To the editor
Shrimp allergic subjects have IgE that binds tropomyosin, the major shrimp allergen (1, 2). Tropomyosin could also induce CD4+ T cell proliferation in shrimp allergic subjects (2, 3). Other shrimp allergens, including arginine kinase (4, 5), sarcoplasmic calcium-binding protein (6) and myosin light chain (7), have also been identified. However, little is known about specific T cells response towards these allergens. In this report, we examined T cell responses toward tropomyosin and arginine kinase in shrimp allergic and non-allergic subjects.
A total of 9 adults with history of allergic symptoms to shrimp and positive immunoCAP to shrimp extract were recruited for this study. Subjects recruited with shrimp allergy were associated with either the HLA-DRB1*03:01 (DR3) or HLA-DRB1*15:01-DRB5*01:01-DQB1*0602 (DR15) haplotype. Twelve shrimp non-allergic adults, including 7 non-atopic controls and 5 peanut allergic subjects without shrimp allergy, with matched HLA class II restriction were also recruited as controls (Table E1). The Tetramer Guided Epitope Mapping (TGEM) approach was used to identify CD4+ T-cell epitope for Pen m 1 and Pen m 2 (tropomyosin and arginine kinase from the black tiger shrimp Penaeus monodon respectively). Pen m 19-28 and Pen m 281-100 were identified as DRB1*03:01-restricted T-cell epitopes. Pen m 1161-180 and Pen m 2193-212 were identified as DRB5*01:01-restricted T-cell epitopes. Finally, Pen m 197-116 and Pen m 2265-284 were identified as DQA*01:02/DQB*06:02-restricted T-cell epitopes (Table E2 and Fig. E1).
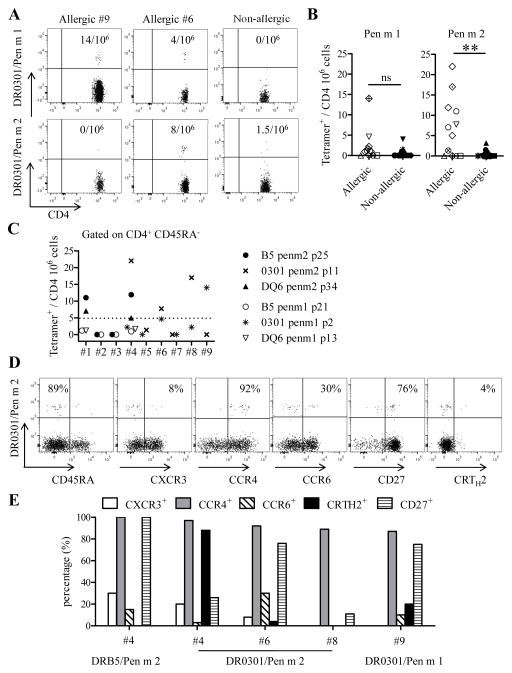
The anti-PE magnetic bead tetramer approach was used to detect shrimp allergen reactive T cells ex vivo in PBMC. Pen m 1 and Pen m 2 epitope-specific memory (CD45RA−) CD4+ T cells were detected in allergic but not in non-allergic adults ex vivo (Fig 1A, B and Fig E2A, E2B). However, the frequency of Pen m 1 epitope-specific CD4+ T cells was low and was not significantly different between adults with or without shrimp allergy (Fig 1B). In contrast, the frequency of Pen m 2 epitope-specific CD4+ T cells was significantly higher in adults with shrimp allergy compared to non-allergic adults (Fig 1B). Regarding each subject individually, Pen m 1 and Pen m 2 epitope-specific memory T cells were detected in 1 out of 9 and 4 out of 9 allergic subjects respectively with frequency higher than 5 cells per million CD4+ T cells (Fig 1C). These allergen specific T cells were not detected in the naïve T cell compartment in allergic or non-allergic subjects (Fig E2C). Detectable Pen m 1 and Pen m 2-specific CD4+ T cells in allergic subjects were associated with a memory phenotype CD45RA− and were CCR4+ CXCR3low, suggesting a TH2 phenotype (Fig 1D–E). Variable expression of CCR6, CRTH2 and CD27 were observed (Fig 1E) and T cells with CRTH2 expression were associated with low expression of CD27, which correspond to a terminally differentiated TH2 phenotype (8).
FIG 1. Frequency and phenotype of Pen m 1 and Pen m 2 epitope-specific CD4+ T cells.
