Abstract
Neisseria meningitidis (the meningococcus) causes significant morbidity and mortality in children and young adults worldwide through epidemic or sporadic meningitis and/or septicemia. In this review, we describe the biology, microbiology, and epidemiology of this exclusive human pathogen. N. meningitidis is a fastidious, encapsulated, aerobic gram-negative diplococcus. Colonies are positive by the oxidase test and most strains utilize maltose. The phenotypic classification of meningococci, based on structural differences in capsular polysaccharide, lipooligosaccharide (LOS) and outer membrane proteins, is now complemented by genome sequence typing (ST). The epidemiological profile of N. meningitidis is variable in different populations and over time and virulence of the meningococcus is based on a transformable/plastic genome and expression of certain capsular polysaccharides (serogroups A, B, C, W-135, Y and X) and non-capsular antigens. N. meningitidis colonizes mucosal surfaces using a multifactorial process involving pili, twitching motility, LOS, opacity associated, and other surface proteins. Certain clonal groups have an increased capacity to gain access to the blood, evade innate immune responses, multiply, and cause systemic disease. Although new vaccines hold great promise, meningococcal infection continues to be reported in both developed and developing countries, where universal vaccine coverage is absent and antibiotic resistance increasingly more common.
Keywords: Neisseria meningitidis, Pathogenesis, Bacterial infections, Microbiology, Epidemiology
1. Introduction
In 1887, Weichselbaum (1) was the first to identify the meningococcus from the cerebrospinal fluid (CSF) of a patient with meningitis. Epidemics of meningococcal meningitis were first described during the early nineteenth century, in 1805 in Geneva, Switzerland by Vieusseux (2), in 1806 in New Bedford, Massachusetts by Danielson and Mann (3) and in the early 1900s in the African meningitis belt (4). The meningococcus was recognized as a habitant of the nasopharynx of healthy individuals (5), especially seen in the setting of military recruits camps (6) at the beginning of the twentieth century. Treatment for meningococcal disease included serum therapy introduced in 1913 by Flexner (7) and sulfonamides introduced in 1937 (8). The emergence of resistance to sulfonamides (9) in the 1960s prompted the development of the first vaccines against meningococci (10).
Despite an understanding of the pathogenesis, the availability of therapeutic and prophylactic antibiotics and immunizations against important serogroups, the meningococcus remains a leading cause worldwide of bacterial meningitis (11). Invasive meningococcal disease results from the interplay of: (1) microbial factors influencing the virulence of the organism, (2) environmental conditions facilitating exposure and acquisition, and (3) host susceptibility factors favoring bacterial acquisition, colonization, invasion, and survival. In the pre-serum therapy and pre-antibiotic eras, 70–85% of meningococcal disease cases were fatal; today, the overall mortality rate in invasive meningococcal disease still remains high, at between 10 and 15% (12). Meningococcal disease is also associated with marked morbidity including limb loss, hearing loss, cognitive dysfunction, visual impairment, educational difficulties, developmental delays, motor nerve deficits, seizure disorders, and behavioral problems (13). In this chapter, we review the biology, microbiology, and epidemiology of the meningococcus.
2. Biology of the Meningococcus
The virulence (14) of N. meningitidis is influenced by multiple factors: capsule polysaccharide expression, expression of surface adhesive proteins (outer membrane proteins including pili, porins PorA and B, adhesion molecules Opa and Opc), iron sequestration mechanisms, and endotoxin (lipooligosaccharide, LOS). N. meningitidis also has evolved genetic mechanisms resulting in a horizontal genetic exchange, high frequency phase, antigenic variation, and molecular mimicry, allowing the organism to successfully adapt at mucosal surfaces and invade the host (14).
2.1. Genetics
Genome sequences for a number of N. meningitidis strains including MC58 (serogroup B, ST-32) (15), Z2491 (serogroup A, ST-4) (16), FAM18 (serogroup C, ST-11), and NMB-CDC (serogroup B, ST-8) have been reported. Based on the sequencing of several genomes, the chromosome is between 2.0 and 2.2 megabases in size and contains about 2,000 genes (17). Except for the IHT-A1 capsule locus, no specific core pathogenome has been identified, suggesting that virulence may be clonal group-dependent. The core meningococcal genome that encodes for essential metabolic functions represents about 70% of the genome. Large genetic islands are present in different strains and are predicted to encode hypothetical surface proteins and virulence factors. The IHT-A1 locus contains the genes for capsule biosynthesis and transport, the IHT-A2 locus is predicted to encode an ABC transporter and a secreted protein, and the IHT-C locus is predicted to encode 30 open reading frames including toxin homologues, a bacteriophage, and potential virulence proteins (18).
The meningococcus shares about 90% homology at the nucleotide level with either N. gonorrhoeae or N. lactamiea. Mobile genetic elements including IS elements and prophage sequences make up to ~10% of the genome. Transfer of DNA occurs between meningococci, gonococci, and commensal Neisseria spp. as well as other bacteria (such as Haemophilus) (19). Several repetitive sequence and polymorphic regions are present, usually in large heterogeneous arrays, suggesting active areas of genetic recombination. Another characteristic of the meningococcal genome is the presence of multiple genetic switches (e.g., slipped-strand misparing, IS element movement), contributing to the expression of pathogen-associated genes (20). In summary, a central characteristic in the evolution of the meningococcus is the plasticity of the genome and the capacity created by this plasticity for diversity of phenotype.
2.2. Capsule
N. meningitidis can be either encapsulated or not. However, N. meningitidis strains causing invasive disease and isolated from sterile sites such as the blood or the CSF are almost always encapsulated. The capsule is essential for the survival of the organism in the blood as it provides resistance to antibody/complement-mediated killing and inhibits phagocytosis (21). Antibodies directed at capsule play a major part in protection against meningococcal disease and capsule forms the basis for licensed polysaccharide (22, 23) and new conjugate-polysaccharide meningococcal vaccines (24) (except for serogroup B) and for the classification of meningococci into serogroups.
The main meningococcal capsular polysaccharides associated with invasive disease are composed of sialic acid derivatives, except for the serogroup A capsule, which consists of repeating units of N-acetyl-mannosamine-1-phosphate. In N. meningitidis, N-acetylneuraminic acid (Neu5Ac), unlike in mammalian cells, is synthesized from N-acetylmannosamine (ManNAc) and phospoenolpyruvate without phosphorylated intermediates (25). Neu5Ac is the most common form of sialic acid in humans and plays an important role in intercellular and/or intermolecular recognition (26). The incorporation of Neu5Ac into meningococcal capsules allows the meningococcus to become less visible to the host immune system (27, 28) because of molecular mimicry. The most striking example is observed in the serogroup B capsule (29), an α(2–8)-linked sialic acid homopolymer identical in structure to the human fetal neural cell-adhesion molecule (NCAM). Such identity is responsible for the particularly poor immune response generated against serogroup B capsule by humans (30).
Capsular genes are located in a single locus cps within the IHT-Al 24 kb virulence island that is divided in three regions A, B, and C (31). Region A contains genes responsible for the synthesis and the polymerization of the polysaccharide. Regions B and C contain genes responsible for translocation of the polysaccharide from the cytoplasm to the surface. “Capsule switching” occurs due to genetic identity of parts of the capsule loci and is the result of horizontal exchange by transformation and recombination in the locus of serogroup specific capsule biosynthesis genes (32). Capsule switching is another mechanism of escape from vaccine-induced or natural protective immunity and a virulence mechanism shown by other encapsulated bacterial pathogens (e.g., Streptococcus pneumoniae). For example, the emergence of W-135 as a cause of outbreaks in 2000–2002 was due to the spread of W-135 ET-37 (ST-11) strains closely related to ET-37 (ST-11) serogroup C strains (33, 34). The phenomenon of capsule switching (32, 35) has raised concerns about the immune pressure that capsule-based vaccination programs may apply. However, so far, capsule switching has not caused significant problems after meningococcal conjugate vaccine introduction (36, 37).
