 to 8 seconds. ACC, anterior cingulate cortex; amyg, amygdala; IFG, inferior frontal gyrus; L, left; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; R, right.
Threat versus Nonthreat Activation
Regional activations to the threat versus nonthreat contrast were observed in bilateral OFC (Montreal Neurological Institute coordinates: −28, 22, −18; 38, 28, −12), which extended to the IFG (i.e., pars orbitalis) (−42, 30, −14; 44, 38, −16) and left amygdala (−16, 0, −20) (Figure 3; Table S1 in Supplement 1). There was also activation in bilateral middle frontal gyrus (MFG) extending to superior frontal gyrus.
Figure 3.
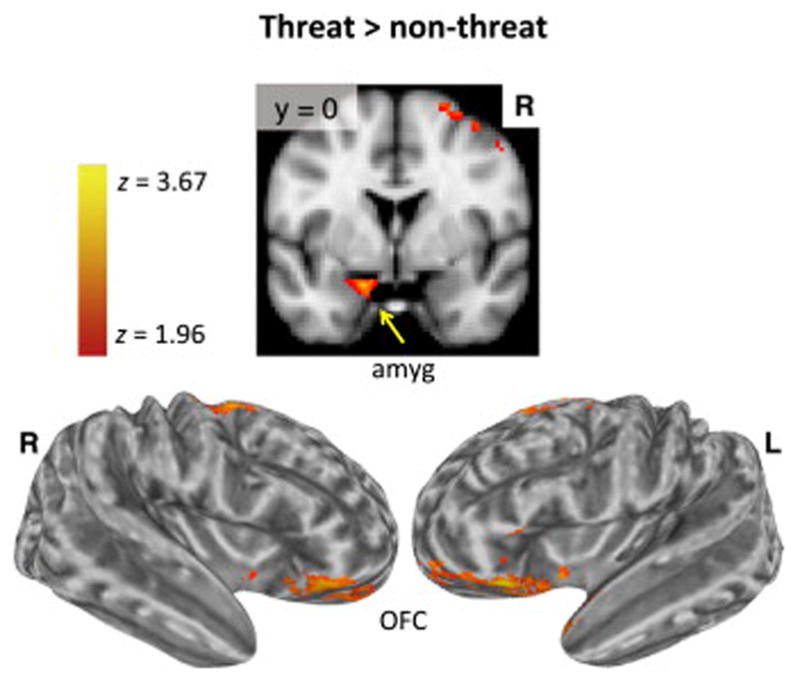
Voxel-wise results: Threat versus nonthreat contrast. The results for the threat versus nonthreat contrast are shown. Activation (z > 1.96, p < .05 cluster corrected) in the left amygdala (amyg) is shown on the volume rending (top row). Displayed on the lateral view of the inflated cortical surface (bottom row), there was activation in orbitofrontal prefrontal cortex (OFC) and inferior frontal gyrus (i.e., pars orbitalis). L, left; R, right.
Amygdala Functional Connectivity
The PPI contrast revealed that threat increased right amygdala connectivity with three clusters including the a priori regions (Figure 4; Table S2 in Supplement 1). As predicted, greater right amygdala connectivity was observed with bilateral IFG (−50, 24, −2; 42, 56, −6), OFC (−42, 18, −8; 50, 24, −14), mPFC (including local maxima in vmPFC [2, 40, −12], ACC [2, 44, 4], and frontopolar cortex [−6, 60, 12]), and left insula (−42, 10, −8). There was also increased connectivity in the left MFG, lateral temporal lobe, precuneus, and cuneus. There were no significant activations for the left amygdala PPI contrast.
Figure 4.
