Abstract
Background
The T-box transcription factor Tbx1, is essential for the normal development of multiple organ systems in the embryo. One of the most striking phenotypes in Tbx1−/− embryos is the failure of the caudal pharyngeal pouches to evaginate from the foregut endoderm. Despite considerable interest in the role of Tbx1 in development, the mechanisms whereby Tbx1 controls caudal pouch formation have remained elusive. In particular, the question as to how Tbx1 expression in the pharyngeal endoderm regulates pharyngeal pouch morphogenesis in the mouse embryo is not known.
Results
To address this question, we produced mouse embryos in which Tbx1 was specifically deleted from the pharyngeal endoderm and as expected, embryos failed to form caudal pharyngeal pouches. To determine the molecular mechanism, we examined expression of Fgf3 and Fgf8 ligands and downstream effectors. Although Fgf8 expression is greatly reduced in Tbx1-deficient endoderm, FGF signaling levels are unaffected. Furthermore, pouch morphogenesis is only partially perturbed by the loss of both Fgf3 and Fgf8 from the endoderm, indicating that neither are required for pouch formation.
Conclusions
Tbx1 deletion from the pharyngeal endoderm is sufficient to cause caudal pharyngeal arch segmentation defects by FGF-independent effectors that remain to be identified.
Keywords: Tbx1, FGF, pharyngeal endoderm, morphogenesis
INTRODUCTION
The T-box transcription factor Tbx1 is essential for the normal development and morphogenesis of several structures and organs in the head, pharynx and chest (Jerome and Papaioannou, 2001; Lindsay et al., 2001; Merscher et al., 2001). Haploinsufficiency for TBX1 is associated with DiGeorge/velocardiofacial syndrome, which is most often caused by microdeletions of chromosome 22q11.2 (Scambler, 2010), although isolated mutations in the TBX1 gene itself have been reported (Yagi et al., 2003; Paylor et al., 2006; Torres-Juan et al., 2007). One of the most striking phenotypic abnormalities in Tbx1−/− mouse embryos is a non-segmented caudal pharyngeal apparatus caused by caudal pharyngeal pouch aplasia, which is responsible for defects such as thymus aplasia (Jerome and Papaioannou, 2001; Lindsay et al., 2001; Vitelli et al., 2002a).
The pharyngeal apparatus is a transient structure in the mid-gestation embryo that gives rise to several essential organs (Graham, 2003). This structure is formed by the evagination of a series of pharyngeal pouches from the foregut endoderm and the invagination of the pharyngeal ectoderm to form ectodermal clefts. As the ectodermal clefts contact the endodermal pouches, the pharyngeal region is divided into distinct segments referred to as pharyngeal arches. Each pharyngeal arch contains a pharyngeal arch artery, surrounded by a mesodermal core and neural crest-derived mesenchyme (Graham, 2003). Previous studies have shown that the pharyngeal arches form in an iterative fashion, with the anterior arches forming first, followed by the progressive addition of more caudal arches (Tamarin and Boyde, 1977; Veitch et al., 1999; Crump et al., 2004). In mouse and humans, five distinct pairs of arches can be distinguished (indicated as I, II, III, IV and VI) that are separated by four pairs of pharyngeal pouches (pp1–pp4) (Graham et al., 2005).
Experiments in chick and zebrafish embryos have suggested that cellular processes that direct morphogenesis of the pharyngeal endoderm to form the pharyngeal pouches are the key event that drives the segmentation of the pharyngeal apparatus (Veitch et al., 1999; Crump et al., 2004). The formation of the pharyngeal pouches also provides a permissive niche for neural crest cell migration. In addition to producing inductive signals that guide the migrating crest into the apparatus (Begbie et al., 1999), the evagination toward and fusion of the pharyngeal endoderm with the ectoderm also appears to provide a physical barrier that can influence neural crest infiltration of the pharyngeal arches (Rizzoti and Lovell-Badge, 2007). Despite this apparently critical role for the pharyngeal endoderm, the mechanisms that control pouch morphogenesis are incompletely understood. Tbx1 appears to be a key player in pharyngeal pouch formation, however, as it is expressed in pharyngeal ectoderm, endoderm and mesoderm, it is not yet known whether Tbx1 in the endoderm directly controls pouch morphogenesis. In addition to Tbx1, experiments in zebrafish have implicated FGFs, in particular Fgf3 and Fgf8, in pouch formation (Crump et al., 2004). Studies in the mouse embryo have shown that pharyngeal pouch formation is disorganized in Fgf8 hypomorphic embryos (Abu-Issa et al., 2002; Frank et al., 2002). Fgf3−/−;Fgf10−/− mouse embryos exhibit severe hypoplasia of the 4th pharyngeal arch (Urness et al., 2011), and embryos homozygous for a hypomorphic Fgfr1 mutation have second pharyngeal arch hypoplasia (Trokovic et al., 2003). Together these studies suggest important roles for FGF signaling in pharyngeal segmentation in the mouse. These FGF ligands are produced in both epithelial (endoderm and ectoderm) and mesodermal tissues in the developing pharyngeal region and are likely involved in mediating complex, cross-regulatory interactions between these tissues during pharyngeal morphogenesis. The function of the endodermal Fgf3 and Fgf8 expression domains have not been established and the role of FGF receptor activation and FGF signaling in the pharyngeal endoderm is not known.
A number of observations have suggested that Tbx1 and FGF signaling are functionally linked. Vitelli et al. showed that Fgf8 expression in the pharyngeal endoderm is lost in Tbx1−/− embryos, indicating that Tbx1 functions upstream of Fgf8. Although 4th pharyngeal arch artery hypoplasia is significantly enhanced in Tbx1+/−;Fgf8+/− embryos compared to Tbx1+/− and Fgf8+/− embryos, pharyngeal segmentation was not assessed in these mutants (Vitelli et al., 2002b). Brown et al. reported that the deletion of Fgf8 in Tbx1-expressing cells with a Tbx1-Cre transgenic line resulted in thymus hypoplasia and a range of cardiovascular defects (Brown et al., 2004). A recent study from Vitelli et al. also suggested that reduced Fgf8 expression contributes to the outflow tract defect in Tbx1−/− embryos (Vitelli et al., 2010). The deletion of a conditional Tbx1 allele from the pharyngeal endoderm using the Foxg1-Cre line generated embryos with an un-segmented caudal pharyngeal region that lacked Fgf3 and Fgf8 expression (Arnold et al., 2006). However, Tbx1 is also upstream of FGF genes in the mesoderm and Tbx1, Fgf8 and Fgf10 genes interact during remodeling of the pharyngeal arch arteries (Aggarwal et al., 2006). Taken together, these studies clearly place Tbx1 upstream of FGF gene expression and indicate that reduced FGF gene expression or signaling are responsible for some of the cardiovascular and thymus phenotypes associated with Tbx1 deficiency but the tissue specific requirements have been unclear. In particular, the functional significance of the loss of Fgf8 gene expression in the pharyngeal endoderm of Tbx1-deficient embryos is not known.