2.3. Cell Envelope
In gram-negative bacteria, such as N. meningitidis, the subcapsular cell envelope consists of an outer membrane (OM), a peptidoglycan layer, and a cytoplasmic or inner membrane (Fig. 1). The OM has an outside layer primarily composed of lipopolysaccharide (LPS), actually a lipooligosaccharide, and proteins and an inside layer composed of phospholipids (38) that contains proteins primarily responsible for regulating the flow of nutrients and metabolic products. The major phospholipid component of Neisseria membranes consists largely of phosphatidylethanolamine (PE), with varying amounts of phosphatidylglycerol (PG), cardiolipin (CL), and phosphatidate (PA) (39). The structure of peptidoglycan of different N. meningitidis strains consists of a maximum of two layers (40) with different variations in the degree of cross-linking and O-acetylation. Typically, the percentage of cross-linking of the meningococcal peptidoglycan is around 40%, similar to other gram-negative bacteria (41). The O-acetylation of peptidoglycan results in resistance to lysozyme and to other muramidases (42). Also, peptidoglycan structures are recognized by components of the innate immune system (43).
Fig. 1.
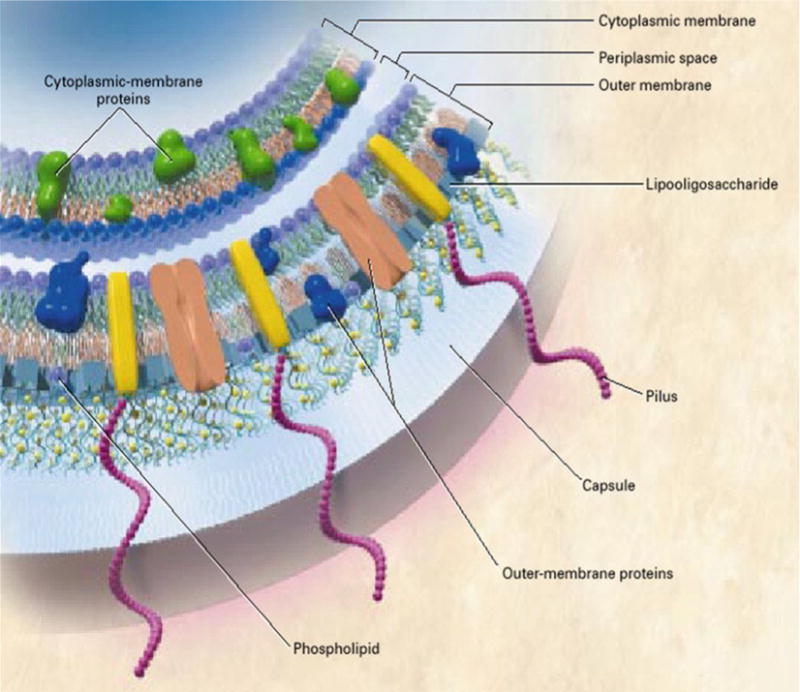
Cross-sectional view of the meningococcal cell membrane. (Copyright© 2001 Massachusetts Medical Society. All rights reserved).
2.4. Lipopoly saccharide (LPS)
Meningococcal LPS or LOS (endotoxin) (44, 45) plays a role in the adherence of the meningococcus (46) and in activation of the innate immune system (47). Meningococcal LOS lacks a repeating O-side chain of LPS found in enteric gram-negative bacilli (46). Meningococcal LPS consists of three parts (48): lipid A containing hydroxy fatty-acid chains and phosphoethanolamine, a core oligosaccharide containing 3-deoxy-D-manno-oct-2-ulosonic acid (KDO) and heptose residues, and highly variable short oligosaccharides. The heptose residues provide linkage to the short oligosaccharide residues of the α-, β-, and γ-chains. Meningococcal lipid A, a disaccharide of pyranasol N-acetyl glucosamine residues, is responsible for much of the biological activity and toxicity of meningococcal endotoxin. Sialic acid can be a terminal component of the α-chain lacto-N-neotetraose and is available from endogenous (sialic acid production) or exogenous (host sialic acid at the cell surfaces) sources. The α-chain structures of meningococcal LOS can mimic the human I and i antigens, an example of host molecular mimicry and immune escape mechanisms (49). Phase and antigenic variations (50), leading to different chain oligosaccharide and inner core composition, dramatically alter the antigenic properties of LOS and form the basis of the classification into different immunotypes (Ll–12) (35, 51). Immunotyping screenings of strains from invasive disease and from carriage have indicated that carriage isolates commonly expressed shorter structures termed L1 and L8, while invasive isolates were characterized by the expression of LOS with long α-chains. Also, ~97% of isolates from a serogroup B epidemic reported in England expressed the L3, 7, and 9 (36) immunotype, but the immunotypes of carriers were more heterogeneous.
Meningococcal LOS binds to a series of host transfer molecules and receptors on monocytic and dendritic cells of the innate immune system, including LPS-binding protein (LBP), CD14, and myeloid differentiation protein 2 (MD2), part of the Toll-like receptor 4 (TLR4) (52, 53) complex. This triggers the secretion of various cytokines (54) (including IL-6 and TNF-α) that at high levels can result in endothelial damage and capillary leakage. There is a direct correlation between LPS levels and severity of meningococcal disease (55, 56). LPS also induces the release of chemokines, reactive oxygen species (ROS), and nitric oxide (NO). In addition, LOS also plays an important role in resistance to other host defenses. Meningococci are resistant to cationic antimicrobial peptides (CAMPs) due to the lipid A phosphoethanolamine structures present on lipid A head groups. CAMPs are present in macrophages and neutrophils, and occasionally produced by epithelial cells at mucosal surfaces. These peptides play an important role in host defense against microbial infection and are key components of the innate immune responses through their nonoxidative killing action and their signaling functions.
2.5. Adhesins
Acquisition of meningococci through exposure to respiratory secretions and attachment on human upper respiratory mucosal surfaces by N. meningitidis are the first steps in establishing a human carrier state and invasive meningococcal disease. Meningococcal carriage occurs in 8–25% (57–59) of the human population with adolescents being the major reservoir. Duration of carriage can vary from days to several months. Meningococcal transmission among humans occurs largely through large respiratory droplets; the acquisition may be asymptomatic or may result in local inflammation. Invasive disease usually occurs 1–14 days after acquisition. Multiple structures, termed major and minor adhesions, facilitate meningococcal adherence.
2.5.1. Major Adhesins
Pili: The adhesive properties of capsulate N. meningitidis are mediated by pili (60), which extend several thousand nm beyond the capsule and initiate binding to epithelial cells (61). Twitching motility generated by pilus retraction is important for passage through the epithelial mucus layer, movement over epithelial surfaces, and microcolony formation (62). Piliated meningococci attach to human nasopharyngeal cells (63) in greater numbers when compared to meningococci devoid of pili. In addition, pili are involved in facilitating the uptake of DNA by meningococci (64) and also enable adherence to endothelial cells and erythrocytes. Meningococcal pili (60) are composed of two major pilin families and undergo both phase and antigenic variation. Neisserial pili can undergo posttranscriptional modifications such as pilin glycosylation (65). Glycosylation (66) may promote secretion of the soluble pilin units that compete for both anti-pili antibodies and host cell receptors allowing protection of the organism.