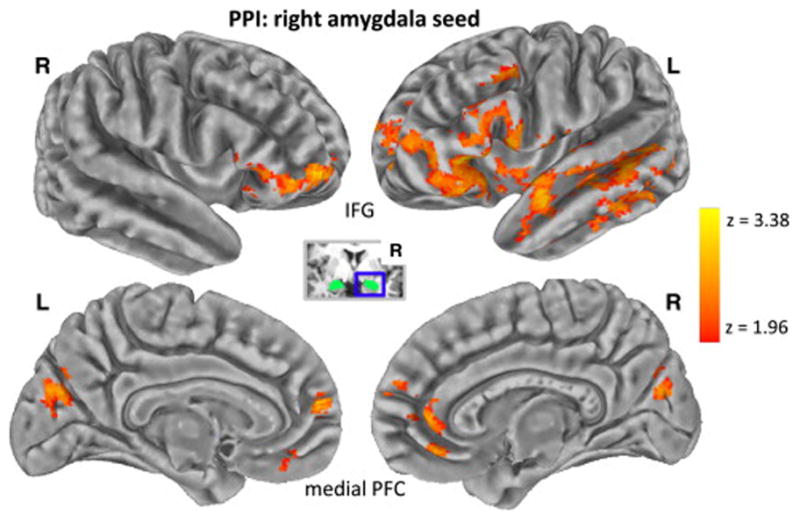
Voxel-wise results: Right amygdala psychophysiological interaction (PPI) analysis. Increased connectivity (z > 1.96, p < .05 cluster corrected) with the right (R) amygdala seed (inset) during the threat versus nonthreat was observed in the following a priori regions: bilateral inferior frontal gyrus (IFG), bilateral orbitofrontal cortex, and medial prefrontal cortex (PFC) with local maxima in ventromedial PFC, anterior cingulate cortex, and frontopolar cortex. Other PPI activation was observed in the left (L) anterior insula, left middle frontal gyrus, left lateral temporal lobe, cuneus, and left precuneus.
Association of Functional Connectivity with Performance
Average rate of rewards earned during the threat condition was positively correlated with right amygdala PPI strength in the mPFC (r = .61, p = .009) and vmPFC (r = .64, p = .005), with a trend toward significance in the right IFG (r = .47, p = .056) but not in the left IFG (r = −.12, p = .63). There were no significant correlations with losses during the threat condition or with either measure during the nonthreat condition (all p values > .11). The correlations between reward performance and right amygdala PPI strength significantly differed between the threat and nonthreat conditions in the mPFC (ZPF = 2.11, p = .035) and vmPFC (ZPF = 2.82, p = .005) (Figure 5).
Figure 5.
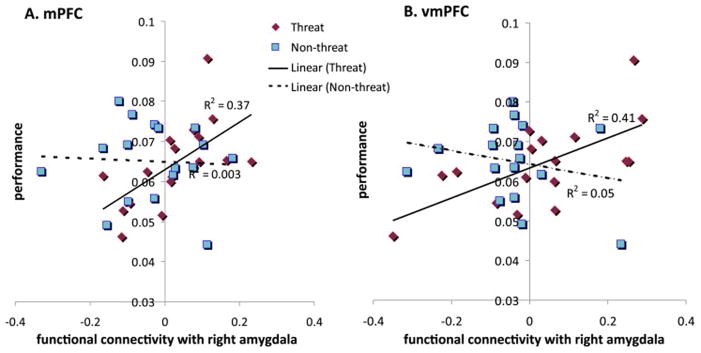
Correlations of functional connectivity and performance by condition. Individual differences analyses were conducted to investigate associations of functional connectivity strength and performance separately in the threat and nonthreat conditions. Scatter plots show the relationship between performance, measured as the average rate of prey captures (y-axis), with functional connectivity, quantified as the average right amygdala psychophysiological interaction beta coefficient (x-axis), for the medial prefrontal cortex (mPFC) and ventromedial prefrontal cortex (vmPFC) assessed during threat (red diamonds) and nonthreat (blue squares). (A) The correlation in the mFC for the threat condition (R2 = .37) was stronger than the correlation for the nonthreat condition (R2 = .003), based on the modified Pearson-Filon (ZPF) statistic = 2.11, p = .035. (B) The correlation in the vmFC for the threat condition (R2 = .41) was stronger than the correlation for the nonthreat condition (R2 = .05), based on the modified Pearson-Filon (ZPF) statistic = 2.82, p = .005.