To address these questions, we set out to ablate Tbx1 expression specifically in the pharyngeal endoderm, leaving other expression domains intact. The effect of Tbx1 deletion on the expression of two FGF ligands, FGF3 and FGF8, and the ability of Tbx1-deficient endoderm to respond to FGF signals were investigated.
RESULTS
Tbx1 expression in the endoderm is required for pharyngeal pouch morphogenesis
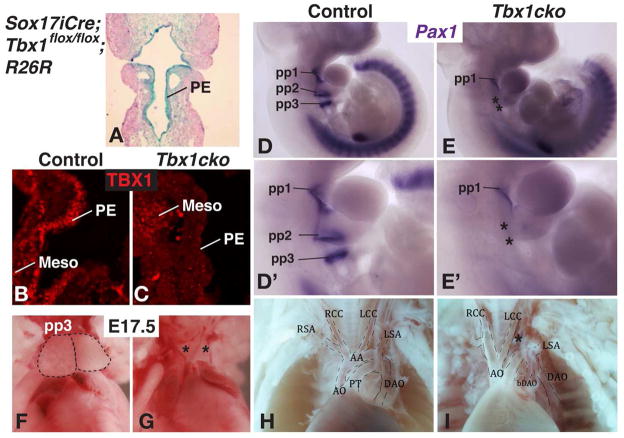
To determine whether Tbx1 expression in the endoderm is required for pouch morphogenesis, a conditional Tbx1 allele was recombined in the foregut endoderm using a Sox17-iCre line (Engert et al., 2009; Simrick et al., 2011). Efficient and pharyngeal endoderm-specific Cre recombinase activity was demonstrated by X-gal staining of Sox17-iCre;Tbx1flox/flox;R26R embryos (Fig. 1A). The absence of Tbx1 protein in the pharyngeal endoderm and maintenance of Tbx1 in pharyngeal mesoderm of Sox17-iCre;Tbx1flox/flox (Tbx1 conditional knockout or Tbx1cko) embryos was confirmed by immunohistochemistry (Fig. 1B,C). In situ hybridization for Pax1 to detect the pharyngeal pouches in whole mount embryos at E10.5, revealed the characteristic Tbx1−/− phenotype of a hypoplastic 1st pouch and absent caudal (2nd and 3rd) pouches (Fig. 1D, D′, E, E′). As expected for embryos lacking the 3rd pharyngeal pouch, which forms the thymus, all Tbx1cko embryos (n=10/10) examined at E17.5 exhibited thymus gland aplasia (Fig. 1F,G). Interrupted aortic arch type B (IAA-B), a phenotype caused by the absence of the left fourth pharyngeal arch artery, was also observed, in agreement with the absence of caudal arches (Fig. 1H,I). Persistent truncus arterious (PTA) was not observed in any of the Tbx1cko embryos analyzed (n=6). These results provide conclusive proof that endodermal Tbx1 is required for caudal pharyngeal pouch formation and that Tbx1 expressed in other pharyngeal tissues cannot compensate for the loss of Tbx1 in the endoderm during this process. However, the absence of a PTA suggests some differences between the Tbx1−/− and these mice.
Figure 1. Tbx1 deletion from the pharyngeal endoderm results in caudal pharyngeal pouch agenesis and thymus aplasia.
A) Frontal section through a Sox17-iCre;Tbx1flox/flox;R26R E9.5 embryo stained with X-gal (blue) and counterstained with Fast Red. Note the strong Cre-activated β-galactosidase activity in the pharyngeal endoderm (PE).
B,C) TBX1 immunohistochemistry on frontal sections through the pharyngeal region of E9.5 mouse embryos. Nuclear TBX1 is present in the pharyngeal endoderm (PE) of the control embryo (A), and absent from the PE of the Tbx1cko embryo (B). TBX1 protein is still present in the mesodermal core (Meso) of the Tbx1cko embryo.
D,E) Wholemount E10.5 embryos after in situ hybridization with an antisense RNA probe to detect Pax1 mRNA, which is expressed in the pharyngeal pouches. Pharyngeal pouches 1–3 (pp1–pp3) are labeled in the control embryo (D). Only pp1 is present in the Tbx1cko embryo, with asterisks indicating agenesis of pp2–pp3 (E).
D′,E′) Magnified views of the pharyngeal apparatus of embryos in D and E.
F,G) Thoracic cavities of E17.5 embryos with the thymus lobes outlined in broken lines in the control (F) and thymus aplasia indicated by asterisks in the mutant (G).
H,I) The heart and its associated vessels imaged in situ within the chest cavity of E17.5 embryos. Interrupted aortic arch type B (IAA-B) is indicated by an asterisk (*).
PTA (scored as a single vessel arising from the heart) was not evident in any Tbx1cKO embryos analyzed, n=6.
PT = Pulmonary trunk, R/LCC = Right/Left common carotid, R/LSA = Right/Left Subclavian, A0 = Aorta, AA = Aortic arch, DAO = Descending aorta, bDAO = branch of the descending aorta.