Opacity proteins: N. meningitidis strains commonly express two types of OM opacity proteins, Opa and Opc. While Opc is only expressed by N. meningitidis and encoded by a single gene, Opa proteins are expressed by both meningococci and gonococci, and encoded by multiple genes. Opa expression is subject to antigenic and phase variation and certain Opa types may predominate in clinical isolates due to their adhesion/virulence properties (67). Opa interacts with multiple members of the CEACAM (carcinoembryonic antigen-related cell-adhesion molecule) family (68); during inflammation high levels of CEACAM are expressed, facilitating Opa interactions and therefore cellular attachment and invasion. Both Opa and Opc (69) also interact with cell-surface associated HSPGs (heparan sulfate proteoglycans) (70).
2.5.2. MinorAdhesins
These molecules are often expressed at low levels in vitro, but may be upregulated in vivo; their potential roles in pathogenesis are not fully defined. Examples of minor adhesins include NadA (neisserial adhesinA), NhhA (Neisseria hia homologue A), App (adhesion and penetration protein), and MspA (meningococcal serine protease A) (71).
2.6. Other Molecules
Iron-binding proteins enable meningococci to acquire iron, a crucial growth factor during colonization and disease (72, 73). Meningococcal iron-acquiring proteins include HmbR (hemoglobin), TbpA and TbpB (transferrin), HbpA and HbpB (lactoferrin), HpnA and HpnB (hemoglobin-haptoglobin complex), and possible siderophore homologues.
N. meningitidis expresses two distinct porins, PorA and PorB, through which small hydrophilic nutrients diffuse into the bacterium via cation or anion selection. OM porins are also involved in host cell interactions and as targets for bactericidal antibodies (74). PorB is the major OM porin that inserts in membranes, induces Ca2+ influx and activates TLR2 and cell apoptosis (75). PorA is a major component of OM vesicle-based vaccines and a target for bactericidal antibodies (76). Of note, with no universal vaccine for serogroup such as PorA and in other proteins B, there is a particular interest in non-capsular OM antigens identified as vaccine targets through several experimental approaches including “reverse vaccinology” (77).
3. Microbiology of the Meningococcus
N. meningitidis is a gram-negative β proteobacterium and member of the bacterial family of Neisseriaceae. N. meningitidis is a fastidious bacterium, dying within hours on inanimate surfaces, and is either an encapsulated or unencapsulated, aerobic diplococcus with a “kidney” or “coffee-bean” shape (Fig. 2). Optimal growth for the organism occurs at 35–37 °C with 5–10% (v/v) carbon dioxide. The organism grows on different media such as blood agar, trypticase soy agar, supplemented chocolate agar, and Mueller-Hinton agar. N. meningitidis colonies on blood agar are grayish, nonhemolytic, round, convex, smooth, moist, and glistening with a clearly defined edge. N. meningitidis, however, tends to undergo rapid autolysis in stationary phase. Colonies are positive by the oxidase test and the result is confirmed with carbohydrate reactions (meningococci oxidize glucose and usually maltose, but not sucrose and lactose).
Fig. 2.
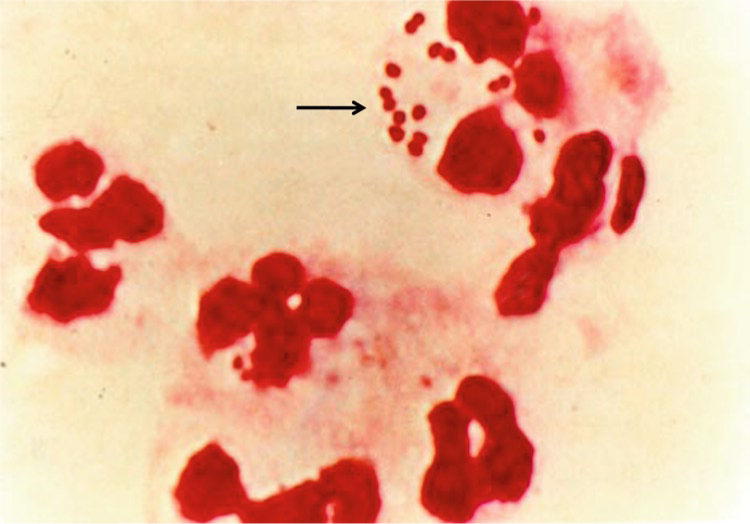
Intracellular gram-negative diplococci and leukocytes in the CSF from a patient with meningococcal meningitis. The arrow denotes diplococci in proximity and within leukocytes. (Copyright© 2001 Massachusetts Medical Society. All rights reserved).
3.1. Classification
Meningococci are classified according to serological typing (78, 79) and serogrouping is the traditional approach. Further classification into serosubtype, serotype, and immunotype is based on PorA, PorB, and LOS structure, respectively (13). At least 13 distinct meningococcal groups have been defined on the basis of their immunological reactivity and structure of the capsule’s polysaccharide (80). These serogroups are the following: A, B, C, E-29, H, I, K, L, W-135, X, Y, Z, and Z’ (29E). Only six serogroups (A, B, C, W-135, X, Y) cause life-threatening disease. Serogroup identification is done by slide agglutination or polymerase chain reaction (PCR) assays, while other meningococcal typing is performed using monoclonal antibodies (mAbs), PCR, and DNA sequencing (81).
3.2. Molecular Typing
Molecular typing is now the preferred approach for identifying clonal groups, closely related strains, strains with the potential to cause outbreaks, and in predicting vaccine coverage and understanding the genome of N. meningitidis. Molecular typing has used multiple techniques (82) including pulsed-field gel electrophoresis (PFGE) (83), multilocus enzyme electrophoresis (MLEE) (84), multilocus sequence typing (MLST) (85), and PCR (86). Currently, MLST is the gold standard for molecular typing (85) and classifies meningococcal strains into different STs (sequence types) based upon polymorphisms in seven housekeeping genes. Many meningococcal STs have been identified, which are independent of the serogroup. Of these, some are disproportionately associated with disease relative to carriage levels and so have been termed hyperinvasive lineages (87). Multilocus and antigen sequence typing data are assembled in large databases accessible via the internet (81).
3.3. Antimicrobial Susceptibility
Antimicrobial susceptibility testing of N. meningitidis should not be performed by disk diffusion, but by either minimal inhibitory concentration (MIC) determination by broth microdilution, or by use of the Etest® strip. To date, antibiotic resistance except for sulfonamides is relatively uncommon in the meningococcus. However, emergence of ciprofloxacin resistant strains (88) was recently observed and a decrease in penicillin susceptibility has been seen with some strains. Laboratory personnel at risk for exposure to aerosolized N. meningitidis should ensure their protective vaccination status is current, and they should work in a biological safety cabinet. Researchers who manipulate invasive N. meningitidis isolates in a manner that could induce aerosolization or droplet formation (i.e., plating, subculturing and serogrouping) on an open bench top and in the absence of effective protection from droplets or aerosols should consider antimicrobial chemoprophylaxis.
