Abstract
Epidemiology reports state temporomandibular joint disorders (TMD) affect up to 25% of the population, yet their etiology and progression are poorly understood. As a result, treatment options are limited and fail to meet the long-term demands of the relatively young patient population. TMD are a class of degenerative musculoskeletal conditions associated with morphological and functional deformities. In up to 70% of cases, TMD are accompanied by malpositioning of the TMJ disc, termed “internal derangement.” Though onset is not well characterized, correlations between internal derangement and osteoarthritic change have been identified. Due to the complex and unique nature of each TMD case, diagnosis requires patient-specific analysis accompanied by various diagnostic modalities. Likewise, treatment requires customized plans to address the specific characteristics of each patient’s disease. In the mechanically demanding and biochemically active environment of the TMJ, therapeutic approaches capable of restoring joint functionality while responding to changes in the joint have become a necessity. Capable of integration and adaptation in the TMJ, one such approach, tissue engineering, carries significant potential in the development of repair and replacement tissues. The following review presents a synopsis of etiology, current treatment methods, and the future of tissue engineering for repairing and/or replacing diseased joint components, specifically the mandibular condyle and TMJ disc. Preceding the current trends in tissue engineering is an analysis of native tissue characterization, toward identifying tissue engineering objectives and validation metrics for restoring healthy and functional structures of the TMJ.
Keywords: TMJ, TMD, TMJ Disc, Condyle, Cartilage, Tissue Engineering
INTRODUCTION
The temporomandibular articulation is composed of bilateral, diarthrodial, temporomandibular joints (TMJs). Each joint is formed by a mandibular condyle and its corresponding temporal cavity (glenoid fossa and articular eminence), as seen in Fig. 1. The TMJ and its associated structures play an essential role in guiding mandibular motion and distributing stresses produced by everyday tasks, such as chewing, swallowing, and speaking. TMJ disorders (TMD) are a class of degenerative musculoskeletal conditions associated with morphological and functional deformities.1, 2 TMD include abnormalities of the intra-articular discal position and/or structure as well as dysfunction of the associated musculature.3 Symptoms and signs include painful joint sounds, restricted or deviating range of motion, and cranial and/or muscular pain known as orofacial pain.
Figure 1.
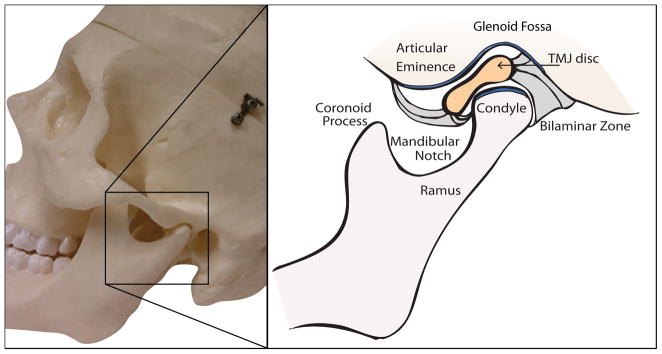
Temporomandibular joint sagittal schematic.
While up to 25% of the population may experience symptoms of TMD,4 only a small percentage of afflicted individuals seek treatment. For instance, studies in the 1980s detected TMD symptoms in 16% to 59% of the population,5 although only 3% to 7% of the adult population actually sought care for pain and dysfunction associated with TMD.6 Furthermore, TMD symptoms occur disproportionately between the sexes with a much higher incidence reported in females; female to male ratios range between 2:1–8:1.4, 7–9 Most patients presenting symptoms are between 20 and 50 yrs of age,9–11 an unusual distribution for a disease that is considered a degenerative disorder.11
Up to 70% of TMD patients suffer from pathology or malpositioning of the TMJ disc, termed “internal derangement” (ID).12 While disease progression is poorly understood, the primary pathology appears to be a degenerative condition, known as osteoarthritis (OA) or osteoarthrosis, depending on whether inflammatory or non-inflammatory states exist, respectively. In a study of patients presenting unilateral TMD pain symptoms during function, palpation, and assisted or unassisted mandibular opening (n=131), it was reported that 54.2% of individuals showed osteoarthritis in the affected joint.13 Asymptomatic patients, whose discs are identified by magnetic resonance imaging (MRI) in the “normal” anatomical position, show minimal morphological change in the condyle and articular eminence in light of normal adaptive processes. In contrast, substantial osseous change is observed in symptomatic patients with ID.14 Osteoarthritic changes observed during TMD include deterioration and abrasion of articular cartilage, and thickening and remodeling of underlying bone.1 In TMD patients, it is readily apparent that once joint breakdown commences, OA can be crippling, leading to morphological deformity and functional obstruction.1
As related to Wilkes’ stages of internal derangement of the TMJ,9 management options vary with respect to the severity of degeneration. Non-invasive and minimally invasive options exist for patients in the early stage of ID progression. Minimally invasive and sub-total reconstruction options exist for intermediate stage patients. Fully invasive, total joint replacements are the only option currently available for patients in late stage ID progression. Unfortunately however, many patients require repeat or follow-up surgery, indicating little promise for the long-term success of this management option.
