Abstract
Dominance hierarchies are an important aspect of group-living as they determine individual access to resources. The existence of dominance ranks in access to space has not been described in socially monogamous, communally nesting prairie voles (Microtus ochrogaster). Here we tested whether dominance could be assessed using the tube test. We also tested whether dominance related to alcohol intake, similar to what has been demonstrated in non-monogamous species. Same-sex pairs of unfamiliar peers were tested in a series of three trials of the tube test, then paired and allowed individual access to alcohol and water for four days, and then tested again in the tube test. For all pairs, the same subjects won the majority of trials before and after alcohol drinking. The number of wins negatively correlated with alcohol intake on the first day of drinking, and positively correlated with levels of Fos in the paraventricular nucleus of the hypothalamus following the tube test in a separate group of voles. Dominance was not related to Fos levels in other brain regions examined. Together, these results indicate that prairie voles quickly establish stable dominance ranks through a process possibly involving the hypothalamus, and suggest that dominance is linked to alcohol drinking.
Keywords: aggression, stress, vasopressin, oxytocin, CRF
Introduction
Social living often necessitates the establishment and maintenance of specific hierarchies. In some species this may allow dominant individuals exclusive or preferential access to resources such as food or reproductively active conspecifics (Clarke & Faulkes, 1998; Sousa, et al., 2005). Often, dominance between two individuals can be determined in a single encounter which may include demonstration of aggressive behavior. Animals can remember this encounter for long periods of time, leading to a stable hierarchy (Adkins-Regan, 2005). In other cases, dominance is asserted or maintained through repeated interactions, such as grooming and other continuous interactions between female primates (Seyfarth, 1976).
Prairie voles (Microtus ochrogaster) are often studied for their well-understood social behaviors. They are socially monogamous, and after forming a bond with a pair-mate, will become aggressive toward other conspecifics of both sexes. It is thought that this selective aggression helps to maintain the pair bond. In addition, prairie voles often form communal nests in the wild, where some litters may remain in their natal nest and help rear future litters of siblings (Getz, Hofmann, & Carter, 1987). Visiting voles are common at nest sites (Getz & Hofmann, 1986), and the precise community structure changes as individuals join or leave the group (Getz & McGuire, 1997). However, little is known about the social dynamics in such groups. While selective aggression towards stranger animals in pair-bonded voles has been well-studied (Gobrogge, Liu, Jia, & Wang, 2007; Gobrogge, Liu, Young, & Wang, 2009; Insel, Preston, & Winslow, 1995; Z. Wang, Hulihan, & Insel, 1997; Winslow, Hastings, Carter, Harbaugh, & Insel, 1993), there are no studies or descriptions of dominance hierarchies in group-living prairie voles. In contrast to the notable selective aggression in bonded voles, non-pair-bonded animals exhibit very little aggression toward other prairie voles (Winslow, et al., 1993; Young, Liu, & Wang, 2008). One study examined the behavior of wild-caught voles that had dispersed from their natal nest into uninhabited territory, which were presumed to be non-pair-bonded, especially as many were juvenile or sub-adult. In a social interaction test of aggressive behaviors, these disperser prairie voles exhibited fewer aggressive, and more avoidance behaviors than controls, and were reasoned to be subordinate (Myers & Krebs, 1971).
Prairie voles have also recently emerged as a promising model for interactions between social relationships and drug and alcohol exposure (Anacker, et al., 2014; Anacker, Loftis, Kaur, & Ryabinin, 2011; Liu, et al., 2010; Liu, Young, Curtis, Aragona, & Wang, 2011). Our recent studies have demonstrated that same-sex prairie vole peers can directly influence each other’s alcohol intake, although the mechanism of this influence remains to be elucidated (Anacker, Loftis, Kaur, et al., 2011; Anacker, Loftis, & Ryabinin, 2011; Anacker & Ryabinin, 2013). In light of our repeated observations that one vole in a pair typically changes its alcohol drinking behavior to match the intake of its cage mate, we have hypothesized that there is a dominant-subordinate relationship which results in the dominant animal influencing the subordinate to alter its drinking level. This idea is in accordance with evidence from other animal models demonstrating that dominant animals are more likely to drink lower amounts of alcohol than subordinates (reviewed in Anacker & Ryabinin, 2010; Blanchard, Hori, Tom, & Blanchard, 1987; McKenzie-Quirk & Miczek, 2008; Wolffgramm & Heyne, 1991), as in our studies it was typically the high drinker that changed its intake to match the low drinker in the pair (Anacker, Loftis, & Ryabinin, 2011; Anacker & Ryabinin, 2013).
The present study was designed to evaluate whether prairie vole pairs establish dominance relationships that can be assessed in laboratory conditions. We used a simple test for dominance that has been used for decades in other laboratory rodents, the tube test (Lindzey, Winston, & Manosevitz, 1961). This test assesses a dyadic interaction in response to limited space, where one subject must yield to the other. There are many other tests of dominance, some discrepancies between them, and controversy about the relation of such tests to ‘dominance’ per se (reviewed in Benton, Dalrymple-Alford, & Brain, 1980; Miczek & Barry, 1975). We chose to use the tube test, a procedure that does not rely on measures of aggressive behavior, since virgin voles are not typically aggressive toward one another and there is no evidence that aggression or resource sharing play a role in communal relationship structures in prairie voles. In these burrowing, nesting animals, space use may be the most ethologically relevant test of dominance behaviors. Moreover, space use is an important element of mating strategy whereby the majority of prairie voles remain in a home range with a bonded partner, while a significant minority adopts a ‘wandering’ strategy, overlapping home ranges with other voles (Ophir, Phelps, Sorin, & Wolff, 2008; Ophir, Wolff, & Phelps, 2008). A previous study of prairie voles in the laboratory used aggressive behavior as a primary measure to determine dominance among male prairie vole dyads, but found that the low level of aggression made the distinction difficult, and several pairs failed to demonstrate a dominance relationship based on the selected criteria (Shapiro & Dewsbury, 1986). This further justifies our use of the tube test, rather than an aggression-dependent measure. In addition, there is more recent evidence from studies of mice that the tube test results correlate with other measures of dominance (Strozik & Festing, 1981; F. Wang, et al., 2011).