DISCUSSION
We examined the influence of threat-induced anxiety on the functional connectivity of the amygdala with brain regions implicated in threat-related distraction. Self-assessment and psychophysiological measures demonstrated that threat exposure increased anxiety and arousal. Threat-induced anxiety did not produce changes in performance based on monetary losses or rewards. The validity of our task was supported by increased activations in the amygdala, OFC, and IFG elicited by threat-induced anxiety. As predicted, threat-induced anxiety modulated the functional connectivity between the amygdala and the IFG, OFC, and mPFC, including vmPFC, ACC, and frontopolar cortex. Sustained threat of unpredictable aversive events generates anticipatory processing that is central to anxiety but has received minimal attention in prior functional connectivity research. Functional connectivity changes provide evidence for cortical-subcortical interactions that help protect goal pursuit in the face of threat and mediate the regulation of anxiety.
Participants were cued before each trial to either warn of the possibility of receiving unpredictable and unavoidable shocks or to signal safety from shock. Subjective ratings from participants, elevated arousal levels from SCR, and a marginally significant decrease in HRV provided converging support in demonstrating that threat cues induced anxiety. Our results are consistent with prior studies showing increased SCR during anticipation of impending shock (35–37). However, most prior studies were limited to passive observation of stimuli or rating of one’s own emotions but lacked concurrent goal-directed activity. In this study, increased arousal to threat was observed while participants were engaged in the goal-directed activity of maximizing monetary gain by capturing prey while avoiding a predator.
Despite increased anxiety and arousal generated by threat, participants performed equally well on the task under threat and nonthreat conditions. Threat processing (in the absence of shock) led to increased functional connectivity observed between the amygdala and ventral PFC. Threat modulated the association between reward performance and functional connectivity of the amygdala with the mPFC and vmPFC. This is consistent with our hypothesis that increased connectivity enables participants to compensate for increased anxiety while maintaining performance, despite threat of shock. This interpretation is complicated by null results for correlations of performance with SCR/HRV and with self-reported anxiety (Supplement 1), which might be expected to show a negative correlation. However, research has shown physiological measures do not always correlate with subjective experiences of anxiety (53). Moreover, the subjective ratings were collected after the scan to minimize interference with the fMRI results. Enhanced functional connectivity may reflect inhibition of the amygdala by the ventral PFC through top-down regulation of anxious arousal to maintain task performance. It is also possible that increased functional connectivity reflects bottom-up modulation by the amygdala or bidirectional modulation or that the task was simple enough to be performed while distracted. Our findings are consistent with prior studies showing increased amygdala connectivity during threat-induced anxiety facilitates inhibitory processing across affective, cognitive, and motor domains in the IFG and the assessment of affective salience and mood regulation in the vmPFC and OFC (2,30,54).
The vmPFC emerged as the only region with consistently increased regional activation across fear extinction, the placebo response, and emotion regulation-based cognitive strategies in a meta-analysis focused on the successful reduction of negative affect (17). This supports the central role of the vmPFC as a domain-general affective regulation system. Moreover, positive amygdala–vmPFC correlation has been demonstrated during extinction recall following fear conditioning (55). The rodent homologue of vmPFC has been linked to activation of inhibitory networks within the amygdala and associated with reduced amygdala-generated affective responses in fear extinction (56). Increased threat-related functional connectivity between the amygdala and vmPFC is consistent with prior research supporting a functional relationship between these regions (56,57). Functional correlates of amygdala–vmPFC interactions may be effectively probed by the current paradigm in anxiety disorders given its sensitivity to detect threat-induced anxiety changes in functional connectivity.
Activation of the IFG and OFC was reported in meta-analyses of cognitive reappraisal and the placebo response but not fear extinction (17). Delgado et al. (58) compared amygdala, vmPFC, and lateral PFC activation using matched paradigms testing fear extinction or deliberate emotion regulation. Whereas fear extinction resulted in amygdala and vmPFC activation but not lateral PFC activation, emotion regulation showed activation in dorsolateral PFC in addition to vmPFC and amygdala. They proposed lateral PFC involvement stemmed from online manipulation of information during emotion regulation as opposed to passive fear extinction. Our functional connectivity findings in IFG and mPFC suggest these regions may be regulating anxiety while maintaining goal-directed activity.