The following review presents disease etiology, diagnosis, and management with an emphasis on the future of tissue engineering for joint reconstruction. Inherently, a discussion of native TMJ tissue characterization precedes review of the current progress in tissue engineering, as native tissue characterization is essential to identifying design objectives and validating progress.
DISEASE ETIOLOGY AND DIAGNOSIS
Remodeling of the load-bearing joints is an essential adaptation process needed for appropriate stress distribution and function. It has been established that, while progressive and regressive, mechanically-induced remodeling is a normal process early on. When the capacity for the joint to remodel has been exceeded, remodeling merges into osteoarthritis.15, 16 Characteristic osteoarthritic changes observed in the TMJ include alterations in shape and overall size of joint components, specifically, flattened fossa, less pronounced articular eminence, decreased condylar volume and thickened disc, see Fig. 2.15 Degenerative remodeling present in pathologic TMJs may result from either decreased adaptive capacity in the articulating structures or from excessive or sustained physical stress to the articulating structures.3, 17, 18 Important to our understanding of TMD etiology, such degenerative changes have been correlated with internal derangement of the TMJ disc.
Figure 2.
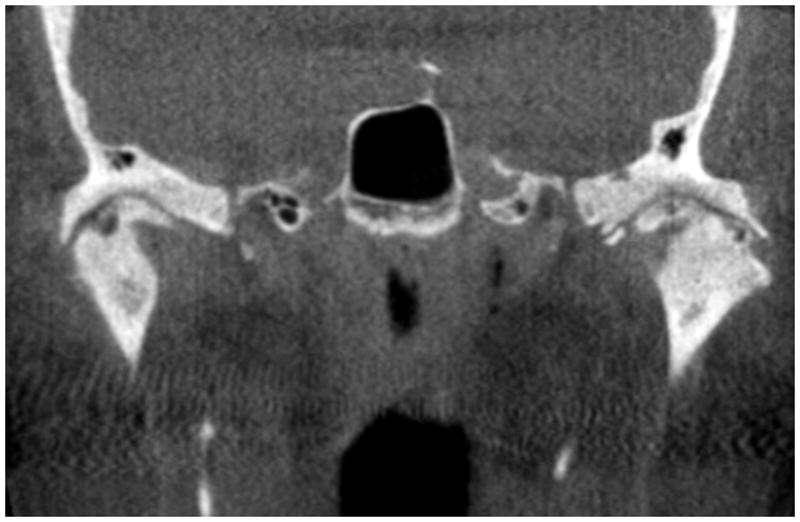
Bilateral TMJ degeneration. Coronal TMJ CT scan depicting signs of osteoarthritis. Superficial erosions and osteophytes present in the left joint (right side) and a sub-chondral cyst present in the right joint (left side).
While the simultaneous or subsequent progression of ID and OA is not completely understood, it is established that a correlation exists between the two. In the previously mentioned study of patients reporting unilateral orofacial pain referred to or within the TMJ during palpation, function, and assisted or unassisted mandibular opening, a significant relationship was identified between MRI diagnosis of TMJ ID and TMJ OA.13 In light of the degenerative changes observed most commonly, including erosion of the articulating surfaces, followed by flattening and reformation, it is considered more plausible that ID precedes OA, rather than the reverse.9, 19, 20 Corroborating this hypothesis, a series of rabbit studies showed surgically induced ID led to degenerative changes in the condylar cartilage.21 In a third possibility, ID and OA are initiated simultaneously in response to a causative event. This possibility has been explored, and it was shown that excessive loading produced by postero-superior displacement of the rabbit mandible can cause simultaneous ID and OA onset in the rabbit TMJ.22 Though studies have yet to determine the cause and effect relationship, a clear correlation exists between displacement of the TMJ disc and development of OA. Until progression is better understood, treatment modalities must address all possible scenarios.