We also assessed whether alcohol experience would alter the initial dominance ranking of pairs. There is extensive evidence that drugs, and alcohol in particular, induce increased aggression both in humans (Bushman & Cooper, 1990; Lipsey, Wilson, Cohen, & Derzon, 1997) and in animal models of aggression (reviewed in Miczek, de Boer, & Haller, 2013; van Erp & Miczek, 1997; Winslow, Ellingboe, & Miczek, 1988). However, measures of aggression are typically not separated from other measures of dominance, and there is a complex relationship between aggression and subsequent alcohol drinking (Funk, Harding, Juzytsch, & Le, 2005; Kudryavtseva, Madorskaya, & Bakshtanovskaya, 1991; van Erp, Tachi, & Miczek, 2001). In prairie voles, amphetamine increases agonistic behavior such as aggression (Gobrogge, et al., 2009), in addition to having detrimental effects on pro-social behaviors such as pair bonding (Liu et al., 2010). In contrast, alcohol drinking did not modulate selective aggression against the opposite sex in this species (Anacker, et al., 2014). Importantly, the interactions between addictive drugs and any other measures of dominance have never been tested in prairie voles.
In male prairie voles, the vasopressin system is critically important to the development of the pair-bond and selective aggression. In particular, activation of vasopressin 1a receptors in the anterior hypothalamus is necessary for the expression of aggression, as an antagonist delivered to this region blocks the pair bond-induced selective aggression, and also blocks the general aggression induced by amphetamine (Gobrogge, et al., 2009). Other regions have been implicated in aggressive behaviors, including the lateral septum, medial amygdala, and nucleus accumbens (De Lorme & Sisk, 2013; van Erp & Miczek, 2007; Y. Wang, He, Zhao, & Li, 2013). Vasopressin-containing fibers innervate these regions from the paraventricular nucleus of the hypothalamus. In contrast, brain regions determining other measures of dominance are poorly understood. The final experiment in the present study was designed to identify brain regions differentially activated following the tube test thereby providing a glimpse into potential neurocircuitry involved in dominant and submissive behaviors.
Methods
Adult prairie voles of both sexes from our colony at the Portland Veterans Affairs Medical Center Veterinary Medical Unit were used in these experiments, and all testing was approved by the Institutional Animal Care and Use Committee.
Experiment 1
Subjects (n=12; 75–95 days of age) were weighed immediately prior to testing. Voles were housed under a 14:10 light:dark cycle, and all testing occurred during the light phase of the day. All subjects were housed with same-sex siblings since weaning.
Pilot studies indicated that the voles would not always enter the apparatus during testing with another subject if they had not been able to explore the tube previously. Therefore, each subject was allowed to explore the apparatus freely for two minutes or until they had passed back and forth at least twice, one day prior to the test. Pairs of prairie voles (2 female dyads, 4 male dyads) previously unknown to each other were paired in the dominance tube test. Each was picked up with a small plastic cup and the open end of the cup was secured to a fitted attachment, one on each end of the tube. Before the test began, gates were in place, preventing the voles from entering the main part of the tube (Fig. 1C). After both subjects were in position and facing the gates, the gates were raised and the voles entered the tube. The subjects met in the middle of the tube and then one continued to move forward, pushing the other back to its starting cup. The trial ended when one vole returned to its starting cup, or when five minutes had elapsed, whichever came first; however, nearly all trials were completed in less than one minute. At this time, the subjects were removed from the apparatus, placed alone in a holding cage and the process was repeated for a total of three trials. While fighting (scratching, biting) never occurred, the test would have been stopped for any given pair if injury occurred.
Figure 1.
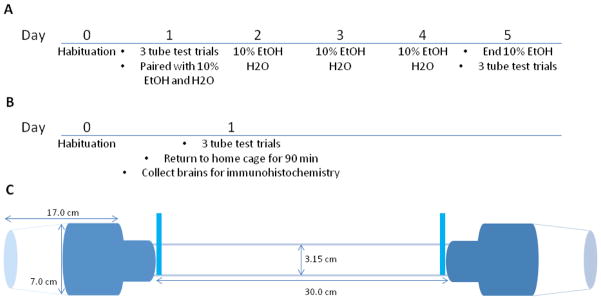
Timeline and tube test apparatus The timeline of events for Experiments 1 (A) and 2 (B) are shown. A schematic of the tube test apparatus, including starting cups, adapters, gates, tube and the relevant dimensions is presented (C).
In each trial the animal that was forced back to its starting cup was designated the loser, and the one who pushed was the winner. The subject with the most wins out of three trials was considered the dominant member of the pair, and the other the subordinate.