3.4. Metabolism
Very little direct work on the metabolism of the meningococcus has been carried out in the past 25 years. In 2007, Bart et al (89) screened the genome of MenB for open reading frames (ORFs) that code for enzymes present in the primary metabolism, yielding a genome-scale metabolic network (90, 91). Their genome-scale flux model was verified using flux balance analysis. According to the model, glucose can be completely catabolized mainly through the Entner-Douderoff pathway (ED), but also through the pentose phosphate pathway (PP), but not the Embden-Meyerhof-Parnas glycolytic pathway (EMP). The ED cleavage synthesizes the major part of pyruvate (67–87%) (92) and the PP pathway accounts for the remainder. N. meningitidis requires glucose, pyruvate, or lactate as sole carbon source (93) with a certain level of environmental CO2 tension to initiate growth (94). During cultivation on any of the carbon sources, secretion of acetate into the medium occurs (95). Studies on lactate utilization showed that lactate can be utilized by different meningococcal lactate dehydrogenases (LDH) (96). All biochemical pathways for amino acid synthesis in N. meningitidis are available (97). Reduced sulfur in the form of cysteine, cystine, or thiosulfate is required by the meningococcus for growth (98).
4. Epidemiology of the Meningococcus
Meningococcal infection is a global but not uniform problem occurring as sporadic, hyper-sporadic, and epidemic disease (Fig. 3). There are an estimated 1.2 million cases of meningococcal infection per year, with a death toll of ~135,000 worldwide. Disease patterns vary widely over time and between geographical areas, age groups, and bacterial serogroups. Most disease is caused by a few genetically defined clonal complexes of N. meningitidis that can emerge and spread worldwide (85).
Fig. 3.

Worldwide serogroup distribution of invasive meningococcal disease. (FEMS Microbiol Rev; used with permission).
4.1. Geographical Areas
United States: large serogroup A outbreaks occurred in the US during the first part of the twentieth century, but since the 1950s serogroup A meningococcal disease outbreaks disappeared in the USA as well as other industrialized countries (13) for unknown reasons. In the USA, the attack rate is now less than one case per 100,000 per year. During the 10-year period of 1998–2007 and according to the Active Bacterial Core surveillance (ABCs), the annual incidence decreased by 64.1%, from 0.92 cases per 100,000 in 1998 to 0.33 cases per 100,000 in 2007 with an average of 0.53 cases per 100,000 per year (99). A decrease in racial disparities was observed as well. The decrease in rates could not be readily explained by a change in environmental factors, such as smoking (100) and crowding, which are known to be risk factors for meningococcal disease. The decrease in rates was observed prior to implementation of the quadrivalent (serogroups A, C, Y, and W-135) meningococcal conjugate vaccine in 2005 in the USA, which for several reasons had low vaccine uptake in the early years, 11.4% uptake in 2006 and 32.4% uptake in 2007 (99). Infants aged <1 year have the highest incidence of meningococcal disease (5.38 cases per 100,000) (99), and a quadrivalent meningococcal vaccine is now recommended in the US for children 9 months to two years at increased risk of meningococcal disease. Today, serogroups C (101), Y (since the mid 1990s) and B cause most disease in the USA (102).
Europe: the attack rates (≥2 per 100,000 per year) in Europe have been higher than those observed in the USA. In the United Kingdom, rates of 5 per 100,000 per year prompted universal vaccination against serogroup C (103). In 1999, the UK was the first country to introduce serogroup C meningococcal conjugate vaccines to control the growing burden of serogroup C disease. This program has been very successful in reducing the incidence of serogroup C meningococcal disease in vaccinated persons (104). A major attribute of these vaccines was their reduction of serogroup C meningococcal carriage, leading to a decrease in the incidence of serogroup C meningococcal disease in the unvaccinated population as a result of herd immunity (105). With the success of the serogroup C immunization campaign in the UK, around 90% of remaining cases of invasive meningococcal disease in that country are now caused by serogroup B. The success of this program led to the subsequent introduction of meningococcal conjugate vaccines into routine immunization programs in other European countries (106), as well as Canada and Australia.
Africa: the term “meningitis belt” was defined by Lapeyssonnie (107) in 1963 when he described the sub-Saharan region from Ethiopia to Senegal, which includes 18 countries with more than 270 million people. The “meningitis belt” is characterized by periodic large epidemics of predominantly meningococcal meningitis. Epidemics occur every 8–10 years and began around 1905. The reasons for development and persistence of these outbreaks are not well understood, but environmental factors such as humidity and dust contribute (108, 109). Meteorological data may provide early warning for an impending epidemic (110) in the belt. While the peak of meningococcal disease coincides with respiratory viral illnesses (111, 112) during winter months in developed countries, meningococcal disease in Africa occurs in the dry season (109). However, mycoplasma infections have been associated with African outbreaks. Rates vary from 20 to 1,000 per 100,000 depending on the year and the presence or absence of epidemics.
Latin America: the epidemiology of meningococcal disease in Latin America is characterized by marked differences between countries (113). The overall incidence of meningococcal disease per year varies from less than 0.1 case per 100,000 in countries like Mexico to 2 cases per 100,000 in Brazil and Chile with major outbreaks. Serogroup A is rare in Latin America and most cases are due to serogroups B and C, with serogroups W-135 and Y now emerging in certain countries (e.g., serogroup Y was the most prevalent serogroup causing disease in Columbia and Venezuela in 2006).
Asia: large outbreaks of serogroup A have historically occurred in China (114), Nepal, India, and Russia but have recently been replaced by localized disease due to serogroups B and C. The incidence in Japan since WWII has fallen to very low levels, ~0.1 case per 100,000. Serogroups B and C are dominant now in Australia with a prolonged serogroup B epidemic in New Zealand occurring in the 1990s.
4.2. Meningococcal Serogroups
Most of the cases of meningococcal disease worldwide are caused by six serogroups (A, B, C, Y, W-135 and recently, in sub-Saharan Africa, X). Serogroup A has been responsible for the largest and most devastating meningococcal outbreaks in sub-Saharan Africa (115). In the largest meningococcal epidemic outbreak recorded in 1996–1997 in Africa, an estimated 300,000 cases and 30,000 deaths occurred in the meningitis belt due to serogroup A infection. Serogroup A was the cause of most meningococcal disease in the first part of the twentieth century in developed countries, but is now rare in the US and Europe.
Serogroup B is typically associated with a lower incidence of disease when compared to serogroup A or C, but prolonged outbreaks of serogroup B disease cause significant morbidity and mortality. Currently, serogroup B is the most important cause of endemic disease in developed countries, causing 30–40% of disease in the US and up to 80% in Europe. Serogroup B polysaccharide is poorly immunogenic (116), but serosubtype-specific vaccines have been developed for countries such as Cuba, New Zealand, and Norway that have experienced prolonged epidemic serogroup B disease. These vaccines are based on strain-specific OM vesicle (OMV) preparations (PorA major target) and were successful in reducing the incidence of local serogroup B outbreaks (117, 118). In New Zealand, the incidence of meningococcal disease increased from 1.6 cases per 100,000 population in 1990 to a peak of 17.4 cases per 100,000 population in 2001, with 85% of cases due to serogroup B. An OMV vaccine against the epidemic strain was introduced in 2004. The incidence decreased to 2.6 cases per 100,000 population by 2007, and the estimated effectiveness of the vaccine was 80% in fully immunized children aged 6 months to 15 years (119). Broadly protective vaccines that prevent both endemic and epidemic serogroup B disease, however, remain a major need worldwide.