(A) Ex vivo frequency of Pen m 1 and Pen m 2 epitope-specific CD45RA− CD4+ T cells in adults with or without shrimp allergy. The frequencies of specific T cells per million CD4+ T cells are as indicated. (B) Comparison of Pen m 1 and Pen m 2-specific CD4+ T cells frequency, for each epitopes described (data point), between 9 allergic adults and 12 non-allergic adults. Different symbols represent different subjects. (C) Frequencies of memory Pen m 1 and Pen m 2-specific CD4+ T cells for 9 allergic subjects. (D) PBMCs of an allergic adult were stained with phycoerythrin-labeled DRB1*03:01/Pen m 281-100 tetramers and a panel of antibodies. (E) Phenotype of Pen m 2 and Pen m 1-specific T cells for five allergic adults. Numbers indicate (Y axis) the percentage of tetramer positive cells that expressed a specific surface marker. Data were compared using Student’s impaired t-test (**: p≤0.01).
The CD154 up-regulation assay was also used to examine the overall T cell responses toward Pen m 1 and Pen m 2 on 7 out of 9 shrimp allergic individuals (available). Strong Pen m 1 and Pen m 2-specific responses correlated with our pMHCII tetramer staining in shrimp allergic individuals (Fig E3). These results confirmed that, in contrast to Pen m 1-derived epitopes, Pen m 2-derived epitopes are strongly immunogenic ex vivo in shrimp allergic subjects.
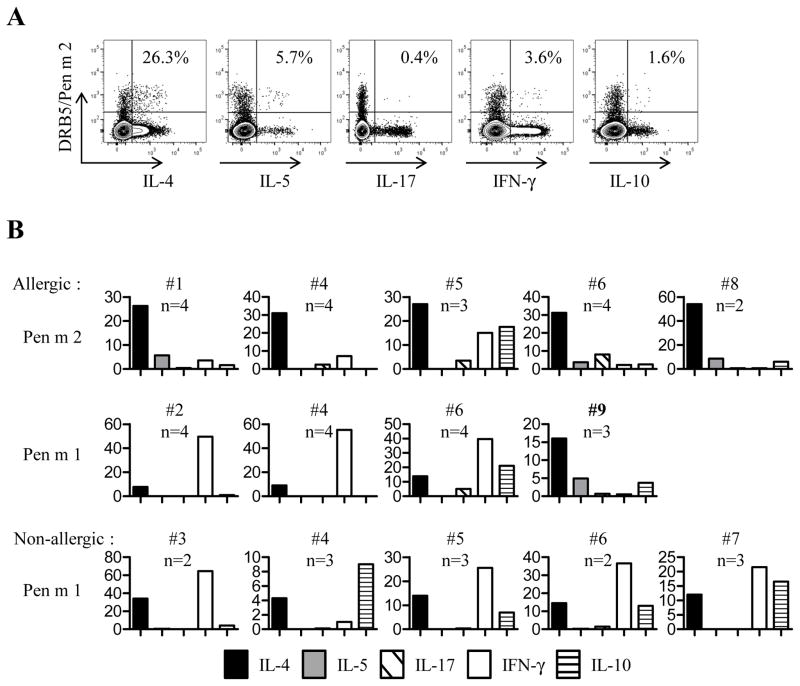
Pen m 1 and Pen m 2-specific T cell lines were generated by stimulating PBMCs from allergic subjects with antigenic Pen m 1 or Pen m 2 peptides for 2 weeks. Pen m 2-specific T cells lines were successfully generated only from allergic subjects (Fig 2 and Fig E4). All these specific T cells lines obtained produced IL-4 and IL-5, confirming the observed ex vivo TH2 phenotype (Fig 2A–B and Fig E4). Although, IL-4 was dominant response, there were Pen m 2-specific T cells lines that produced IL-17.
FIG 2. Intracellular cytokines staining of Pen m 1 and Pen m 2 epitope-specific T cells lines.
(A) Cytokine profile of a DRB5*01:01/Pen m 2 epitope-specific T cell line generated from allergic adult PBMCs. Percentages of tetramer positive cells that expressed the cytokine of interest are as indicated. (B) Graphs represent cytokine profiles of Pen m 1 and Pen m 2 epitope-specific T cells lines from allergic adults (top 2 rows) and from non-allergic subjects (bottom row). N indicated the number of allergen specific cell lines examined in each subject, and the average percentage of tetramer positive cells which produced the indicated cytokine is shown (Y axis).
We were also able to generate Pen m 1-specific T cells lines from allergic and non-allergic adults (Fig 2B and Fig E4). Only Pen m 1-specific T cells lines derived from allergic adult #9 (who present a significant accumulation of specific T cell ex vivo) produced IL-4 and IL-5, confirming the observed ex vivo TH2 phenotype (Fig 1E). All other Pen m 1-specific T cells lines, derived from either allergic or non-allergic adults, produced IFN-γ and or IL-10 (Fig 2B and Fig E4).