Immediately following the dominance tube test, pairs were placed in a new cage where they were separated by a wire mesh divider that has been described previously (Anacker, Loftis, Kaur, et al., 2011; Anacker, Loftis, & Ryabinin, 2011; Hostetler, Anacker, Loftis, & Ryabinin, 2012) in order to measure each subject’s drinking levels. Each vole had access to a bottle containing 10% ethanol in tap water, and another containing water. The volumes were checked and refilled every 24 hours, and measurements were used to calculate daily ethanol intake (grams per kilogram body weight) and ethanol preference (volume of ethanol solution/total fluid volume consumed). Following the fourth day of ethanol intake, pairs were tested in three additional trials of the dominance tube test and dominant/subordinate status was assessed again.
The dominance status was compared for each pair before and after alcohol intake. Body weight and age for each status were assessed by t-test, as were ethanol preference and dose consumed for the first day of intake and the average of the four days. Drinking preference and levels were analyzed for correlation with number of wins in the tube test using Pearson’s correlation. The association between number of wins and alcohol intake was further assessed by treating each pair as an individual data point, in order to avoid the effect of subjects that did not have independent scores; the difference in alcohol intake for each pair (subordinate – dominant) was compared between pairs where the dominant subjects won three out of three trials and those that won two out of three trials in the initial test, using a Mann-Whitney U-test, since the small sample size did could not be considered normally distributed.
Experiment 2
As in Experiment 1, adult prairie voles (n=12; 3 female pairs, 3 male pairs; 63–128 days of age) housed with same-sex siblings were habituated to the apparatus and the next day tested with a stranger in three trials of the dominance tube test, and dominance status was determined. Prior to the trials the subjects either had a small patch of fur shaved with an electric hair trimmer, or were mock shaved, for later identification. They were returned to their home cages and after 90 minutes they were euthanized by carbon dioxide inhalation and brains were removed and processed for c-Fos immunohistochemistry.
The brains were preserved in 4% paraformaldehyde in phosphate buffered saline (PBS) for 24 hours, followed by 20% sucrose in PBS with sodium azide for 24 hours and then stored in 30% sucrose in PBS with sodium azide. Brains were sliced in 40um floating sections on a cryostat and sections containing the nucleus accumbens, lateral septum, medial amygdala, anterior hypothalamus, paraventricular nucleus of the hypothalamus and centrally-projecting Edinger-Westphal nucleus were used for immunohistochemistry. These regions were chosen based on their role in social dominance behaviors and/or alcohol intake. Diaminobenzidine (DAB)-immunohistochemistry was performed as has been described previously (Anacker, Loftis, Kaur, et al., 2011), using a 1:2000 dilution of the mouse c-Fos antibody produced in rabbit (Santa Cruz Biotechnology, Santa Cruz, CA, 1:2000).
The number of Fos-positive cells was counted for each region by a trained experimenter blind to the status of the subjects, and the number was averaged across two slices per region. The numbers of Fos-positive cells in subordinate and dominant animals were analyzed by t-test for each brain region. Fos levels in subjects that won 0, 1, 2, or 3 trials were analyzed by ANOVA, and regions of interest were tested post hoc by Pearson’s correlation. The association between number of wins and Fos levels was further assessed by treating each pair as an individual data point, in order to avoid the effect of subjects that did not have independent scores; the difference in Fos-positive cell number for each pair (dominant – subordinate) was compared between pairs where the dominant subjects won three out of three trials and those that won two out of three trials, using a Mann-Whitney U-test, since the small sample size could not be considered normally distributed.
Following analysis of the DAB immunohistochemistry, all slices containing the PVN from three animals that won all three trials were subjected to double label immunohistochemistry to detect co-expression of Fos with oxytocin (OT), vasopressin (AVP) or corticotropin releasing factor (CRF) utilizing a modified protocol from our laboratory (Anacker, et al., 2014; Ryabinin, Galvan-Rosas, Bachtell, & Risinger, 2003). Unless noted otherwise, all steps were performed in 0.3% Triton-X in tris-buffered saline (TBS) and preceded by three washes in TBS. The sections were rinsed for 30 min in 1% sodium borohydride in TBS, and blocked in 5% normal donkey serum (NDS; Jackson Laboratories; 017-000-121) for 45 minutes. The tissue was then incubated with 1:1000 goat polyclonal c-Fos antibody (Santa Cruz; SC-52G) and 1:20,000 rabbit polyclonal antibodies for either OT (Peninsula Laboratories; T-4084), AVP (Peninsula Laboratories; T-4563) or CRF (Santa Cruz; SC-1759) in blocking solution overnight. An additional block in 5% normal donkey serum (NDS) for 45 min was followed by a one-hour incubation with AlexaFluor 555 anti-goat (raised in donkey) (Invitrogen; A21432). Slices were extensively washed with TBS prior to a one-hour incubation with AlexaFluor 488 anti-rabbit (raised in donkey) (Invitrogen; A21206). Finally, slices were washed with PBS, mounted on gelatinized slides and coverslipped with Prolong Gold with DAPI (Invitrogen; P36930). Co-localization of immunoreactivity was assessed manually using a Leica DM4000 microscope.
Results
Experiment 1
In the initial dominance tube test, status was determined for each pair in three trials. In half of the pairs, the dominant vole won all of three trials, and in the other half of the pairs the dominant vole won two out of three. Importantly, for all pairs the dominant vole in the first test was also the dominant vole in the second test taking place four days later, after having access to ethanol. During the second test, five of the six dominant voles won two of three trials, and one won all three.