Tbx1 deletion in the endoderm only partially eliminates Fgf3 and Fgf8 expression
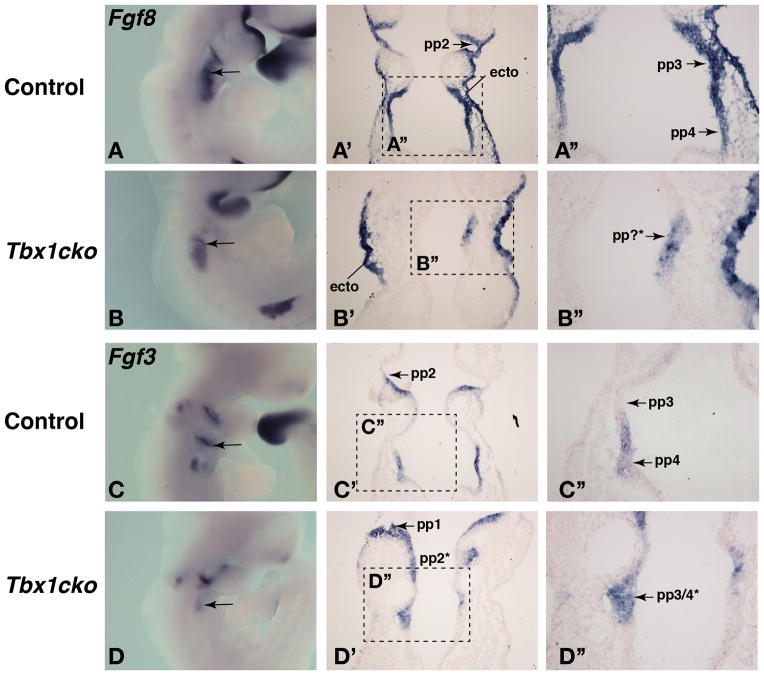
As previous studies suggested that Fgf3 and Fgf8 gene expression in the pharyngeal endoderm requires Tbx1 (Arnold et al., 2006), we visualized FGF gene expression in Tbx1cko embryos by in situ hybridisation. In agreement with Arnold et al., Fgf3 and Fgf8 expression appeared greatly reduced in the pharyngeal endoderm of Tbx1cko embryos examined after whole mount in situ hybridization (Fig. 2A–D). However, faint Fgf3 and Fgf8 expression could be detected, suggesting that expression was reduced or more diffuse, rather than completely absent (Fig. 2A–D, arrows point to expression sites in the caudal pharyngeal region). In situ hybridization analyses on sections indicated that Fgf8 expression was substantially reduced in the endoderm, but not entirely absent (Fig. 2A′,A″,B′,B″). As expected, Fgf8 expression in the pharyngeal ectoderm was not changed in these mutants (Fig. 2B′, ecto). Fgf3 expression was maintained in the pharyngeal endoderm of Tbx1cko embryos (Fig. 2C′,C″,D′,D″).
Figure 2. Fgf8, but not Fgf3 gene expression is reduced in the Tbx1-deficient endoderm.
A,B) Wholemount Fgf8 in situ hybridization on E9.5 embryos, anterior to the right. Arrows point to expression domains in the caudal pharyngeal region.
A′,B′) Fgf8 gene expression visualized by in situ hybridization on frontal sections through the pharyngeal region of E9.5 embryos. Fgf8 expression in pp2 of the control (A′) and pharyngeal ectoderm (ecto) of both embryos is indicated.
A″,B″) Magnified views of the areas indicated in panels A and B. Fgf8 expression in the evaginating pp3 and pp4 are indicated in the control (A″) and a small patch of cells with low Fgf8 expression is visible in a presumptive pouch (pp?*) of the mutant endoderm (B″).
C,D) Wholemount Fgf3 in situ hybridization on E9.5 embryos, anterior to the right. Arrows point to expression domains in the caudal pharyngeal region.
C′,D′) Fgf3 gene expression visualized by in situ hybridization on frontal sections through the pharyngeal region of E9.5 embryos. Fgf3 expression in pp2 of the control (C′) and pp1 and the presumptive pp2 (pp2*) of the mutant embryo (D′) are indicated.
C″,D″) Magnified views of the areas indicated in panels C and D. Fgf3 expression in the evaginating pp4 is indicated in the control (C″) and equivalent levels of Fgf3 expression in a presumptive pouch (pp3/4*) of the mutant endoderm can be seen (D″).
The loss of endodermal Tbx1 is not sufficient to disrupt normal FGF signaling levels in the pharyngeal apparatus
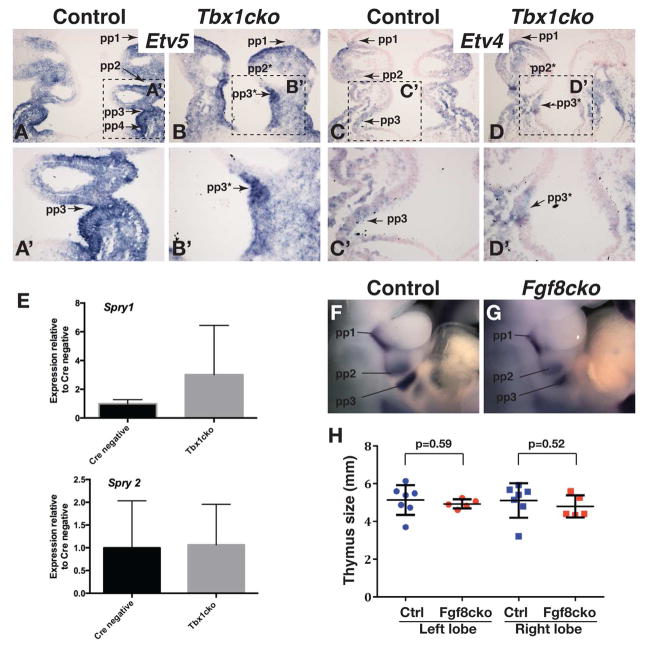
Although previous studies have shown that the expression of several genes encoding FGF ligands are altered in Tbx1-deficient embryos, few reports on the effects of these changes on downstream FGF signaling are available (Vitelli et al., 2010; Simrick et al., 2012). Therefore, to determine whether the reduced expression of Fgf8 in the endoderm affected FGF signaling in the Tbx1cko pharyngeal apparatus, we analysed the expression of two transcriptional read-outs of FGF signaling, Etv4 and Etv5 (Klein et al., 2008). Both of these genes are expressed at high levels in the posterior pouch endoderm of the 1st and 2nd pouches and strongly expressed in the evaginating 3rd and 4th pouches (Fig. 3A,A′,C,C′). Although distinct caudal pouches could not be distinguished in Tbx1cko embryos, presumptive “pouches” could be identified based on gene expression. The expression levels of FGF target genes in these presumptive pouches of Tbx1cko embryos were not markedly different from stage-matched, littermate controls (Fig. 3B,B′,D,D′). As in situ hybridisation does not provide a quantitative measure of gene expression levels, we also quantified the abundance of two additional read-outs of FGF signaling, Spry1 and Spry2 by quantitative RT-PCR. The abundance of Spry1 and Spry2 transcripts in RNA extracted from micro-dissected pharyngeal regions confirmed that gene expression was not significantly altered in Tbx1cko embryos (Fig. 3E). This observation is in contrast to the severely reduced Spry1 and Spry2 expression in the pharyngeal region of Tbx1−/− embryos (Simrick et al., 2012). Taken together, these observations indicate that the near-complete loss of Fgf8 expression in the endoderm of Tbx1cko embryos is not sufficient to cause an over-all reduction in FGF signaling levels in the developing pharyngeal region at the time of caudal pouch formation. Importantly, these data suggest that the failure of pharyngeal segmentation in Tbx1cko embryos cannot be attributed to a reduction in FGF signaling levels in the pharyngeal endoderm.