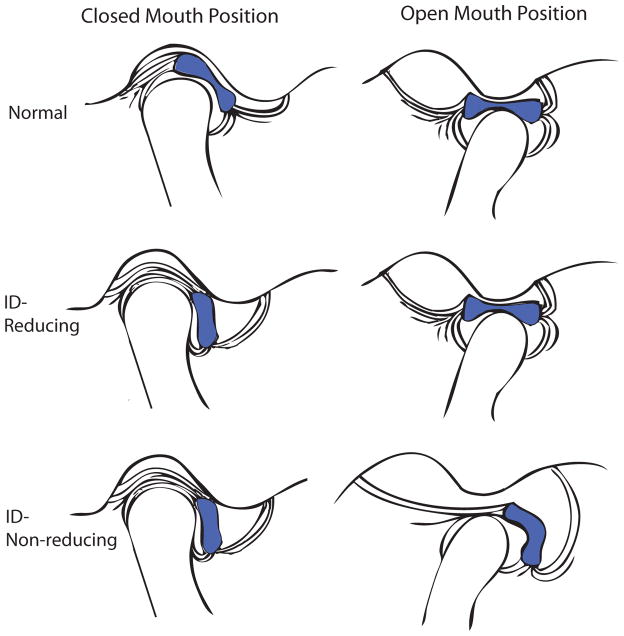
Although the onset of TMD is poorly understood, Wilkes9 has established a five stage system for classifying the progression of internal derangement based on clinical and imaging criteria. A schematic depicting anterior disc displacement, as described by Wilkes’ stages, may be seen in Fig. 3. In Stage I, clinical observations include painless clicking early in opening and late in closing with unrestricted mandibular motion. Imaging observations indicate slight forward displacement of the disc, with passive incoordination as the disc returns to the “normal” anatomical position (ID-reducing). Osseous contours appear normal. In Stage II, symptoms include occasional pain with clicking, intermittent locking, and orofacial pain. Imaging shows slight deformation of the disc and slight forward displacement but as in Stage I, the disc reduces to the “normal” position at maximal opening. Osseous contours again appear normal. Stage III, on the other hand, is associated with frequent orofacial pain as locking becomes more frequent and mandibular motion becomes restricted. When imaged, the disc is clearly displaced anteriorly to its “normal” anatomical position. Moderate disc thickening is also apparent. Early in Stage III the disc reduces at maximal opening but fails to do so as the stage progresses (ID-non-reducing). In this case, at maximal opening (terminal translation) the disc deforms in response to the condyle pushing forward and downward on it. The osseous contours, however, remain normal in appearance. In Stage IV, contours begin to change. Clinical symptoms include chronic pain and restricted mandibular motion. Observed during imaging, the displaced disc is markedly thickened and does not reduce upon maximal opening. Imaging also shows evidence of abnormal bony contours on the condyle and articular eminence. Stage V, the most advanced stage, is associated with similar clinical and imaging observations as Stage IV, but with more significant progression. Patients with Stage V degeneration experience chronic pain, crepitus, and significantly restricted range of motion. Imaging shows gross deformation and thickening of the non-reducing, anteriorly displaced disc, as well as degenerative changes. These changes include abrasion of the articular cartilage and disc surfaces, as well as thickening and remodeling of the underlying bone.
Figure 3.
Internal derangement of the TMJ. Normal: Normal anatomical position of articulating disc with respect to condyle and surfaces of articulation. ID-Reducing: Anteriorly displaced disc returning to normal anatomical position upon maximal opening (Wilkes Stage II-early Stage III). ID-Non-reducing: Anteriorly displaced disc during closed and maximal opening positions with disc thickening present (Wilkes late Stage III-Stage IV).
Clinical observations demonstrate that numerous factors may play a role in the progression of TMD and associated degenerative changes. Thus, each TMD case much be treated uniquely. Such factors include the independent or interrelated roles of trauma, parafunction, unstable occlusion, functional overloading, and increased joint friction.3, 17, 18, 23, 24 The respective roles of each of these potential components are controversial, however, as direct cause and effect relationships have not been determined with consistency. For example, overloading the joint through excessive or unbalanced stress may result in the onset and progression of OA as well as ID. However, contributions are difficult to establish due to the significant time necessary for degeneration to occur in the face of small changes in loads. Also demonstrating the lack of causal relationships, while some patients with dental malocclusions do progress to clinically significant TMD, many do not. It is clear that little is known about the independent or interrelated roles of each of these factors. If treatment is to include reconstruction with biological tissues, we must attempt to recognize and address all factors potentially contributing to joint degeneration. Consequently, each patient needs to be analyzed uniquely and treatment approaches customized to address specific characteristics of the disease.