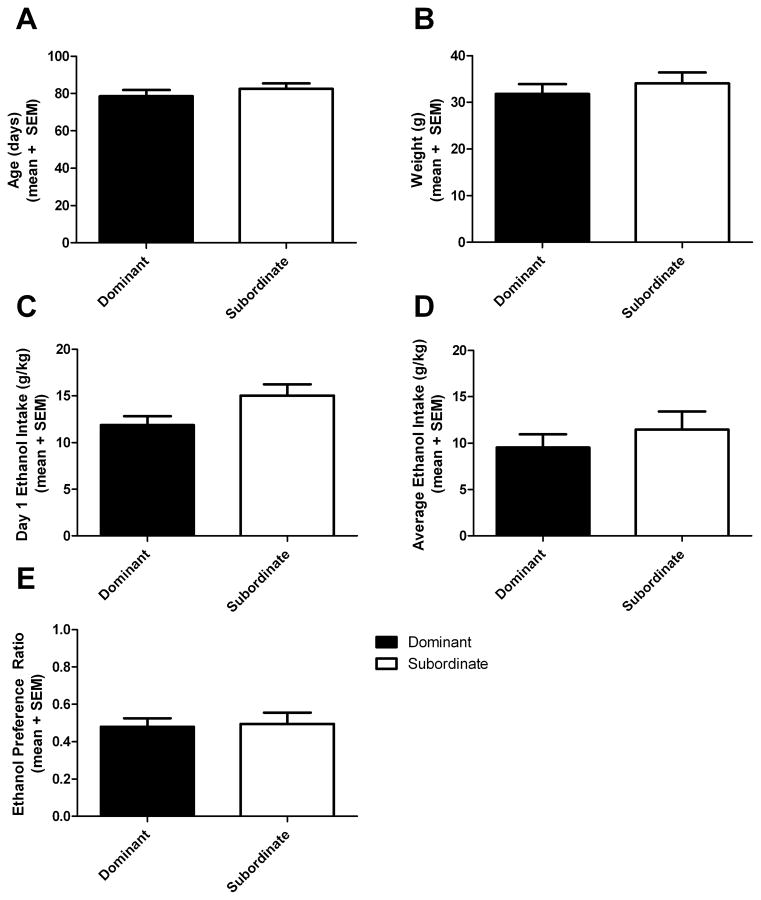
There was no apparent effect of age or weight on dominance status: in half of the pairs the dominant animal was older, and in four of six pairs the dominant animal weighed less than the subordinate. Average age and weight as a function of dominance status are shown in Fig. 2 A–B, and t-tests showed no significant differences (age: t(10)=0.874, p=0.402; weight: t(10)=0.719, p=0.488). There was no noticeable effect of sex on the determination of dominance: female pairs were equally likely to exhibit strong dominance expression (win three out of three trials) or weaker dominance expression (win two out of three trials) compared to male pairs.
Figure 2.
Age, weight, ethanol intake and preference in dominant and subordinate voles.
There was no difference in age (A) or weight (B) between dominant and subordinate voles. There was a trend toward a lower dose of ethanol consumed by dominant voles compared to subordinates on the first day of intake (C), no significant effect when the average intake over four days was analyzed (D), and there was no effect of rank on preference for ethanol (E).
During ethanol drinking (Fig 2 C–D), there was a trend toward a significant difference in the level of ethanol consumed in the first 24 hours of access: dominant voles drank lower doses than subordinates (t(10)=2.051, p=0.067). However, there was no difference between dominant and subordinate animals in ethanol preference (t(10)=0.232, p=0.822) or dose consumed (t(10)=0.790, p=0.448) when averaged across the four days. In four out of six pairs, the dominant subjects drank lower doses than subordinates on average over the four day period.
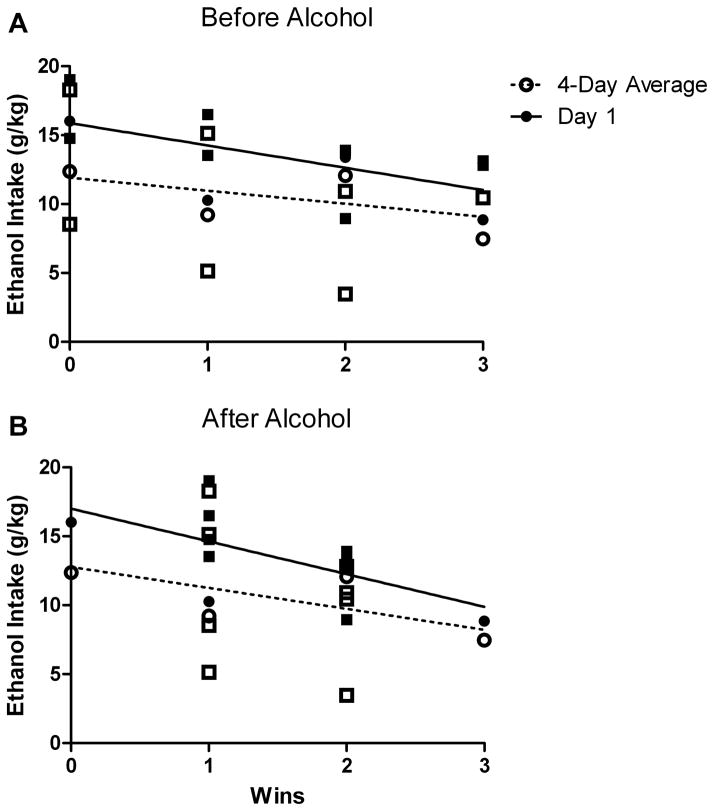
There was a negative correlation between the number of wins in both the first (r=−0.625, p=0.030, n=12) and last (r=−0.626, p=0.029, n=12) dominance tube test and the dose of alcohol consumed on the first day (Fig 3), but no significant correlation with preference, and no significant correlation with the average dose or preference across four days. The negative correlation from the last day of the tube test was driven by the pair of females that scored 3-0 in the tube test. There was no effect of the strength of dominance (winning three out of three versus two out of three trials in the first tube test) on the difference between dominant and subordinate alcohol intake on the first day of alcohol intake (U(4)=2, p=0.40).