Figure 3. Normal levels of FGF signaling is maintained in Tbx1cko embryos.
A–D) The expression of two FGF signaling reporter genes, Etv5 (A,B) and Etv4 (C,D) in frontal sections through E9.5 pharyngeal apparatus are compared between control (A,C) and mutant (B,D) embryos. Pharyngeal pouches (pp1–pp4) and presumptive pouches (pp2*, pp3*) are indicated.
A′–D′) Magnified views of the areas indicated in panels A–D. Note the equivalent levels of gene expression in evaginating and presumptive 3rd pouches of control and mutant embryos.
E) Relative qRT-PCR quantification of FGF-regulated transcripts in the E9.5 pharyngeal region.
F,G) Pax1 in situ hybridization to visualize pharyngeal pouches in control (F) and Fgf8cko (G) embryos at E10.5. Note that of all pharyngeal pouches are present in the mutant.
H) Estimated sizes of thymus lobes at E17.5 as measured by thymus circumference from wholemount pictures. Note the absence of any significant thymus hypoplasia in Fgf8cko embryos.
Fgf8 deletion in the pharyngeal endoderm is not sufficient to prevent pharyngeal pouch formation
A prediction from the results presented thus far is that the deletion of Fgf8 from the pharyngeal endoderm would not be sufficient to cause pharyngeal segmentation defects. To test this, we generated Sox17-iCre;Fgf8flox/flox (Fgf8cko) embryos. Pax1 in situ hybridisation at E10.5 revealed normal pharyngeal segmentation in Fgf8cko embryos (Fig. 3F,G). Although pouch formation was not completely abolished in Fgf8cko mutants as in Tbx1cko embryos, the caudal pouches appeared slightly hypoplastic in some embryos, although we considered these to be within normal inter-embryonic variation (compare the size of pp3 in Fig. 3F with G; Table 1). To determine whether Fgf8 deletion resulted in thymus hypoplasia, Fgf8cko embryos were examined at E17.5. No thymus hypoplasia was observed, consistent with normal third pharyngeal pouch development (Fig. 3H).
Table 1.
Summary of pharyngeal apparatus defects in conditional FGF-deficient embryos
| Genotype | 2nd arch hypoplasia | 3rd pouch hypoplasia | ||||
|---|---|---|---|---|---|---|
| Unilateral | Bilateral | Total incidence/pouch | Unilateral | Bilateral | Total incidence/pouch | |
| Cre-negative | 0/12 | 0/12 | 0/24 | 0/12 | 0/12 | 0/24 |
| Fgf8cko | 0/3 | 0/3 | 0/6 | 0/3 | 0/3 | 0/6 |
| Fgf3+/cko; Fgf8+/cko | 0/2 | 0/2 | 0/4 | 0/2 | 0/2 | 0/4 |
| Fgf3Δ/cko; Fgf8Δ/+ | 1/4 (L) | 0/4 | 1/8 | 0/4 | 0/4 | 0/8 |
| Fgf3Δ/+; Fgf8Δ/cko | 2/2 (R) | 0/2 | 2/4* | 1/2 (L) | 0/2 | 1/4 |
| Fgf3Δ/cko; Fgf8Δ/cko | 1/4 (L) | 2/4 | 5/8** | 1/4 (R) | 1/4 | 3/4* |
| Fgfr1;2cko | 0/2 | 2/2 | 4/4** | 0/2 | 2/2 | 4/4** |
Phenotypes determined from whole mount images of E10–10.5 embryos after in situ hybridization with a Pax1 antisense probe.
Left side affected.
Right side affected.
p<0.05,
p<0.001 (compared to Cre-negative control); Fisher’s exact test.
Endodermal Fgf3 and Fgf8 function redundantly during pharyngeal segmentation
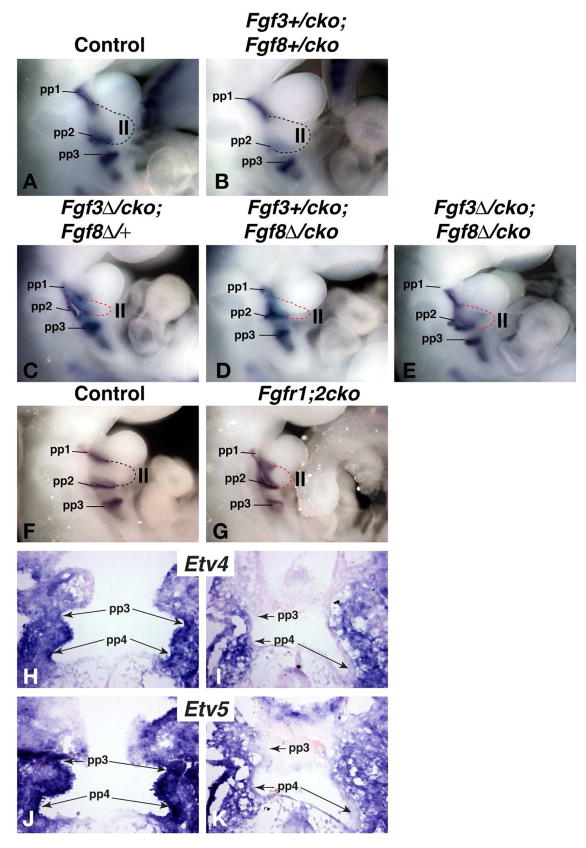
The observations that Fgf3 expression is maintained in the pharyngeal endoderm of Tbx1cko embryos (Fig. 2D′) and that pharyngeal segmentation proceeds normally in Fgf8cko embryos (Fig. 3G), raise the possibility that the Fgf3 expression that remains in the Tbx1cko endoderm compensates for the loss of Fgf8. To address this possibility, we analysed embryos in which different combinations of Fgf3 or Fgf8 conditional alleles were deleted in the endoderm with the Sox17-iCre line (Suppl. Fig. 1). Significant abnormalities in the pharyngeal apparatus were present when at least three FGF alleles were deleted from the endoderm (Compare Fig. 4C–E with Fig. 4A,B). The most consistent anomaly was hypoplasia of the 2nd pharyngeal arch (Table 1). In some cases the second arch hypoplasia was so severe that the 1st and 2nd pharyngeal pouches, visualized by Pax1 expression, appeared fused together (see Fig. 4D).