Recent publications show that tropomyosin can induce CD4+ T cell proliferation in shrimp-allergic patients (2, 3). However, it is unclear whether these proliferating cells are TH2 cells. Pen m 1 epitopes described in this current work include similar amino acid sequence as these previous studies. Only one allergic subject in our cohort presents detectable Pen m 1-specific T cells with a TH2 profile. The TH1-like profile of Pen m 1-specific T cells lines from the majority of shrimp allergic and non-allergic subjects suggest Pen m 1-specific T cells may not play a major role in mediating the TH2 responses in shrimp allergy in subjects with either the DR3 or DR15 haplotype. This TH1-like profile was previously observed in the T cell response against alder allergen in non-allergic subjects, which suggest a non-allergic response (8).
Pen m 1 from the black tiger shrimp, Penaeus monodon, has high amino acid sequence homology to other shrimp tropomyosin (Lit v 1, Pen a 1 and Met e 1) and other crustaceans tropomyosin. Moreover, shrimp tropomyosin has high sequence homology (>80%) to tropomyosin in cockroaches and house dust mites. IgE-cross-reactivity between shrimp and insect/dust mites’ tropomyosin was reported (9). Interestingly, all adults with shrimp allergy in our cohort were also sensitized to house dust mites (Table E1). Hence, it is possible that insect/dust mites’ tropomyosin sensitizes subjects to shrimp allergen in the absence of Pen m 1-reactive CD4+ T cells.
The current study suggests an important pathogenic role of Pen m 2-reactive T cells in shrimp allergic subjects with either the DR3 or DR15 haplotype. For further study of T cell responses to shrimp allergens, it is essential to examine T cell responses to tropomyosin, arginine kinase, sarcoplasmic calcium-binding protein and myosin light chain in a larger cohort. Understanding T cell responses to all these shrimp allergens is a prerequisite for rational design of future immunotherapy vaccine.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING: Supported by National Institutes of Health (NIH) contract HHSN272200700046C
We thank Jennifer Heaton, Lisa Myers Bulmash and Gladys Doronio for help with subject recruitment. We also thank Diana Sorus for assistance in preparing the article. And we thank Nadia Torres-Chinn for help with blood processing.
ABBREVIATIONS
- CRTH2
Chemoattractant receptor-homologous molecule expressed on TH2 cells
- MHCII
Major histocompatibility complex class II molecules
- Pen m
Penaeus monodon
- PBMCs
Peripheral blood mononuclear cells
- TGEM
Tetramer-guided epitope mapping
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. Journal of immunology. 1993;151(10):5354–63. [PubMed] [Google Scholar]
- 2.Wang S, Delgado JC, Ravkov E, Eckels DD, Georgelas A, Pavlov IY, et al. Penaeus monodon tropomyosin induces CD4 T-cell proliferation in shrimp-allergic patients. Human immunology. 2012;73(4):426–31. doi: 10.1016/j.humimm.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravkov EV, Pavlov IY, Martins TB, Gleich GJ, Wagner LA, Hill HR, et al. Identification and validation of shrimp-tropomyosin specific CD4 T cell epitopes. Human immunology. 2013;74(12):1542–9. doi: 10.1016/j.humimm.2013.08.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu CJ, Lin YF, Chiang BL, Chow LP. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. Journal of immunology. 2003;170(1):445–53. doi: 10.4049/jimmunol.170.1.445. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Orozco KD, Aispuro-Hernandez E, Yepiz-Plascencia G, Calderon-de-la-Barca AM, Sotelo-Mundo RR. Molecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus vannamei. International archives of allergy and immunology. 2007;144(1):23–8. doi: 10.1159/000102610. [DOI] [PubMed] [Google Scholar]
- 6.Shiomi K, Sato Y, Hamamoto S, Mita H, Shimakura K. Sarcoplasmic calcium-binding protein: identification as a new allergen of the black tiger shrimp Penaeus monodon. International archives of allergy and immunology. 2008;146(2):91–8. doi: 10.1159/000113512. [DOI] [PubMed] [Google Scholar]
- 7.Ayuso R, Grishina G, Bardina L, Carrillo T, Blanco C, Ibanez MD, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. The Journal of allergy and clinical immunology. 2008;122(4):795–802. doi: 10.1016/j.jaci.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. The Journal of allergy and clinical immunology. 2012;129(2):544–51. 51 e1–7. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopata AL, O’Hehir RE, Lehrer SB. Shellfish allergy. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2010;40(6):850–8. doi: 10.1111/j.1365-2222.2010.03513.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.