Figure 3.
Number of dominance trial wins negatively correlates with ethanol consumption
There was a significant negative correlation between the number of dominance tube test trials won before (A) or after (B) four days of ethanol intake and the average dose of ethanol consumed on the first day of access (filled symbols), but not the average intake over four days (open symbols). Males are indicated by squares and females by circles.
Experiment 2
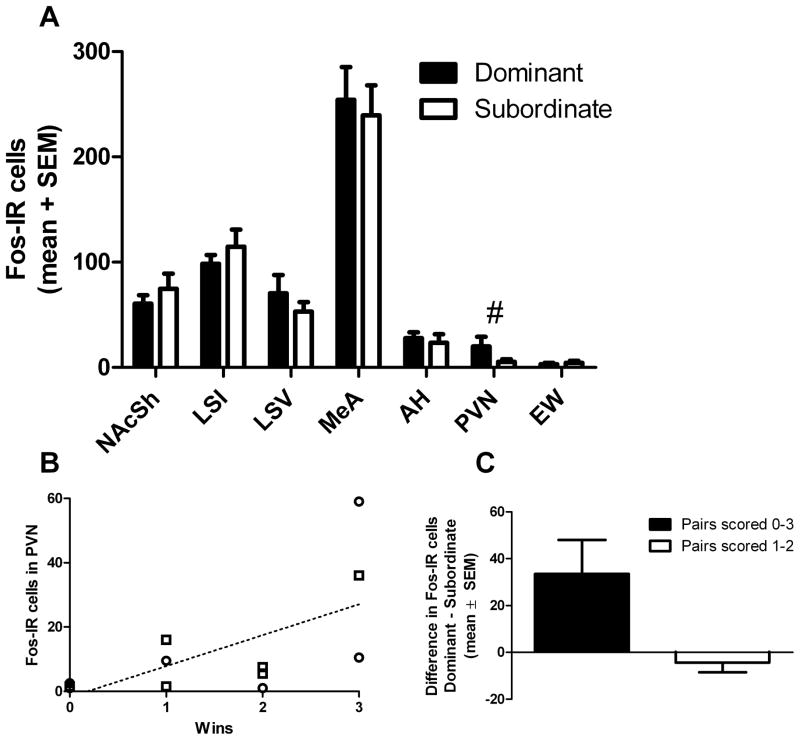
The number of Fos-immunoreactive cells was not significantly different between dominant and subordinate subjects in any region examined (Fig. 4A; nucleus accumbens shell: t=0.851, p=0.415; intermediate lateral septum: t=0.880, p=0.399; ventral lateral septum: t=0.882, p=0.398; medial amygdala: t=0.350, p=0.734; anterior hypothalamus t=0.427; p=0.679; paraventricular nucleus of the hypothalamus: t=1.514, p=0.161; centrally projecting Edinger-Westphal nucleus: t=0.510, p=0.621).
Figure 4.
Effect of dominance on Fos levels
There was no significant effect of dominance rank on the number of Fos-immunoreactive (Fos IR) cells in any region examined, but there was a trend for an effect of dominance on Fos IR cells in the PVN (# p=0.067) (A). There was a significant positive correlation between the number of dominance trial wins and the number of Fos-positive cells in the PVN (B). Males are indicated by squares and females by circles. Between pairs, there was a trend toward a significant effect of the strength of the dominance (i.e. 3 wins versus 2 wins in the tube test) on the number of Fos IR cells in the PVN (C). Abbreviations: NAc: nucleus accumbens; LSI: intermediate lateral septum; LSV: ventral lateral septum; MeA: medial amygdala; AH: anterior hypothalamus; PVN: paraventricular nucleus of the hypothalamus; EW: centrally-projecting Edinger-Westphal nucleus)
The number of Fos-immunoreactive cells was not significantly different in any region examined dependent on the number of trials won, although there was a trend for a higher level of c-Fos immunoreactivity in the paraventricular nucleus of the hypothalamus of dominant animals (F(3,7)=3.782, p=0.067). A post-hoc correlation between the number of trials won and the number of Fos-positive cells in the PVN (Fig 4B) revealed a significant positive correlation (r=0.637, p=0.026, n=12). There was a trend toward a significant effect of the strength of the dominance level on Fos-positive staining (Fig 4C), where the difference in cell number between the dominant and subordinate was greater in pairs in which the dominant subject won three out of three trials than those in which the dominant subject won two out of three trials (U(4)=0, p=0.076).
Double-label immunohistochemistry performed on the PVN of dominant animals did not reveal any co-labeling of Fos-positive nuclei with CRF or AVP. There were very low levels of co-localization of Fos with OT (Fig. 5).
Figure 5.