These presumed modulatory influences of threat upon functional connectivity should be considered in light of known anatomical connections between the PFC and nuclei within the amygdala. The IFG has indirect connections to the amygdala, whereas the vmPFC and OFC have direct connections (23,59). The spatial extent of the IFG clusters showing increased amygdala connectivity during threat-induced anxiety also included the orbital and medial PFC network. The right IFG cluster included the pars orbitalis and OFC, and the left IFG cluster extended to mPFC, which included vmPFC and left frontopolar cortex. Ventral PFC regions are proposed to promote emotion regulation through structural connections with the amygdala (23). Increased functional connectivity during threat may be facilitated by direct cortical amygdala connections in OFC and vmPFC and an indirect connection between the IFG and amygdala that is routed through the OFC and/or vmPFC. Modulatory influences of threat may be driven by connections with the basolateral amygdala complex implicated in associative fear learning (60). However, further research is needed to test whether basolateral amygdala complex functional connectivity exerts such modulatory influences during threat-induced anxiety.
Shocks evoked activation in the insula, amygdala, cingulate cortex, IFG, thalamus, and postcentral gyrus consistent with the pain matrix and reliably observed in prior studies of unconditioned fear and pain (61–63). Activation to shocks was observed in a dorsal region corresponding to the central nucleus of the amygdala, which orchestrates defensive responses to environmental threats and is consistent with the finding of Mobbs et al. (9) showing activation of dorsal amygdala to proximal but not distal threat (64). Greater activation was elicited by the threat compared with nonthreat condition in the amygdala, OFC, which extends to the pars orbitalis of the IFG, and dorsolateral PFC (dlPFC). This pattern is consistent with the experience and anticipation of pain stimuli, which have been shown to produce overlapping activation in the prefrontal cortex (63). Increased activation of these regions is consonant with their function of integrating emotional and cognitive domains (15,65). Greater dlPFC activation and increased amygdala connectivity in the MFG during threat are consistent with evidence that the dlPFC, a region underlying attentional control, facilitates goal-directed behavior by indirectly modulating the amygdala’s response to threat stimuli during goal distraction, possibly through connections with the temporal cortex (66,67).
Connectivity-based neuroimaging studies in humans are needed to test hypotheses regarding abnormal cortical–subcortical interactions in anxiety and mood disorders, which are predicated on animal models of anxiety disorders that have established functional alterations of brain networks (59,68–70). Amygdala, insular cortex, and ventral PFC circuits are reliably altered during emotional and cognitive processing in anxiety disorders (5–7,71,72). Moreover, the diminished sense of control generated by stress and the lack of predictability from environmental threats have been examined in translational research, particularly in the onset and chronicity of posttraumatic stress disorder, panic disorder, and depression (11–13,73). Given our findings in this nonclinical sample, our task is well suited to testing functional connectivity changes in populations that are exposed to unpredictable and uncontrollable aversive life events and traumas, which have been linked to increased rates of posttraumatic stress disorder, depression, and other psychopathology (74–76) and lack of treatment response in depression (77). By combining reward contingencies with a threat manipulation, we simultaneously tested multiple constructs of the proposed research domain criteria (78), including negative and positive affect systems and their interactions.
Limitations
Although we used a long trial duration (32 seconds) to increase statistical power, the presence of correlated regressors (i.e., psychological, physiological, and PPI regressors) reduces statistical power in the GLM for the PPI contrast (79). Study limitations include modest sample size and the inability to determine directional information and whether modulatory interactions reflect direct and/or indirect pathways using the PPI method.
Conclusion
In summary, this study demonstrated amygdala functional connectivity changes associated with the processing of threat among PFC brain regions implicated in the regulation of anxiety. Interpretation of our amygdala–ventral PFC connectivity results was bolstered in light of psychophysiological findings and nonhuman primate research demonstrating structural connections among these regions. Confirmatory findings in healthy adults provide a foundation for translational applications of our predator-prey paradigm. Such applications might elucidate the neural underpinnings of sustained psychological state changes linked to anxiety that persist beyond the presence of precipitating stimuli and impair goal-directed behavior and daily functioning.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Neurological Disorders and Stroke Grant NS-41328 (GM) and by the Yale University Faculty of Arts and Sciences (FAS) Imaging Fund. Dr. Morey was supported by Department of Veterans Affairs (DVA) 1I01CX000748-01A1 and I01CX000120-01 and the Mid-Atlantic Mental Illness, Research, and Education Center. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Mental Health (ALG). ALG is currently affiliation with the Section on Development and Affective Neuroscience, National Institute of Mental Health, Bethesda, Maryland.