Serogroup C is responsible for part of the reported endemic disease and localized epidemic outbreaks in developed countries, accounting for 30% of disease in the US (101) and Europe. Serogroup C has occasionally caused large epidemics. Serogroup Y has emerged in the US and caused more than a quarter of the disease due to meningococci in the US in the last decade (102). Compared with the early-1990s, when the proportion of serogroup Y cases was 2% during 1989–1991, the rates increased to 32.6% in 1996 (102) and serogroup Y still caused 26% of meningococcal disease cases in 2007 (99). Serogroup Y causes meningococcal pneumonia in older adults, but is also responsible for a large proportion of meningococcaemia and meningitis among infants less than 6 months of age (99). Serogroup Y has also been seen recently in South Africa, South America, and Israel.
Serogroup W-135 has emerged in the last 20 years as a cause of epidemic disease and like serogroup A previously, it has been also important especially in relationship to the Hajj pilgrimage (120). Since the 1990s, W-135 meningococcal infection has affected the health of these travelers to Saudi Arabia and their contacts in countries throughout the world (121–123). Saudi authorities require Hajj pilgrims to show evidence of immunization with the tetrava-lent meningococcal vaccines (A, C, W-135 and Y) and not just with mono- or bivalent meningococcal vaccines. Serogroup W-135 has also emerged in South America (124) and in Africa; Burkina Faso (125–128) in particular witnessed a large outbreak of serogroup W-135 infection in 2002. Serogroup X has recently been found responsible for meningococcal cases and outbreaks in certain African countries such as Kenya (129), Niger (130), and Ghana (131).
4.3. Age Groups
The meningococcus remains a common cause of bacterial meningitis in children and young adults in the USA (132), now mostly affecting children less than 2 years of age (102, 133). Two-thirds of meningococcal disease in the first year of life in the US occurs in infants less than 6 months of age (134). Worldwide, the rates of meningococcal disease are also highest for young children due to waning protective maternal antibody, but in epidemic outbreaks, older children and adolescents can have high rates of disease. Fifty percent of cases in infants in the US are due to serogroup B; serogroup C is mostly seen in adolescents and serogroups B and Y in older adults. Even though peak incidence occurs among infants and adolescents; one-third to one-half of sporadic cases are seen in adults older than 18 years.
4.4. Clonal Complexes
The advances in molecular typing have created a better understanding of the epidemiology and population biology of the meningococcus. The genetic diversity of the meningococcus, even though extensive, is highly structured. Groups of genetically closely related meningococci are grouped into clonal complexes. A minority of clonal complexes, the so-called hyperinvasive lineages, are relatively stable with life spans of many decades and with global geographic spread (135). The hyperinvasive lineages are responsible for a disproportionate number of cases of disease with specific phenotype features (Table 1). While the ST-1, ST-4, and ST-5 complexes are restricted nearly exclusively to strains of serogroup A, other clonal complexes, like the ST-11 complex, may be associated with various serogroups. For example, the emergence of W-135 as a cause of outbreaks in 2000–2002 was the result of the spread of W-135, ST-11 strains closely related to the ST-11 serogroup C strains. Other “virulent” clonal complexes include the ST-269 complex, a significant cause of serogroup B disease since the 1990s in the UK and now recognized worldwide; the ST-8, ST-32, and ST41/44 complexes associated with serogroup B worldwide; the ST-23 complex associated with serogroup Y disease in the US and now in other countries.
Table 1.
Epidemiology and genotypic features of meningococcal clonal complexes
| Epidemiology | |||||
|---|---|---|---|---|---|
| Clonal complex Protype Strain Disease/carriage ratio (136) |
ST-32 MC58 3.5 |
ST-8 NMB-CDC 24.5 |
ST-4 Z2491 19.5 |
ST-11 FAM18 6.6 |
ST-23 M6049 0.8 |
| Feature/Genotype | |||||
| IHTA Capsule synthesis | Yes (B, C) | Yes (B) | Yes (A) | Yes (C, W-135) | Yes (Y, W-135, NG) |
| IHTB/C/D | Yes | No | No | No | No |
| IHTE Lamboid bacteriophage | No | Yes | No | Yes | No |
| Pnml or 2 Mu-like prophage | Yes | No | Yes | No | Variable |
| MDA Disease associated phage | Yes | Yes | Yes | Yes | Variable |
| hmbR locus | Yes | Yes | Yes | Yes | No |
ST sequence type, IHT islands of horizontal transfer
5. Conclusions
The human species is the only natural host for the meningococcus. The meningococcus has evolved multiple mechanisms to be able to transmit from, adapt to, and colonize predominantly human upper respiratory tract mucosal surfaces. Certain clonal groups of meningococci have also evolved the capacity (e.g., expression of certain capsular polysaccharides) to cause invasive disease. N. meningitidis remains a global threat causing sporadic cases, case clusters, epidemics, and pandemics, although new conjugate and protein-based vaccines hold great promise and are already influencing the incidence of meningococcal disease.
Acknowledgments
We would like to thank Lane Pucko for her help in preparing this review. Research is supported by NIH/NIAID grants (R01 AI33517 and R01 AI40247) to D.S.S. and “Atlanta Clinical and Translational Science Institute” (UL1RR025008; KL2FF025009; TL1RR025010) and Georgia Research Alliance (GRA.VAC.09.K) grants to N.G.R and D.S.S.
References
- 1.Weichselbaum A. Ueber die Aetiologie der akuten meningitis cerebrospinalis. Fortschr Med. 1887;5:573. [Google Scholar]
- 2.Vieusseux M. Memoire sur la maladie qui a régné à Genéve au printemps de 1805. J Med Clin Pharm. 1805;11:163–82. [Google Scholar]
- 3.Danielson L, Mann E. A history of a singular and very noted disease, which lately made its appearance in Medfield. Med Agricultural Reg. 1806;1:65–9. [Google Scholar]
- 4.Greenwood B. Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–53. doi: 10.1016/s0035-9203(99)90106-2. [DOI] [PubMed] [Google Scholar]
- 5.Kiefer F. Zur differential Diagnose des Erregers der epidemischen Cerebrospinal meningitis und der. Gonorrhoea Berl Klin Wochenschr. 1896;33:628. [Google Scholar]
- 6.Glover J. The cerebrospinal fever epidemic of 1917 at “X” depot. J R Army Med Corps. 1918;30:23. [Google Scholar]
- 7.Flexner S. The results if the serum treatment in thirteen hundred cases of epidemic meningitis. J Exp Med. 1913;17:553. doi: 10.1084/jem.17.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwentker F, Gelman S, Long P. The treatment of meningococcic meningitis with sulfonamide. Preliminary report. JAMA. 1937;108:1407. doi: 10.1001/jama.251.6.788. [DOI] [PubMed] [Google Scholar]
- 9.Schoenback E, Phair J. The sensitivity of meningococci to sulfadiazine. Am J Hyg. 1948;47:177–86. doi: 10.1093/oxfordjournals.aje.a119194. [DOI] [PubMed] [Google Scholar]
- 10.Artenstein MS, Gold R, Zimmerly JG, et al. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970;282:417–20. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- 11.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococca-emia, and Neisseria meningitidis. Lancet. 2007;369:2196–210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 12.Sharip A, Sorvillo F, Redelings MD, et al. Population-based analysis of meningococcal disease mortality in the United States: 1990–2002. Pediatr Infect Dis J. 2006;25:191–4. doi: 10.1097/01.inf.0000202065.03366.0c. [DOI] [PubMed] [Google Scholar]