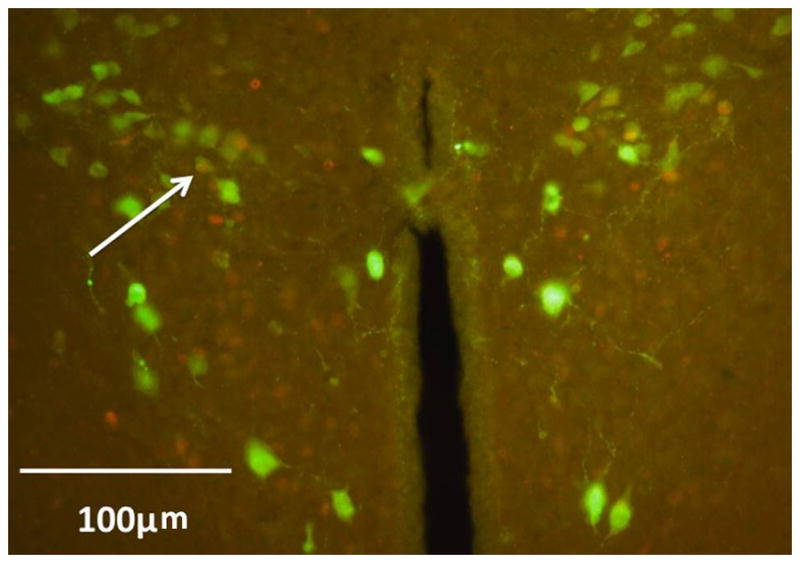
Double-label of Fos and oxytocin
Representative image of the PVN of a dominant male vole exhibiting high Fos-immunoreactivity. Fos-positive nuclei are shown in red, with OT-positive cell bodies shown in green. Very few cells exhibit co-localization; arrow indicates an example of co-labeling of Fos and OT.
Discussion
This study for the first time presents evidence that same-sex pairs of prairie voles exhibit a dominance relationship that is established quickly and lasts over time. The results of the tube test are most likely to relate specifically to space use, which may be particularly important among this burrowing, communally nesting species. This finding suggests that in group-living prairie voles, there may be established hierarchies that influence behaviors from resource allocation to mating (Getz, Dluzen, & McDermott, 1983) to dispersal (Myers & Krebs, 1971), even in the absence of aggressive behaviors. This relationship was independent of the relative age and weight of the subjects, and therefore may relate to other behavioral traits specific to individual animals. When aggression was used as a primary indicator of dominance, weight also was not significantly different between dominant and subordinate male prairie voles (Shapiro & Dewsbury, 1986). Similar results to the present study have been demonstrated in mice using the tube test. Different strains of mice exhibited dominance tendencies independent of weight, and at least half the time the dominant subject won all trials in the tube test (Lindzey, et al., 1961), as was observed here in voles. C57BL/6 mice living in groups also demonstrated stability in tube test rankings over time, though not as reliably as the voles in the present study (F. Wang, et al., 2011).
Dominance ranking is typically studied in male rodents as they usually exhibit more aggression. Aggression in prairie voles has most often been studied in male-female dyads; males typically exhibit more aggressive behavior than females (Getz & Carter, 1980), but in some cases females regularly initiate aggressive encounters with males (Getz, Carter, & Gavish, 1981). These may not indicate aggression levels toward a same-sex conspecific. One study that examined aggression in same-sex dyads observed more aggressive behaviors in males than females, although the effect of sex was not statistically tested (Harper & Batzli, 1997). Our experiments observed strong and weak dominance in both male and female vole pairs.
In Experiment 1 we found that the dominant voles exhibited a trend toward consuming significantly less alcohol than subordinate voles in the 24-hour period following the tube test, and that there was a significant negative correlation between the number of trials won and the level of alcohol intake on that first day of alcohol access. While it is not possible to definitively say whether the drinking is a consequence of the dominance testing experience or a pre-existing trait that corresponds with dominance level, there are a number of other studies relating dominance rank with alcohol drinking that can help to understand the relationship (reviewed in Anacker & Ryabinin, 2010). In colonies of rats with established dominance hierarchies, for example, the dominant male typically drinks the least, and subordinate males and females drink the most (Blanchard, et al., 1987; Wolffgramm & Heyne, 1991). The same pattern holds true for non-human primates (McKenzie-Quirk & Miczek, 2008). Studies in mice demonstrated that losers of aggressive contacts also drank more than winners (Kudryavtseva, et al., 1991), and in hamsters, repeated social defeat led to increased food intake (Foster, Solomon, Huhman, & Bartness, 2006). In contrast, studies examining the effect of forced subordination by introduction to an older, larger, aggressive conspecific demonstrated that alcohol drinking levels decreased following the defeat in rats (Funk, et al., 2005; van Erp, et al., 2001). If the drinking levels observed in the current study were a result of the test experience, we would have expected the subordinate subjects to have lower alcohol intake levels. Taken together, these findings support the idea that high levels of dominance correspond with lower levels of alcohol intake.
This conclusion is of particular interest given our previous work demonstrating that in adult same-sex prairie vole pairs, typically the higher drinker decreases its alcohol intake to match the level of the lower drinker, which we have speculated may be due to the low drinker being the dominant member of the pair (Anacker, Loftis, & Ryabinin, 2011; Anacker & Ryabinin, 2013). Those prior studies may explain why the difference in alcohol intake between dominant and subordinate voles in the present study was not significant after the first day or when all days were averaged: the subordinate voles decreased their alcohol intake to the level of the dominant subjects following the first day of alcohol intake.