Resulting from the diverse nature of TMD symptoms, patient evaluation often requires a physical examination along with various imaging modalities. As previously mentioned, there exists a population of individuals experiencing unilateral or bilateral disc displacement (presence or absence of joint noises) and minimal osseous change, but these individuals have not progressed to clinically relevant TMD.14, 26–28 Therefore, various diagnostic modalities, including clinical and radiological examination, may be necessary to identify the stage of degeneration in patients presenting with possible TMD symptoms. Steadfast rules remain to be established regarding imaging for TMD diagnosis under the current Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD). As result, TMD identification may involve any combination of the following modalities: MRI, conventional and computed tomography (CT), plain and panoramic radiography, arthrography, a thorough history, and physical examination. CT is considered most beneficial for imaging bone and OA, while MRI is considered most beneficial in imaging soft tissues, including the disc and its joint relation.25, 26 Patient evaluation, together with various imaging modalities, may help to elucidate a patient’s stage of degeneration, aiding in diagnosis and treatment planning.
CLINICAL MANAGEMENT
For patients seeking management of TMD symptoms, it has been established that non-invasive modalities should first be explored. However, the complicated nature of the TMJ, along with the debilitating nature of late stage disease, has created a demand for more invasive solutions. An analysis of current non-invasive, minimally invasive, and fully invasive management options now follows. The ultimate goals of the presented modalities are to: 1) increase mandibular range of motion, 2) decrease joint and masticatory muscle pain and inflammation, and 3) prevent further degenerative change in articulating tissues, including direct or indirect joint damage.3
Non-Invasive
The non-invasive modalities implemented most commonly include physical therapy, occlusal splints and/or adjustments, and pharmacologics. Beginning first with physical therapy, electrophysical modalities and manual/exercise techniques are used to relieve pain in the joint and masticatory muscles, and improve range of motion.27 Physical therapists may complement these techniques with behavioral changes by drawing awareness to the patient’s posture, diet, and stress-related habits. Electrophysical modalities include transcutaneous electric nerve stimulation (TENS), ultrasound, and laser.28 Such modalities are implemented to reduce inflammation, increase local blood flow, and promote muscle relaxation.28 Current research does not point to any significant decrease in pain in electrophysically treated patients. In fact, one study of 23 bruxists showed a significant increase in range of motion and a decrease in muscular activity with muscular awareness relaxation training over the TENS treatment group.29 Manual therapies designed to increase mobility and reduce pain have shown promise and are often used in conjunction with exercise techniques. Such exercise techniques work to strengthen and improve mobility in the masticatory and cervical spine muscles.30 Furthermore, these techniques offer the potential to “re-teach” and rehabilitate the musculature. This observation is especially noted in patients exhibiting stress-related habits.31 Along with exercise techniques, postural exercises may aid in alignment of the craniomandibular system. Intended to relieve pain associated with TMD and improve range of motion, physical therapy treatment plans must be patient-specific and may involve a combination of modalities.
Also non-invasive, occlusal splints and occlusal adjustments work to establish balance in the occlusion and TMJs. The occlusion, or bite position, is a third and important element in the joint system and is the element often addressed by general dentists. Adjustments and splints may be used to achieve the most stable and least joint- traumatizing bite position. The ultimate goal of splints and adjustments is to minimize pain in the joint and masticatory muscles by establishing stability. Furthermore, as reviewed by Ingawale and Goswami,32 splints may be used to control bruxism, which has been associated with tooth attrition, malocclusion, myofacial pain, and masticatory muscle strain, fatigue, and fibrosis. The literature has shown mixed results associated with splint use. These results are not surprising considering that the role of malocclusion in TMD progression remains poorly understood. Occlusal splints and adjustments may be suggested to reestablish balance in the joint system, but the long-term effectiveness of this therapy remains controversial.32
Regarding pharmacologic agents, commonly prescribed non-steroidal anti-inflammatory drugs (NSAIDs) offer advantages in reducing inflammation. Research, however, is needed to exploit long-term use and to identify whether the advantages in management of pain and inflammation outweigh the negative side effects.33 Muscle relaxants may also be prescribed for treatment of muscle pain and/or spasm.34 However, studies have failed to demonstrate that muscle relaxants are any more effective in pain relief than NSAIDs.35 To improve their benefit, muscle relaxants are often used in combination with NSAIDs. NSAIDs may therefore be recommended for their anti-inflammatory and analgesic benefits yet further research is needed to elucidate the benefits and risks of both short and long-term use.