- 13.Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med. 2001;344:1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 14.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B71–7. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tettelin H, Saunders NJ, Heidelberg J, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–15. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 16.Parkhill J, Achtman M, James KD, et al. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–6. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 17.Schoen C, Blom J, Claus H, et al. Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc Natl Acad Sci USA. 2008;105:3473–8. doi: 10.1073/pnas.0800151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotopp JC, Grifantini R, Kumar N, et al. Comparative genomics of Neisseria meningitidis: core genome, islands of horizontal transfer and pathogen-specific genes. Microbiology. 2006;152:3733–49. doi: 10.1099/mic.0.29261-0. [DOI] [PubMed] [Google Scholar]
- 19.Davidsen T, Tonjum T. Meningococcal genome dynamics. Nat Rev Microbiol. 2006;4:11–22. doi: 10.1038/nrmicro1324. [DOI] [PubMed] [Google Scholar]
- 20.Hilse R, Hammerschmidt S, Bautsch W, et al. Site-specific insertion of IS1301 and distribution in Neisseria meningitidis strains. J Bacteriol. 1996;178:2527–32. doi: 10.1128/jb.178.9.2527-2532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uria MJ, Zhang Q, Li Y, et al. A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J Exp Med. 2008;205:1423–34. doi: 10.1084/jem.20072577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–8. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snape MD, Pollard AJ. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect Dis. 2005;5:21–30. doi: 10.1016/S1473-3099(04)01251-4. [DOI] [PubMed] [Google Scholar]
- 25.Blacklow RS, Warren L. Biosynthesis of sialic acids by Neisseria meningitidis. J Biol Chem. 1962;237:3520–6. [PubMed] [Google Scholar]
- 26.Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–55. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 27.Estabrook MM, Griffiss JM, Jarvis GA. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–4. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahler CM, Martin LE, Shih GC, et al. The (alpha2–>8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66:5939–47. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobb RI, Tzeng YL, Choudhury BP, et al. Requirement of NMB0065 for connecting assembly and export of sialic acid capsular polysaccharides in Neisseria meningitidis. Microbes Infect. 2010;12:476–87. doi: 10.1016/j.micinf.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmer SM, Stephens DS. Serogroup B meningococcal vaccines. Curr Opin Investig Drugs. 2006;7:733–9. [PubMed] [Google Scholar]
- 31.Frosch M, Weisgerber C, Meyer TF. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci USA. 1989;86:1669–73. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swartley JS, Marfin AA, Edupuganti S, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–6. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguilera JF, Perrocheau A, Meffre C, et al. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg Infect Dis. 2002;8:761–7. doi: 10.3201/eid0808.010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghunathan PL, Jones JD, Tiendrebeogo SR, et al. Predictors of immunity after a major serogroup W-135 meningococcal disease epidemic, Burkina Faso, 2002. J Infect Dis. 2006;193:607–16. doi: 10.1086/499822. [DOI] [PubMed] [Google Scholar]
- 35.Harrison LH, Shutt KA, Schmink SE, et al. Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era-United States, 2000–2005. J Infect Dis. 2010;201:1208–24. doi: 10.1086/651505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso JM, Gilmet G, Rouzic EM, et al. Workshop on vaccine pressure and Neisseria meningitidis, Annecy, France, 9–11 March 2005. Vaccine. 2007;25:4125–9. doi: 10.1016/j.vaccine.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol. 2002;51:717–22. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- 38.Nikaido H. Microdermatology: cell surface in the interaction of microbes with the external world. J Bacteriol. 1999;181:4–8. doi: 10.1128/jb.181.1.4-8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman MM, Kolli VS, Kahler CM, et al. The membrane phospholipids of Neisseria meningitidis and Neisseria gonorrhoeae as characterized by fast atom bombardment mass spectrometry. Microbiology. 2000;146(Pt8):1901–11. doi: 10.1099/00221287-146-8-1901. [DOI] [PubMed] [Google Scholar]
- 40.Antignac A, Rousselle JC, Namane A, et al. Detailed structural analysis of the pep-tidoglycan of the human pathogen Neisseria meningitidis. J Biol Chem. 2003;278:31521–8. doi: 10.1074/jbc.M304749200. [DOI] [PubMed] [Google Scholar]
- 41.Quintela JC, Caparros M, de Pedro MA. Variability of peptidoglycan structural parameters in gram-negative bacteria. FEMS Microbiol Lett. 1995;125:95–100. doi: 10.1111/j.1574-6968.1995.tb07341.x. [DOI] [PubMed] [Google Scholar]
- 42.Clarke AJ, Dupont C. O-acetylated peptidoglycan: its occurrence, pathobiological significance, and biosynthesis. Can J Microbiol. 1992;38:85–91. doi: 10.1139/m92-014. [DOI] [PubMed] [Google Scholar]
- 43.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 44.Jennings HJ, Johnson KG, Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983;121:233–1. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- 45.Gamian A, Beurret M, Michon F, et al. Structure of the L2 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem. 1992;267:922–5. [PubMed] [Google Scholar]
- 46.Kahler CM, Stephens DS. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin) Crit Rev Microbiol. 1998;24:281–334. doi: 10.1080/10408419891294216. [DOI] [PubMed] [Google Scholar]
- 47.Plant L, Sundqvist J, Zughaier S, et al. Lipooligosaccharide structure contributes to multiple steps in the virulence of Neisseria meningitidis. Infect Immun. 2006;74:1360–7. doi: 10.1128/IAI.74.2.1360-1367.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zughaier SM, Lindner B, Howe J, et al. Physicochemical characterization and biological activity of lipooligosaccharides and lipid A from Neisseria meningitidis. J Endotoxin Res. 2007;13:343–57. doi: 10.1177/0968051907084435. [DOI] [PubMed] [Google Scholar]
- 49.Mandrell RE, Griffiss JM, Macher BA. Lipo oligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168:107–26. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jennings MP, Srikhanta YN, Moxon ER, et al. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology. 1999;145:3013–21. doi: 10.1099/00221287-145-11-3013. [DOI] [PubMed] [Google Scholar]
- 51.Mandrell RE, Zollinger WD. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977;16:471–5. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zughaier SM, Tzeng YL, Zimmer SM, et al. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect Immun. 2004;72:371–80. doi: 10.1128/IAI.72.1.371-380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zughaier S, Steeghs L, van der Ley P, et al. TLR4-dependent adjuvant activity of Neisseria meningitidis lipid A. Vaccine. 2007;25:4401–9. doi: 10.1016/j.vaccine.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braun JM, Blackwell CC, Poxton IR, et al. Proinflammatory responses to lipooligosaccharide of Neisseria meningitidis immunotype strains in relation to virulence and disease. J Infect Dis. 2002;185:1431–8. doi: 10.1086/340501. [DOI] [PubMed] [Google Scholar]
- 55.Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 56.Brandtzaeg P, Bryn K, Kierulf P, et al. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J Clin Invest. 1992;89:816–23. doi: 10.1172/JCI115660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenfield S, Sheehe PR, Feldman HA. Meningococcal carriage in a population of “normal” families. J Infect Dis. 1971;123:67–73. doi: 10.1093/infdis/123.1.67. [DOI] [PubMed] [Google Scholar]