Figure 4. Endodermal deletion of Fgf3 and Fgf8, or Fgfr1 and Fgfr2 disrupt normal pharyngeal morphogenesis.
A–E) Visualization of pharyngeal pouches in E10.5 embryos by Pax1 in situ hybridization. Fgf3+/cko;Fgf8+/cko embryos (B) have normal-sized second pharyngeal arches (II, outlined in broken lines), compared to control embryos (A). Embryos with only one remaining Fgf3 or Fgf8 allele (C,D) or complete loss of all Fgf3 and Fgf8 alleles in the endoderm (E), exhibit 2nd arch and 3rd pharyngeal pouch (pp3) hypoplasia (also see Table 1).
F,G) Wholemount Pax1 in situ hybridization of E10 embryos reveals pharyngeal phenotypes identical to Fgf3;8cko embryos in embryos that lack both Fgfr1 and Fgfr2 in the endoderm (G).
H–K) Comparison of Etv4 and Etv5 expression in control (H,J) and Fgfr1;2cko (I,K) embryos at E9.5, reveal a significant reduction of FGF signaling levels in the FGFR-deficient pharyngeal endoderm.
In contrast to Tbx1cko embryos, caudal pharyngeal pouches were still present when both Fgf3 and Fgf8 were deleted, although the pouches were clearly hypoplastic in these embryos (Fig. 4E, Table 1). These data indicate that Fgf3 expression in the endoderm to some extent compensates for the loss of Fgf8 expression. However, the combined deletion of Fgf3 and Fgf8 from the endoderm does not phenocopy Tbx1cko embryos. Rather, the phenotypes observed in Fgf3;Fgf8cko embryos are reminiscent of other examples in which FGF signaling and related pathways have been disrupted in the pharyngeal region (Trokovic et al., 2003; Trokovic et al., 2005; Rizzoti and Lovell-Badge, 2007; Kameda et al., 2009). In particular, 2nd arch hypoplasia has been attributed to defects in FGF-dependent patterning of the pharyngeal epithelia.
FGF signaling in the endoderm is required for normal development of the pharyngeal apparatus
If our hypothesis that the caudal pouch aplasia in Tbx1cko mutants are not caused by the reduced FGF signaling within the pharyngeal endoderm, rendering the endoderm unresponsive to FGF signals, should result in a phenotype similar to FGF mutants and not pouch aplasia as observed in Tbx1cko embryos. We therefore investigated whether the simultaneous deletion of the two main FGF receptors expressed in the pharyngeal region, Fgfr1 and Fgfr2 (Trokovic et al., 2005), with Sox17-iCre phenocopied Tbx1cko or Fgf3;Fgf8cko mutants. The analysis of pouch formation in these embryos indicated that the near-loss of FGF signaling in the pharyngeal endoderm resulted in 2nd arch and caudal (3rd pouch) hypoplasia (Fig. 4F,G, Table 1). To assess the impact of deleting Fgfr1 and Fgfr2 in the endoderm on FGF signaling in this tissue, we visualized Etv4 and Etv5 expression in these Fgfr1/2cko embryos. Etv4 and Etv5 expression were markedly reduced in the endoderm (Fig. 4H–K, indicating that 1) these genes are bona fide readouts of FGF signaling in the pharyngeal endoderm and 2) that deleting Fgfr1 and Fgfr2 was sufficient to dramatically reduce the responsiveness of this tissue to FGF ligands.
In conclusion, our analysis of mutant lines in which either multiple FGF ligands or FGF receptors were deleted in the endoderm confirm that FGF signaling is required for normal pharyngeal development and indicates that the pharyngeal endoderm itself needs to be responsive to FGF signaling for this process. Taken together with other published reports, our observations indicate that the abnormalities in pharyngeal development in embryos in which FGF signaling has been disrupted, are strikingly similar to each other, and involves hypoplasia of the 2nd pharyngeal arch, irregular pouch formation and pouch hypoplasia (Trokovic et al., 2003; Trokovic et al., 2005; Hoch and Soriano, 2006; Kameda et al., 2009). Most critically, the pharyngeal abnormalities in FGF pathway mutants are phenotypically distinct from the characteristic loss of caudal pouch formation observed in Tbx1−/− and endodermal Tbx1cko embryos (Fig. 1D). This conclusion is in agreement with the findings reported above which suggest that the defects in caudal pouch formation and pharyngeal segmentation in Tbx1-deficient embryos are not associated with significant perturbations in FGF signaling.
The pharyngeal endoderm maintains its capacity to proliferate in the absence of Tbx1
The analysis of cellular defects in embryos in which Tbx1 was conditionally ablated at different developmental time points, has revealed a significant deficit in the proliferation of the pharyngeal endoderm by E10 (Xu et al., 2005). To determine whether this effect on cell proliferation is directly caused by loss of Tbx1 function in the endoderm, we visualized mitotic cells with an antibody to Ser10-phospho-histone H3 and calculated the mitotic index in the pharyngeal region of Tbx1cko embryos in the same way as Xu et al. (Xu et al., 2005). We failed to detect any significant difference in cell proliferation in the mutant endoderm (Fig. 5). This finding suggests that a drastic change in cell proliferation at E9.5 could not explain the failure of pharyngeal pouch evagination observed in Tbx1cko embryos at this stage of development.
Figure 5. Tbx1 deletion from the endoderm does not affect cell proliferation.
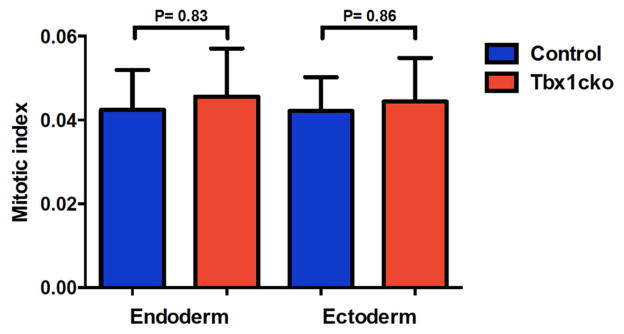
Mitotic indices (number of proliferating (phosphor-histone H3-positive) cells/total cell number) in pharyngeal endoderm and ectoderm of control and Tbx1cko embryos at E9.5. More than 2000 cells were counted for each cell type in each embryo. The data is representative of two independent experiments.