The results of Experiment 2 inform us about possible mechanisms by which the tube test relates to behavior, which may be investigated more in the future. As the number of trials won correlated with the level of alcohol intake in Experiment 1, they also correlated with the level of Fos immunoreactivity in the paraventricular nucleus of the hypothalamus (PVN). This region produces several neuropeptides that are involved in the stress response and social behaviors. The most obvious candidate for regulating dominance in this brain region is CRF, release of which ultimately results in the release of the stress hormone corticosterone through the hypothalamic-pituitary-adrenal (HPA) axis. If the increased Fos in the PVN of dominant voles was a sign of increased HPA axis activity, then it would appear that the more dominant voles experienced more stress in the tube test than did those who won fewer trials. This idea is in agreement with a meta-analysis of stress hormone levels in different species of primates revealing several species in which the levels were higher in dominant individuals, or equal to subordinates, contrary to the traditional view that subordinates are more likely to exhibit hypercortisolemia (Abbott, et al., 2003). However, there was no co-labeling of Fos and CRF in the PVN, indicating that the tube test experience was not activating a stress response via the HPA axis. AVP is also produced in the PVN, and can also activate the HPA axis. However, similar to CRF, there was no co-labeling of Fos and AVP in the PVN, indicating that the tube test experience was not affecting vasopressin-containing neurons in this brain region. Since AVP has been shown to regulate aggression (Gobrogge, et al., 2007; Gobrogge, et al., 2009; Gobrogge & Wang, 2011; Winslow, et al., 1993), our data could serve as evidence for different mechanisms regulating dominance in the tube test versus aggression.
OT is another neuropeptide produced in the PVN, which has wide-ranging effects on stress-related and social behaviors, including dominance and aggression. For example, dominant female rhesus macaques have higher serum levels of OT than subordinates (Michopoulos, Checchi, Sharpe, & Wilson, 2011; Michopoulos, Higgins, Toufexis, & Wilson, 2012), the receipt of aggression in a social defeat procedure induces OT release in the lateral septum of male rats (Ebner, Wotjak, Landgraf, & Engelmann, 2000), and intracerebroventricular administration of OT increases aggressive behavior in dominant (but not subordinate) squirrel monkeys (Winslow & Insel, 1991). These findings suggest that activation of OT cells in the PVN could contribute to the assertion of dominance observed in prairie voles in the present study. However, the observed co-localization of Fos and OT was minimal and it is unclear whether the activation of those cells would generate a biologically relevant response.
Importantly, there were many Fos-positive nuclei that were not co-localized with any of the three peptides assayed in Experiment 2. The locations of Fos-positive nuclei were spread throughout the PVN, likely including both magnocellular and parvocellular neurons, and could be linked to multiple other neurotransmitters or peptides. Likely candidates include cocaine- and amphetamine-regulated transcript, thyroid releasing hormone, or somatostatin (Richard & Timofeeva, 2009). Identification of the precise neurochemical nature of these neuronal populations is beyond the scope of this study, and should be addressed in the future.
One drawback to the current study is that there was no comparison group of subjects that entered the tube test but did not experience the dominance test. Therefore, while there was a correlation between wins in the test and Fos levels, we cannot be certain that the effect was due to the interaction experience rather than a reaction to the tube itself. On the other hand, c-Fos levels tend to habituate quickly to novelty (Melia, Ryabinin, Schroeder, Bloom, & Wilson, 1994; Radulovic, Kammermeier, & Spiess, 1998), and our voles had a previous encounter with the apparatus, which should have minimized novelty-induced c-Fos expression.
In conclusion, we have demonstrated that prairie voles form quick and lasting dominance relationships within pairs, and their ranks correspond with alcohol intake levels. The establishment of dominance also correlates with activation of the PVN. It will be important for future studies to determine the mechanistic relationship between dominance, stress, and alcohol intake, and their effects on one another. This knowledge may be helpful for extending understanding of the human condition as well, most importantly for learning what conditions lead to changes in alcohol intake.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism under Grants AA019793 to AER and AA020126 to AMJA.