We thank Mr. Jeffrey Hoerle for his assistance in programming and Ms. Kirsten Koons, Dr. George He, and Mr. William Walker for their assistance in data acquisition and analysis.
Footnotes
DISCLOSURES
Drs. Gold, Morey, and McCarthy have no biomedical financial interests or potential conflicts of interest to declare related to the present study.
Presented at the Annual Meeting of the Society for Neuroscience, November 15, 2011, Washington, DC.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.03.030.
References
- 1.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 4.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 5.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, et al. When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, et al. From threat to fear: The neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: An animal model. Psychol Bull. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 12.Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Dolcos F, Iordan AD, Dolcos S. Neural correlates of emotion–cognition interactions: A review of evidence from brain imaging investigations. J Cogn Psychol (Hove) 2011;23:669–694. doi: 10.1080/20445911.2011.594433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 18.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 22.Anticevic A, Repovs G, Barch DM. Resisting emotional interference: Brain regions facilitating working memory performance during negative distraction. Cogn Affect Behav Neurosci. 2010;10:159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray R, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev. 2011;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, et al. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- 26.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies [published online ahead of print June 13] Cereb Cortex. 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 28.Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 29.Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17:1591–1594. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- 30.Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: Intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. Neuroimage. 2009;47:705–712. doi: 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 32.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat Protoc. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: Implications for depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR, et al. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage. 2011;55:401–410. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopacz FM, 2nd, Smith BD. Sex differences in skin conductance measures as a function of shock threat. Psychophysiology. 1971;8:293–303. doi: 10.1111/j.1469-8986.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 37.Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 38.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler T, Pan H, Tuescher O, Engelien A, Goldstein M, Epstein J, et al. Human fear-related motor neurocircuitry. Neuroscience. 2007;150:1–7. doi: 10.1016/j.neuroscience.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 40.Kumari V, ffytche DH, Das M, Wilson GD, Goswami S, Sharma T. Neuroticism and brain responses to anticipatory fear. Behav Neurosci. 2007;121:643–652. doi: 10.1037/0735-7044.121.4.643. [DOI] [PubMed] [Google Scholar]
- 41.Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 42.Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, et al. Anxiety reduction through detachment: Subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- 43.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. New York: New York State Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- 44.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 45.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 46.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(suppl 1):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 47.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 49.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 50.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 51.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 52.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, et al. Response to learned threat: An fMRI study in adolescent and adult anxiety. Am J Psychiatry. 2013;170:1195–1204. doi: 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: A neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry. 2011;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: A single-trial fMRI study. Pain. 2007;128:20–30. doi: 10.1016/j.pain.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 63.Seifert F, Schuberth N, De Col R, Peltz E, Nickel FT, Maihöfner C. Brain activity during sympathetic response in anticipation and experience of pain. Hum Brain Mapp. 2013;34:1768–1782. doi: 10.1002/hbm.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.LeDoux J. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 65.Pessoa L. Emergent processes in cognitive-emotional interactions. Dialogues Clin Neurosci. 2010;12:433–448. doi: 10.31887/DCNS.2010.12.4/lpessoa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell DG. The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behav Brain Res. 2011;217:215–231. doi: 10.1016/j.bbr.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 67.Bishop SJ. Neural mechanisms underlying selective attention to threat. Ann N Y Acad Sci. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- 68.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- 71.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 72.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 73.Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hollis F, Isgor C, Kabbaj M. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience. 2013;249:232–241. doi: 10.1016/j.neuroscience.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 76.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(suppl 1):18–28. [PubMed] [Google Scholar]
- 77.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 78.Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, et al. Developing constructs for psychopathology research: Research domain criteria. J Abnorm Psychol. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 79.O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