DISCUSSION
Reviewing the link between Tbx1 and FGF signaling in the endoderm
Previous studies have provided compelling evidence that Tbx1 functions upstream of a number of FGF genes (Vitelli et al., 2002b; Brown et al., 2004; Aggarwal et al., 2006; Arnold et al., 2006; Vitelli et al., 2010). In particular, the observation that Fgf8 expression is lost in the pharyngeal endoderm of Tbx1−/− embryos, taken together with the demonstration that Tbx1 and Fgf8 null alleles interact during pharyngeal development, provided strong evidence for a functional link between Tbx1 and the FGF signaling pathway (Vitelli et al., 2002b). However, to what extent the reduced FGF signaling in Tbx1-deficient embryos is responsible for the phenotypes associated with Tbx1 deficiency has not been examined fully.
In the present study we describe the generation of endoderm-specific Tbx1 conditional mutants, and confirm Fgf8 downregulation in Tbx1-deficient endoderm. Despite this Fgf8 downregulation, we found that FGF signaling was maintained at normal levels in the endoderm. Furthermore, we could show that Fgf8 deletion from the endoderm was not sufficient to cause caudal pouch agenesis. These observations indicate that the loss of Fgf8 expression in the endoderm of Tbx1-deficient embryos is by itself not responsible for the caudal pharyngeal pouch agenesis present in these embryos. The deletion of both Fgf3 and Fgf8, or Fgfr1 and Fgfr2, were sufficient to downregulate FGF signaling in the endoderm and cause pharyngeal phenotypes. However, these “FGF” phenotypes were qualitatively distinct from the Tbx1 phenotype, such that no caudal pouch aplasia was observed. Taken together, these findings suggest that the mechanisms whereby Tbx1 in the endoderm controls caudal pouch formation are distinct from its effects on FGF signaling. Our recent demonstration that Tbx1 haplo-insufficiency can also affect the ability of the embryo to resist developmental alterations caused by increased FGF signaling, further suggest that the relation between Tbx1 and the FGF pathway may be more complex than originally anticipated (Simrick et al., 2012).
Tissue-specific requirements for Tbx1 during pharyngeal segmentation
The tissue-specific roles of Tbx1 have been dissected using a number of Cre lines with different tissue specificities. Together with a previous study, the data presented here identifies a critical role for endodermal Tbx1 in caudal pharyngeal pouch formation (Arnold et al., 2006). The pharyngeal ectoderm appears to not be required for pouch morphogenesis and pharyngeal segmentation (Zhang et al., 2005; Calmont et al., 2009). Intriguingly, Zhang et al. showed that the deletion of Tbx1 in the mesoderm was sufficient to cause caudal pharyngeal segmentation defects and thymus aplasia. Furthermore, restoring Tbx1 expression in the mesoderm of Tbx1 hypomorphic embryos partially rescued the caudal pouch phenotypes although it failed to rescue thymus aplasia (Zhang et al., 2006b). Thus, it appears that mesodermal Tbx1 has a role in pouch formation. However, the exact relation between the mesoderm and endoderm during this process remain incompletely understood.
FGF signaling in the endoderm is critical for 2nd arch expansion
Our analysis of various compound, endoderm-specific conditional FGF mutants confirms crucial roles for FGF signaling in pharyngeal development. The most consistent phenotypic feature of these mutants is second arch hypoplasia, in agreement with a previous study (Trokovic et al., 2003). Our observation that the deletion of both Fgf3 and Fgf8 from the pharyngeal endoderm resulted in similar phenotypes to the deletion of FGF receptors from the endoderm requires further investigation. As FGF3 has high affinity for FGF receptor isoforms expressed in epithelia (FGFR1(IIIb) and FGFR2(IIIb)), it is conceivable that FGF3 may regulate pharyngeal pouch formation in an autocrine fashion. However, FGF8 preferentially signals to FGFR2(IIIc) and FGFR4, suggesting that it may regulate endodermal behavior indirectly through a relay via the mesenchyme (Zhang et al., 2006a). Our current experimental system cannot distinguish between these possibilities, especially given the high level of functional redundancy between the different FGF ligands present in the developing pharyngeal region.
In summary, our study confirms that endodermal Tbx1 expression is necessary for caudal pouch formation. Whilst FGF signaling in the pharyngeal endoderm is also required for normal pouch morphogenesis, the deletion of Tbx1 from the endoderm does not affect FGF signaling levels. Thus, we conclude that Tbx1 directs pouch formation in an FGF-independent manner.
EXPERIMENTAL PROCEDURES
Mouse husbandry, embryo collection and genotyping
The Sox17-2A-iCre (Sox17-iCre) transgenic line was used to delete a conditional Tbx1flox allele in the pharyngeal endoderm (Arnold et al., 2006; Engert et al., 2009). Sox17-iCre;Tbx1flox/+ males were mated with Tbx1flox/flox females. Sox17-iCre;Tbx1flox/flox embryos lacked Tbx1 in the endoderm and are referred to as Tbx1 conditional knockout (Tbx1cko) embryos. Cre-negative Tbx1flox/+and Tbx1flox/flox littermates were used as controls in all experiments. In addition to Tbx1, Fgf8 (Meyers et al., 1998; Moon and Capecchi, 2000), Fgf3 (Urness et al., 2011), Fgfr1 (Xu et al., 2002) and Fgfr2 (Yu et al., 2003) conditional alleles were deleted in the pharyngeal endoderm by a Sox17-iCre line using the same breeding strategy, with the exception that males were heterozygous for two conditional alleles e.g. Sox17-iCre;Fgfr1flox/+;Fgfr2flox/+; and females homozygous for two conditional alleles e.g. Fgfr1 flox/flox;Fgfr2 flox/flox. During the analysis of Fgf3;Fgf8 conditional mutants, Sox17-iCre;Fgf3Δ/+;Fgf8Δ/+males (carrying an Fgf3 and Fgf8 null (Δ) allele), were mated with Fgf3 flox/flox;Fgf8 flox/flox or Fgf3Δ/flox;Fgf8flox/flox females. Mouse lines were maintained on a mixed (C57BL/6J; FVB/N) genetic background. Sox17-iCre;Tbx1flox/+, Sox17-iCre;Fgf8flox/+ and Sox17-iCre;Fgfr1flox/+;Fgfr2flox/+ embryos displayed no overt pharyngeal pouch or endocrine abnormalities indicating that Sox17-iCre alone did not cause pharyngeal phenotypes. Noon of the day when a vaginal plug was detected was considered E0.5. Embryos were staged more precisely by counting somites and only stage-matched embryos were compared in experiments. Embryos were fixed and processed as appropriate (see below), or pharyngeal tissues were microdissected for RNA extraction. Genotyping of the various alleles were performed by PCR using yolk sac DNA as template and primer pairs described in the original publications.