We appreciate the suggestions of Ajay Kumar who reviewed this manuscript prior to submission.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Hormones and Animal Social Behavior. Princeton: Princeton University Press; 2005. [Google Scholar]
- Anacker AMJ, Ahern TH, Hostetler CM, Dufour BD, Smith ML, Cocking DL, et al. Drinking alcohol has sex-dependent effects on pair bond formation in prairie voles. Proc Natl Acad Sci U S A. 2014;111(16):6052–6057. doi: 10.1073/pnas.1320879111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011;16(1):92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Loftis JM, Ryabinin AE. Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res. 2011;35(10):1884–1890. doi: 10.1111/j.1530-0277.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Ryabinin AE. Biological contribution to social influences on alcohol drinking: evidence from animal models. Int J Environ Res Public Health. 2010;7(2):473–493. doi: 10.3390/ijerph7020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Ryabinin AE. Identification of subpopulations of prairie voles differentially susceptible to peer influence to decrease high alcohol intake. Front Pharmacol. 2013;4:84. doi: 10.3389/fphar.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Dalrymple-Alford JC, Brain PF. Comparisons of measures of dominance in the laboratory mouse. Anim Behav. 1980;28(4):1274–1279. [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28(4):437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Cooper HM. Effects of alcohol on human aggression: an integrative research review. Psychol Bull. 1990;107(3):341–354. doi: 10.1037/0033-2909.107.3.341. [DOI] [PubMed] [Google Scholar]
- Clarke FM, Faulkes CG. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1998;265(1404):1391–1399. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorme KC, Sisk CL. Pubertal testosterone programs context-appropriate agonistic behavior and associated neural activation patterns in male Syrian hamsters. Physiol Behav. 2013;112–113:1–7. doi: 10.1016/j.physbeh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Res. 2000;872(1–2):87–92. doi: 10.1016/s0006-8993(00)02464-1. [DOI] [PubMed] [Google Scholar]
- Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1284–1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183(3):341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS. Social organization in Microtus ochrogaster populations. Biologist. 1980;62(1):56–69. [Google Scholar]
- Getz LL, Carter CS, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Getz LL, Dluzen D, McDermott JL. Suppression of reproductive maturation in male-stimulated virgin female microtus by a female urinary chemosignal. Behav Processes. 1983;8(1):59–64. doi: 10.1016/0376-6357(83)90043-8. [DOI] [PubMed] [Google Scholar]
- Getz LL, Hofmann JE. Social organization in free-living prairie voles, microtus ochrogaster. Behav Ecol Sociobiol. 1986;18(4):275–282. [Google Scholar]
- Getz LL, Hofmann JE, Carter CS. Mating system and population fluctuations of the prairie vole, microtus ochrogaster. Amer Zool. 1987;27(3):909–920. [Google Scholar]
- Getz LL, McGuire B. Communal nesting in prairie voles (Microtus ochrogaster): formation, composition, and persistence of communal groups. Can J Zool. 1997;75:525–534. [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502(6):1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106(45):19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Wang ZW. Genetics of aggression in voles. Adv Genet. 2011;75:121–150. doi: 10.1016/B978-0-12-380858-5.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SJ, Batzli GO. Are staged dyadic encounters useful for studying aggressive behaviour of arvicoline rodents? Can J Zool. 1997;75(7):1051–1058. [Google Scholar]
- Hostetler CM, Anacker AMJ, Loftis JM, Ryabinin AE. Social housing and alcohol drinking in male-female pairs of prairie voles (Microtus ochrogaster) Psychopharmacology (Berl) 2012;224(1):121–132. doi: 10.1007/s00213-012-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57(4):615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Madorskaya IA, Bakshtanovskaya IV. Social success and voluntary ethanol consumption in mice of C57BL/6J and CBA/Lac strains. Physiol Behav. 1991;50(1):143–146. doi: 10.1016/0031-9384(91)90511-l. [DOI] [PubMed] [Google Scholar]
- Lindzey G, Winston H, Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB, Cohen MA, Derzon JH. Is there a causal relationship between alcohol use and violence? A synthesis of evidence. Recent Dev Alcohol. 1997;13:245–282. doi: 10.1007/0-306-47141-8_14. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, et al. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci U S A. 2010;107(3):1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J Neurosci. 2011;31(22):7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 2008;201(1):137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14(10):5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Horm Behav. 2011;59(4):528–535. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Barry H., 3rd What does the tube test measure? Behav Biol. 1975;13(4):537–539. doi: 10.1016/s0091-6773(75)91249-3. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Boer SF, Haller J. Excessive aggression as model of violence: a critical evaluation of current preclinical methods. Psychopharmacology (Berl) 2013;226(3):445–458. doi: 10.1007/s00213-013-3008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JH, Krebs CJ. Genetic, behavioral, and reproductive attributes of dispersing field voles microtus pennsylvanicus and microtus ochrogaster. Ecol Monogr. 1971;41(1):53–78. [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim Behav. 2008;73(3):1143–1154. [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc Natl Acad Sci U S A. 2008;105(4):1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci. 1998;18(18):7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D, Timofeeva E, editors. Encyclopedia of Neuroscience. Academic Press; 2009. [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003;165(3):296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. Social relationships among adult female baboons. Anim Behav. 1976;24(4):917–938. [Google Scholar]
- Shapiro L, Dewsbury D. Male dominance, female choice and male copulatory behavior in two species of voles (Microtus ochrogaster and Microtus montanus) Behavioral Ecology and Sociobiology. 1986;18(4):267–274. [Google Scholar]
- Sousa MB, Albuquerque AC, da Albuquerque FS, Araujo A, Yamamoto ME, de Arruda MF. Behavioral strategies and hormonal profiles of dominant and subordinate common marmoset (Callithrix jacchus) females in wild monogamous groups. Am J Primatol. 2005;67(1):37–50. doi: 10.1002/ajp.20168. [DOI] [PubMed] [Google Scholar]
- Strozik E, Festing MF. Whisker trimming in mice. Lab Anim. 1981;15(4):309–312. doi: 10.1258/002367781780953040. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Increased aggression after ethanol self-administration in male resident rats. Psychopharmacology (Berl) 1997;131(3):287–295. doi: 10.1007/s002130050295. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology (Berl) 2007;191(3):679–688. doi: 10.1007/s00213-006-0637-3. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Tachi N, Miczek KA. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001;12(5):335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334(6056):693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wang Y, He Z, Zhao C, Li L. Medial amygdala lesions modify aggressive behavior and immediate early gene expression in oxytocin and vasopressin neurons during intermale exposure. Behav Brain Res. 2013;245:42–49. doi: 10.1016/j.bbr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767(2):321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Ellingboe J, Miczek KA. Effects of alcohol on aggressive behavior in squirrel monkeys: influence of testosterone and social context. Psychopharmacology (Berl) 1988;95(3):356–363. doi: 10.1007/BF00181947. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci. 1991;11(7):2032–2038. doi: 10.1523/JNEUROSCI.11-07-02032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav. 1991;38(2):389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148(4):401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]