Minimally Invasive
Minimally invasive modalities for management of TMD symptoms include sodium hyaluronate and corticosteroid injections, arthrocentesis, and arthroscopy. Injections of corticosteroids and high molecular weight sodium hyaluronate in the superior joint space are designed to treat osteoarthritic symptoms. With research indicating both regenerative and degenerative responses to such injections, their use remains controversial.34 The pathophysiology of the disease indicates there may be more significant potential for these injections in early stages of degeneration when inflammation first begins to exacerbate tissue catabolism.3, 36
Similar to intra-articular injections, arthrocentesis and arthroscopic surgery are minimally invasive techniques requiring entrance into the joint capsule to lubricate articulating surfaces and reduce inflammation. During arthrocentesis, a sterile needle is used to drain fluid from the joint.37 After draining, the joint is flushed of debris and inflammatory cytokines using a sterile solution.37 During the procedure, the physician may also attempt to restore some range of motion with mandible manipulation.38 Through arthroscopic surgery, a slightly more invasive procedure, the surgeon may break intra-articular adhesions that may be preventing disc reduction in ID patients.39 With joint visualization during surgery, arthroscopy offers advantages in TMD stage diagnosis and identification of OA. While arthroscopic surgery and arthrocentesis may be used to lubricate joint surfaces and reduce inflammation, further research is needed to identify long-term advantages especially in the absence of disc repositioning or replacement.38, 40
Invasive
For the 5% of TMD patients whose nonsurgical methods fail, open joint surgery may be necessary to restore mandibular motion and mitigate orofacial pain.41 Most commonly, open joint surgery may include discectomy, reshaping or reconstruction of the articulating surfaces, and implantation of autologous or alloplastic materials.42 Total joint replacement, the most invasive option, may become necessary when joint degeneration and pain exceed the potentials of the less invasive surgical methods. Condylar replacements in clinical use include autologous costochondral grafts, but autologous full joint replacements are not currently available. Alloplastic joint replacement systems, including total joint prostheses and hemiarthroplasties, have been in development since the 1960s. The currently available systems have, however, seen substantial modifications since their inception.
Discectomy and Disc Replacement
In TMD patients presenting with limited range of motion, discectomy offers one means of regaining mandibular motion and reducing orofacial pain, and may be followed by disc replacement. Discectomy has been shown in 5 and 10 yr post-operative follow-ups to increase mandibular motion in patients previously showing no improvement with non-invasive management modalities.43, 44 Radiographic changes in these long-term studies indicate evidence of osteophytes and flattening of articular surfaces in such joints.43–45 Though the mechanism is poorly understood, some authors conclude such changes are indicators of adaptive change rather than degenerative disorders.43–45 In some patients, however, OA-like changes continue to exacerbate, necessitating the development of autologous and alloplastic disc substitutes. Such substitutes, including subcutaneous fat grafts and alloplasts, are aimed at providing a protective cushion for the articulating surfaces of the joint during rotation and translation. Unfortunately, previous attempts with alloplastic disc replacements have often failed.46, 47 Likewise, fat grafts may not sufficiently protect the articulating surfaces. Often, following implantation, the graft is displaced posterior to the condyle.48 The lack of clinical success associated with disc replacement therapies may be the result of varying responses to the respective materials used. For example, with certain alloplasts, most notably the composite Teflon-Proplast implant, degradation of the implant material led to particulate debris that stimulated an osteolytic local foreign body reaction. It was observed that this response eventually led to resorption of the condylar head and fossa, producing perforations in the middle cranial fossa. Other more inert materials, such as silicone-based disc implants, produced a fibrotic response resulting in capsule formation around the implant. Progression of this reaction led to restricted movement of the joint due to the development of an intra-articular scar band. A similar response has also been noted with the use of interpositional fat grafts. If the fat becomes de-vitalized, it undergoes replacement with fibrous tissue and the resultant scar reduces movement of the joint. Patient experience with disc replacement demonstrates the unanswered need for autologous tissue replacements, capable of function in the complex loading environment of the TMJ. While discectomy may be implemented to improve mandibular range of motion, patients experiencing continued joint degeneration reveal the need for a functional, non-pathogenic disc replacement.