- 58.Stephens DS. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet. 1999;353:941–2. doi: 10.1016/S0140-6736(98)00279-7. [DOI] [PubMed] [Google Scholar]
- 59.Caugant DA, Hoiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994;32:323–30. doi: 10.1128/jcm.32.2.323-330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinner RW, Spellman PA, Stephens DS. Evidence for functionally distinct pili expressed by Neisseria meningitidis. Infect Immun. 1991;59:3169–75. doi: 10.1128/iai.59.9.3169-3175.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Virji M, Alexandrescu C, Ferguson DJ, et al. Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol Microbiol. 1992;6:1271–9. doi: 10.1111/j.1365-2958.1992.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 62.Merz AJ, So M. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu Rev Cell Dev Biol. 2000;16:423–57. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- 63.Stephens DS, McGee ZA. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981;143:525–32. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- 64.Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria – structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66:613–35. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kahler CM, Martin LE, Tzeng YL, et al. Polymorphisms in pilin glycosylation Locus of Neisseria meningitidis expressing class II pili. Infect Immun. 2001;69:3597–604. doi: 10.1128/IAI.69.6.3597-3604.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marceau M, Forest K, Beretti JL, et al. Consequences of the loss of O-linked glycosylation of meningococcal type IV pilin on piliation and pilus-mediated adhesion. Mol Microbiol. 1998;27:705–15. doi: 10.1046/j.1365-2958.1998.00706.x. [DOI] [PubMed] [Google Scholar]
- 67.Callaghan MJ, Jolley KA, Maiden MC. Opacity-associated adhesin repertoire in hyperinvasive Neisseria meningitidis. Infect Immun. 2006;74:5085–94. doi: 10.1128/IAI.00293-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Virji M, Watt SM, Barker S, et al. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–39. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 69.Virji M, Makepeace K, Ferguson DJ, et al. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992;6:2785–95. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 70.Virji M, Evans D, Hadfield A, et al. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol. 1999;34:538–51. doi: 10.1046/j.1365-2958.1999.01620.x. [DOI] [PubMed] [Google Scholar]
- 71.Hill DJ, Griffiths NJ, Borodina E, et al. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin Sci (Lond) 2010;118:547–64. doi: 10.1042/CS20090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perkins-Balding D, Ratliff-Griffin M, Stojiljkovic I. Iron transport systems in Neisseria meningitidis. Microbiol Mol Biol Rev. 2004;68:154–71. doi: 10.1128/MMBR.68.1.154-171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schryvers AB, Stojiljkovic I. Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol. 1999;32:1117–23. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 74.Tzeng YL, Stephens DS. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2000;2:687–700. doi: 10.1016/s1286-4579(00)00356-7. [DOI] [PubMed] [Google Scholar]
- 75.Massari P, Ram S, Macleod H, et al. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 2003;11:87–93. doi: 10.1016/s0966-842x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 76.Vermont CL, van Dijken HH, Kuipers AJ, et al. Cross-reactivity of antibodies against PorA after vaccination with a meningococcal B outer membrane vesicle vaccine. Infect Immun. 2003;71:1650–5. doi: 10.1128/IAI.71.4.1650-1655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine. 2001;19:2688–91. doi: 10.1016/s0264-410x(00)00554-5. [DOI] [PubMed] [Google Scholar]
- 78.Frasch CE, Zollinger WD, Poolman JT. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–10. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 79.Slaterus KW. Serological typing of meningococci by means of micro-precipitation. Antonie Van Leeuwenhoek. 1961;27:305–15. doi: 10.1007/BF02538460. [DOI] [PubMed] [Google Scholar]
- 80.Branham S. Serological relationships among meningococci. Bact Rev. 1953;17:175–88. doi: 10.1128/br.17.3.175-188.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vogel U. Molecular epidemiology of meningococci: Application of DNA sequence typing. Int J Med Microbiol. 2010;300(7):415–20. doi: 10.1016/j.ijmm.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 82.Caugant DA, Froholm LO, Bovre K, et al. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci USA. 1986;83:4927–31. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bevanger L, Bergh K, Gisnas G, et al. Identification of nasopharyngeal carriage of an outbreak strain of Neisseria meningitidis by pulsed-field gel electrophoresis versus phenotypic methods. J Med Microbiol. 1998;47:993–8. doi: 10.1099/00222615-47-11-993. [DOI] [PubMed] [Google Scholar]
- 84.Weis N, Lind I. Epidemiological markers in Neisseria meningitidis: an estimate of the performance of genotyping vs phenotyping. Scand J Infect Dis. 1998;30:69–75. doi: 10.1080/003655498750002330. [DOI] [PubMed] [Google Scholar]
- 85.Maiden MC, Bygraves JA, Feil E, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–5. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mothershed EA, Sacchi CT, Whitney AM, et al. Use of real-time PCR to resolve slide agglutination discrepancies in serogroup identification of Neisseria meningitidis. J Clin Microbiol. 2004;42:320–8. doi: 10.1128/JCM.42.1.320-328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yazdankhah SP, Kriz P, Tzanakaki G, et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol. 2004;42:5146–53. doi: 10.1128/JCM.42.11.5146-5153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu HM, Harcourt BH, Hatcher CP, et al. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med. 2009;360:886–92. doi: 10.1056/NEJMoa0806414. [DOI] [PubMed] [Google Scholar]
- 89.Baart GJ, Zomer B, de Haan A, et al. Modeling Neisseria meningitidis metabolism: from genome to metabolic fluxes. Genome Biol. 2007;8:R136. doi: 10.1186/gb-2007-8-7-r136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Price ND, Papin JA, Schilling CH, et al. Genome-scale microbial in silico models: the constraints-based approach. Trends Biotechnol. 2003;21:162–9. doi: 10.1016/S0167-7799(03)00030-1. [DOI] [PubMed] [Google Scholar]
- 91.Papin JA, Price ND, Wiback SJ, et al. Metabolic pathways in the post-genome era. Trends Biochem Sci. 2003;28:250–8. doi: 10.1016/S0968-0004(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 92.Jyssum K, Borchgrevink B, Jyssum S. Glucose catabolism in Neisseria meningitidis. 1. Glucose oxidation and intermediate reactions of the Embden-Meyerhof pathway. Acta Pathol Microbiol Scand. 1961;53:71–83. [PubMed] [Google Scholar]
- 93.Frantz ID. Growth requirements of the meningococcus. J Bacteriol. 1942;43:757–61. doi: 10.1128/jb.43.6.757-761.1942. 1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chapin Carbon dioxide in the primary cultivation of the gonococcus. J Infect Dis. 1918;19:558–61. [Google Scholar]
- 95.Grossowicz N. Growth requirements and metabolism of Neisseria intracellularis. J Bacteriol. 1945;50:109–15. doi: 10.1128/jb.50.1.109-115.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Erwin AL, Gotschlich EC. Cloning of a Neisseria meningitidis gene for L-lactate dehydrogenase (L-LDH): evidence for a second meningococcal L-LDH with different regulation. J Bacteriol. 1996;178:4807–13. doi: 10.1128/jb.178.16.4807-4813.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leighton MP, Kelly DJ, Williamson MP, et al. An NMR and enzyme study of the carbon metabolism of Neisseria meningitidis. Microbiology. 2001;147:1473–82. doi: 10.1099/00221287-147-6-1473. [DOI] [PubMed] [Google Scholar]