In situ hybridization (ISH)
Embryos were fixed overnight at 4°C in 4% paraformaldehyde in RNAse-free PBS. For ISH on sections, embryos were dehydrated through a series of ethanol and histoclear washes and embedded in wax. Alternate 7μm sections were cut and dried overnight at 42°C. ISH was performed according to standard methods (Yaguchi et al., 2009). For ISH on whole embryos, E10.5 embryos were dissected in RNAse free PBS, fixed overnight in 4% PFA at 4°C, processed and hybridised with digoxigenin-labelled antisense RNA probes as previously described (Wilkinson et al., 1989). The probes used for ISH have all been previously described as follows: Fgf3 (Robinson et al., 1998), Fgf8 (Crossley and Martin, 1995), Etv4, Etv5 (Klein et al., 2006) and Pax1 (Deutsch et al., 1988).
Morphological analysis of thymi
The thoracic cavity of E17.5 embryos were opened and photographed under a stereomicroscope to score the presence/absence of thymus lobes above the heart. Image J was used to measure the circumference of each thymus lobe as an estimate of thymus size. Statistical analysis (Student’s t-test) was done using Graph Pad software.
Immunohistochemistry (IHC)
Immunohistochemistry was performed on 7μm, wax-embedded sections that were cleared of wax in Xylene and rehydrated through a series of ethanol:PBS (100%, 95%, 90%, 70%) washes. After a final wash in PBS, antigen retrieval was performed by microwaving the slides in a 10mM sodium citrate solution, followed by a wash in PBT2 (0.2% Tween-20 in PBS). A blocking solution of 10% goat serum in PBT2 was applied for a minimum of 1 hour before application of the primary antibody (diluted in a solution of 5% goat serum in PBT2) and incubation at 4°C overnight in a humidified chamber. Primary antibodies and dilutions were: rabbit anti-phospho-Histone H3(Ser10) (PH3) (Cell Signaling 9701,1:250 dilution) to identify cells in mitosis, and mouse anti-E-cadherin (Fitzgerald #02660, 1:200) to label epithelial tight junctions. Subsequently, the primary antibody was removed and the slides washed in PBT2 three times before application of an Alexafluor-conjugated secondary antibody, (Invitrogen, diluted 1:250 in a solution of 5% goat serum in PBT2), in a humidified chamber for a minimum of 1 hour. The secondary antibody solution was removed and the slides washed in PBT2. After a final wash in PBS with Hoechst 33342 (Invitrogen, H3570/23363w, 1:50,000), slides were mounted in AF100 mounting solution (Citifluor).
Proliferation assay
To determine the level of proliferation in the pharyngeal epithelia, tissue sections were labeled with antibodies raised against PH3 and E-cad. PH3+ cells were counted in the pharyngeal endoderm and ectoderm (more than 2000 E-cad positive cells per tissue), and a mitotic index (MI) calculated as described (Xu et al., 2005):
Bar graphs and p-values (calculated using the Student’s t-test) were produced with Graph Pad software.
Quantitative RT-PCR
The pharyngeal apparatus (from pharyngeal arch I–VI) was microdissected, heart and neural tube tissues removed, and total RNA extracted and genomic DNA removed using the Absolutely RNA Microprep Kit (Agilent Technologies). A total of 200ng of RNA was used for first-strand DNA synthesis with nanoScript Precision RT kit (PrimerDesign Ltd.) according to the manufacturer’s specifications. cDNA synthesis reactions without reverse transcriptase enzyme (no RT) were used as controls for q-RT-PCR. qRT-PCR was performed on a RotorGene Q cycler (Qiagen) using Precision qPCR MasterMix kit (PrimerDesign Ltd.). Appropriate normalising genes were detected using qbasePLUS software (Biogazelle) with geNorm reference kits (PrimerDesign Ltd.) All reactions were performed in triplicate and normalised to GAPDH. Cq threshold values were determined manually and all were at least 5 Cq values below no RT controls. Cq values were calculated relative to stage-matched control samples using Microsoft Excel software and graphs were produced using GraphPad Prism software. Primers were designed using primer blast (NCBI) and are as follows: Gapdh as a normaliser (AGGTCGGTGTGAACGGATTTG and TGTAGACCATGTAGTTGAGGTCA), Spry1 (GCTCGTGGCTGTCCATCT and GAAACACGTGAGTCCCTTGC) and Spry2 (TGGTGCAAAGCCGCGATCAC and GCAAAGGCTGCGACCCGTTG).
Supplementary Material
Embryos that lack TBX1 in the pharyngeal endoderm fail to form caudal pharyngeal pouches.
Fgf8 expression is greatly reduced in TBX1-deficient endoderm.
The deletion of Fgf8 from the endoderm does not perturb pouch development.
TBX1 and FGF signaling deficiency in the endoderm result in distinct phenotypes.
Acknowledgments
We are grateful to Heiko Lickert (Sox17-iCre), Gail Martin (Fgf8flox), Chuxia Deng (Fgfr1flox), and David Ornitz (Fgfr2flox) for sharing mouse strains, Gail Martin, Ivor Mason and Nancy Manley for providing probes for in situ hybridization studies. We would like to thank Samantha Martin, Chaoying Li and Hagen Schmidt for technical assistance.
All animal experiments were approved by the UK Home Office and by the University of Utah IACUC. This work was supported by a grant from the Medical Research Council (G0601104) to MAB.
References
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Aggarwal VS, Liao J, Bondarev A, Schimmang T, Lewandoski M, Locker J, Shanske A, Campione M, Morrow BE. Dissection of Tbx1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet. 2006;15:3219–3228. doi: 10.1093/hmg/ddl399. [DOI] [PubMed] [Google Scholar]
- Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977–987. doi: 10.1242/dev.02264. [DOI] [PubMed] [Google Scholar]
- Brown CB, Wenning JM, Lu MM, Epstein DJ, Meyers EN, Epstein JA. Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev Biol. 2004;267:190–202. doi: 10.1016/j.ydbio.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Calmont A, Ivins S, Van Bueren KL, Papangeli I, Kyriakopoulou V, Andrews WD, Martin JF, Moon AM, Illingworth EA, Basson MA, Scambler PJ. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in the pharyngeal ectoderm. Development. 2009;136:3173–3183. doi: 10.1242/dev.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004;131:5703–5716. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- Deutsch U, Dressler GR, Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988;53:617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Engert S, Liao WP, Burtscher I, Lickert H. Sox17-2A-iCre: a knock-in mouse line expressing Cre recombinase in endoderm and vascular endothelial cells. Genesis. 2009;47:603–610. doi: 10.1002/dvg.20540. [DOI] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. Development of the pharyngeal arches. Am J Med Genet A. 2003;119:251–256. doi: 10.1002/ajmg.a.10980. [DOI] [PubMed] [Google Scholar]
- Graham A, Okabe M, Quinlan R. The role of the endoderm in the development and evolution of the pharyngeal arches. J Anat. 2005;207:479–487. doi: 10.1111/j.1469-7580.2005.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch RV, Soriano P. Context-specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development. 2006;133:663–673. doi: 10.1242/dev.02242. [DOI] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Kameda Y, Ito M, Nishimaki T, Gotoh N. FRS2alpha is required for the separation, migration, and survival of pharyngeal-endoderm derived organs including thyroid, ultimobranchial body, parathyroid, and thymus. Dev Dyn. 2009;238:503–513. doi: 10.1002/dvdy.21867. [DOI] [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore BS, Lopez M, Pandita RK, Lia M, Carrion D, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26:455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Watanabe Y, Smyth G, Miyagawa-Tomita S, Meyers E, Klingensmith J, Camenisch T, Buckingham M, Moon AM. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development. 2008;135:3599–3610. doi: 10.1242/dev.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, Sparks C, Choi CH, Oghalai J, Curran S, Murphy KC, Monks S, Williams N, O’Donovan MC, Owen MJ, Scambler PJ, Lindsay E. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci U S A. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoti K, Lovell-Badge R. SOX3 activity during pharyngeal segmentation is required for craniofacial morphogenesis. Development. 2007;134:3437–3448. doi: 10.1242/dev.007906. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, Overbeek PA, Chepelinsky AB. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol. 1998;198:13–31. doi: 10.1006/dbio.1998.8879. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. 22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development. Pediatr Cardiol. 2010;31:378–390. doi: 10.1007/s00246-009-9613-0. [DOI] [PubMed] [Google Scholar]
- Simrick S, Lickert H, Basson MA. Sprouty genes are essential for the normal development of epibranchial ganglia in the mouse embryo. Dev Biol. 2011;358:147–155. doi: 10.1016/j.ydbio.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simrick S, Szumska D, Gardiner JR, Jones K, Sagar K, Morrow B, Bhattacharya S, Basson MA. Biallelic expression of Tbx1 protects the embryo from developmental defects caused by increased receptor tyrosine kinase signaling. Dev Dyn. 2012;241:1310–1324. doi: 10.1002/dvdy.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarin A, Boyde A. Facial and visceral arch development in the mouse embryo: a study by scanning electron microscopy. J Anat. 1977;124:563–580. [PMC free article] [PubMed] [Google Scholar]
- Torres-Juan L, Rosell J, Morla M, Vidal-Pou C, Garcia-Algas F, de la Fuente MA, Juan M, Tubau A, Bachiller D, Bernues M, Perez-Granero A, Govea N, Busquets X, Heine-Suner D. Mutations in TBX1 genocopy the 22q11.2 deletion and duplication syndromes: a new susceptibility factor for mental retardation. Eur J Hum Genet. 2007;15:658–663. doi: 10.1038/sj.ejhg.5201819. [DOI] [PubMed] [Google Scholar]
- Trokovic N, Trokovic R, Mai P, Partanen J. Fgfr1 regulates patterning of the pharyngeal region. Genes Dev. 2003;17:141–153. doi: 10.1101/gad.250703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokovic N, Trokovic R, Partanen J. Fibroblast growth factor signalling and regional specification of the pharyngeal ectoderm. Int J Dev Biol. 2005;49:797–805. doi: 10.1387/ijdb.051976nt. [DOI] [PubMed] [Google Scholar]
- Urness LD, Bleyl SB, Wright TJ, Moon AM, Mansour SL. Redundant and dosage sensitive requirements for Fgf3 and Fgf10 in cardiovascular development. Dev Biol. 2011;356:383–397. doi: 10.1016/j.ydbio.2011.05.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch E, Begbie J, Schilling TF, Smith MM, Graham A. Pharyngeal arch patterning in the absence of neural crest. Curr Biol. 1999;9:1481–1484. doi: 10.1016/s0960-9822(00)80118-9. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Lania G, Huynh T, Baldini A. Partial rescue of the Tbx1 mutant heart phenotype by Fgf8: genetic evidence of impaired tissue response to Fgf8. J Mol Cell Cardiol. 2010;49:836–840. doi: 10.1016/j.yjmcc.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli F, Morishima M, Taddei I, Lindsay EA, Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet. 2002a;11:915–922. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development. 2002b;129:4605–4611. doi: 10.1242/dev.129.19.4605. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, McMahon AP. Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989;105:131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Xu H, Cerrato F, Baldini A. Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development. 2005;132:4387–4395. doi: 10.1242/dev.02018. [DOI] [PubMed] [Google Scholar]
- Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, Lindsay EA, Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Xu X, Qiao W, Li C, Deng CX. Generation of Fgfr1 conditional knockout mice. Genesis. 2002;32:85–86. doi: 10.1002/gene.10028.abs. [DOI] [PubMed] [Google Scholar]
- Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, Ichida F, Joo K, Kimura M, Imamura S, Kamatani N, Momma K, Takao A, Nakazawa M, Shimizu N, Matsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- Yaguchi Y, Yu T, Ahmed MU, Berry M, Mason I, Basson MA. Fibroblast growth factor (FGF) gene expression in the developing cerebellum suggests multiple roles for FGF signaling during cerebellar morphogenesis and development. Dev Dyn. 2009;238:2058–2072. doi: 10.1002/dvdy.22013. [DOI] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006a;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, Vincentz J, Furuta Y, Ma L, Martin JF, Baldini A, Lindsay E. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huynh T, Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development. 2006b;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.