- 98.Port JL, DeVoe IW, Archibald FS. Sulphur acquisition by Neisseria meningitidis. Can J Microbiol. 1984;30:1453–7. doi: 10.1139/m84-232. [DOI] [PubMed] [Google Scholar]
- 99.Cohn AC, MacNeil JR, Harrison LH, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50:184–91. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 100.Fischer M, Hedberg K, Cardosi P, et al. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16:979–83. doi: 10.1097/00006454-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 101.Jackson LA, Schuchat A, Reeves MW, et al. Serogroup C meningococcal outbreaks in the United States. An emerging threat. JAMA. 1995;273:383–9. [PubMed] [Google Scholar]
- 102.Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J Infect Dis. 1999;180:1894–901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 103.Cartwright K, Noah N, Peltola H. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European advisory board meeting Vienna, Austria, 6–8 October, 2000. Vaccine. 2001;19:4347–56. doi: 10.1016/s0264-410x(01)00205-5. [DOI] [PubMed] [Google Scholar]
- 104.Trotter CL, Andrews NJ, Kaczmarski EB, et al. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 105.Maiden MC, Ibarz-Pavon AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31:101–7. doi: 10.1111/j.1574-6976.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 107.Lepeyssonnie La méningite cérébro-spinale en Afrique. Bull WHO. 1963;28:53–114. [PMC free article] [PubMed] [Google Scholar]
- 108.Molesworth AM, Cuevas LE, Connor SJ, et al. Environmental risk and meningitis epidemics in Africa. Emerg Infect Dis. 2003;9:1287–93. doi: 10.3201/eid0910.030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greenwood BM, Bradley AK, Wall RA, et al. Meningococcal disease and season in sub-Saharan Africa. Lancet. 1985;2:829–30. doi: 10.1016/s0140-6736(85)90812-8. [DOI] [PubMed] [Google Scholar]
- 110.Lewis R, Nathan N, Diarra L, et al. Timely detection of meningococcal meningitis epidemics in Africa. Lancet. 2001;358:287–93. doi: 10.1016/S0140-6736(01)05484-8. [DOI] [PubMed] [Google Scholar]
- 111.Artenstein MS, Rust JH, Jr, Hunter DH, et al. Acute respiratory disease and meningococcal infection in army recruits. JAMA. 1967;201:1004–7. [PubMed] [Google Scholar]
- 112.Young LS, LaForce FM, Head JJ, et al. A simultaneous outbreak of meningococcal and influenza infections. N Engl J Med. 1972;287:5–9. doi: 10.1056/NEJM197207062870102. [DOI] [PubMed] [Google Scholar]
- 113.Safadi MA, Cintra OA. Epidemiology of meningococcal disease in Latin America: current situation and opportunities for prevention. Neurol Res. 2010;32:263–71. doi: 10.1179/016164110X12644252260754. [DOI] [PubMed] [Google Scholar]
- 114.Wang JF, Caugant DA, Li X, et al. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People’s Republic of China. Infect Immun. 1992;60:5267–82. doi: 10.1128/iai.60.12.5267-5282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hart CA, Cuevas LE. Meningococcal disease in Africa. Ann Trop Med Parasitol. 1997;91:777–85. doi: 10.1080/00034989760536. [DOI] [PubMed] [Google Scholar]
- 116.Wyle FA, Artenstein MS, Brandt BL, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–21. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 117.Oster P, Lennon D, O’Hallahan J, et al. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191–6. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez AP, Dickinson F, Baly A, et al. The epidemiological impact of anti-meningococcal B vaccination in Cuba. Mem Inst Oswaldo Cruz. 1999;94:433–0. doi: 10.1590/s0074-02761999000400002. [DOI] [PubMed] [Google Scholar]
- 119.Galloway Y, Stehr-Green P, McNicholas A, et al. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int J Epidemiol. 2009;38:413–8. doi: 10.1093/ije/dyn228. [DOI] [PubMed] [Google Scholar]
- 120.Dull PM, Abdelwahab J, Sacchi CT, et al. Neisseria meningitidis serogroup W-135 carriage among US travelers to the 2001 Hajj. J Infect Dis. 2005;191:33–9. doi: 10.1086/425927. [DOI] [PubMed] [Google Scholar]
- 121.Lingappa JR, Al-Rabeah AM, Hajjeh R, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis. 2003;9:665–71. doi: 10.3201/eid0906.020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilder-Smith A, Goh KT, Barkham T, et al. Hajj-associated outbreak strain of Neisseria meningitidis serogroup Wl 35: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis. 2003;36:679–83. doi: 10.1086/367858. [DOI] [PubMed] [Google Scholar]
- 123.Hahne SJ, Gray SJ, Jean F, et al. W135 meningococcal disease in England and Wales associated with Hajj 2000 and 2001. Lancet. 2002;359:582–3. doi: 10.1016/s0140-6736(02)07716-4. [DOI] [PubMed] [Google Scholar]
- 124.Efron AM, Sorhouet C, Salcedo C, et al. W135 invasive meningococcal strains spreading in South America: significant increase in incidence rate in Argentina. J Clin Microbiol. 2009;47:1979–80. doi: 10.1128/JCM.02390-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koumare B, Ouedraogo-Traore R, Sanou I, et al. The first large epidemic of meningococcal disease caused by serogroup W135, Burkina Faso, 2002. Vaccine. 2007;25(Suppl 1):A37–41. doi: 10.1016/j.vaccine.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 126.Traore Y, Njanpop-Lafourcade BM, Adjogble KL, et al. The rise and fall of epidemic Neisseria meningitidis serogroup W135 meningitis in Burkina Faso, 2002–2005. Clin Infect Dis. 2006;43:817–22. doi: 10.1086/507339. [DOI] [PubMed] [Google Scholar]
- 127.Decosas J, Koama JB. Chronicle of an outbreak foretold: meningococcal meningitis W135 in Burkina Faso. Lancet Infect Dis. 2002;2:763–5. doi: 10.1016/s1473-3099(02)00455-3. [DOI] [PubMed] [Google Scholar]
- 128.Ouedraogo-Traore R, Hoiby EA, Sanou I, et al. Molecular characteristics of Neisseria meningitidis strains isolated in Burkina Faso in 2001. Scand J Infect Dis. 2002;34:804–7. [PubMed] [Google Scholar]
- 129.Materu S, Cox HS, Isaakidis P, et al. Serogroup X in meningococcal disease, Western Kenya. Emerg Infect Dis. 2007;13:944–5. doi: 10.3201/eid1306.070042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boisier P, Nicolas P, Djibo S, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–63. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 131.Gagneux SP, Hodgson A, Smith TA, et al. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. J Infect Dis. 2002;185:618–26. doi: 10.1086/339010. [DOI] [PubMed] [Google Scholar]
- 132.Harrison LH, Dwyer DM, Maples CT. Risk of meningococcal infection in college students. JAMA. 1999;281:1906–10. doi: 10.1001/jama.281.20.1906. [DOI] [PubMed] [Google Scholar]
- 133.Kaplan SL, Schutze GE, Leake JA, et al. Multicenter surveillance of invasive meningococcal infections in children. Pediatrics. 2006;118:e979–84. doi: 10.1542/peds.2006-0281. [DOI] [PubMed] [Google Scholar]
- 134.Shepard CW, Rosenstein NE, Fischer M. Neonatal meningococcal disease in the United States, 1990 to 1999. Pediatr Infect Dis J. 2003;22:418–22. doi: 10.1097/01.inf.0000066876.77453.04. [DOI] [PubMed] [Google Scholar]
- 135.Caugant DA. Genetics and evolution of Neisseria meningitidis: importance for the epidemiology of meningococcal disease. Infect Genet Evol. 2008;8:558–65. doi: 10.1016/j.meegid.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 136.Caugant DA, Maiden MCJ. Meningococcal carriage and disease – population biology and evolution. Vaccine. 2009;27:B64–B70. doi: 10.1016/j.vaccine.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]