Joint Reconstruction
Several techniques have been proposed for reconstruction of portions of the joint or the entire joint itself. For sub-total reconstruction, a hemiarthroplasty may be used to replace the superior articulating joint surface.47 During reconstruction, joint adhesions are lysed and a vitallium alloy fossa-eminence prosthesis, manufactured by TMJ Implants, is implanted to replace the temporal component of the joint. As reviewed by McLeod et al.,49 a hemiarthroplasty can produce successful results in patients where the condyle is unaffected by severe degenerative changes. Importantly though, condylar change often accompanies degenerative change in the temporal component of articulation. In this case, total joint reconstruction may be necessary.
Total Joint Reconstruction
Reconstruction of the entire joint is indicated when a substantial portion of the joint is lost. Such loss can result from joint removal due to pathology, joint destruction due to trauma, or significant degeneration in the articulating surfaces of the joint, resulting in skeletal changes and malocclusion. Severe degeneration is seen in acute, local osteoarthritis, and in patients with systemic conditions such as rheumatoid disease, where progressive bone and cartilage loss occurs. If immune-mediated processes are not present, a costochondral graft permits a comprehensive reconstructive option in which autologous costochondral segments replace the condyle with a biological graft. The costochondral graft has histological and morphological similarities to the condyle. Further, as a native tissue, its inherent adaptability and lack of immunogenic potential offer significant advantages over alloplastic materials.50–52 The results of costochondral grafting, however, are varied. When used to treat defects caused by pathology or trauma, excellent functional results are seen, even in the presence of significant long-term resorption of the graft. It appears that compensatory changes in the associated musculature and the dentition accommodate for loss of the graft. When costochondral grafts are used to reconstruct patients with TMD, on the other hand, results are less than ideal. Loss of vertical height produced by graft resorption leads to a recurrence of both joint and muscle pain. Alloplastic alternatives appear to be better suited for the treatment of these patients and those with immune-mediated degenerative processes. The three currently available FDA approved alloplastic total joint replacement systems include The Christensen Total Joint system, the TMJ Concepts system, and the Biomet Microfixation prosthetic total joint. A review of the history and current use of alloplastic devices is available in the literature.53 Implant lifetimes are in the range of 10–15 yrs,32 and considering the average age of TMD patients, secondary surgery is often necessary. Specifically, early degradation and local debris may require follow-up or repeat surgery. When a substantial portion of the joint is lost, costochondral or alloplastic systems may be used for reconstruction, but, the young patient population and the dynamic environment of the TMJ necessitate improved treatment options. Based on previous experiences, an ideal replacement system will meet the functional demands of the joint system and maintain its integrity and functionality throughout the duration of the patient’s lifetime.
Currently, the repair and replacement of pathologic TMJ tissues remains an unmet need and tissue engineering presents long-term promise for meeting this demand. Considering the absence of symptoms in some ID patients, and the success of costochondral grafts despite graft resorption in certain patients, it is clear that the TMJ and associated musculature represent an adaptive environment capable of constant remodeling. While in the past 10 yrs significant strides have been taken in the development of joint reconstruction systems, the need remains for tissue replacements capable of adaptation, possessing the biochemical, biomechanical, and geometric properties of healthy TMJ tissues. This challenge may be met using tissue engineering techniques to produce joint components with the ability to adapt to mechanical and chemical stimuli produced by functional articulation.
CURRENT PROGRESS IN TISSUE ENGINEERING
Engineering tissue replacements for the diseased structures of the TMJ may offer a permanent, natural solution to regain function in the joint and eliminate problematic and often painful TMD symptoms. Though tissue engineering of the TMJ is in its infancy, significant steps have been taken toward understanding appropriate cell sources, biochemical and biomechanical signals, and scaffolding for developing condylar and discal cartilage. Engineering tissues matching the native geometric, biochemical, and biomechanical properties of healthy joint tissues requires a thorough understanding of native tissue characteristics. The following sections will outline design objectives and current strategies for condylar as well as discal tissue engineering, as depicted in Fig. 4.
Figure 4.
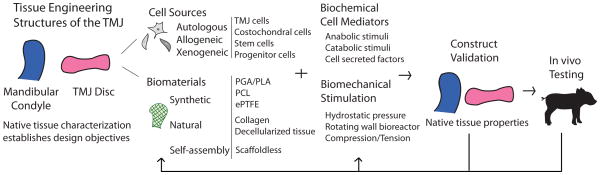
TMJ tissue engineering strategy. Tissue engineering approach to repairing or replacing the mandibular condyle and TMJ disc.
Condylar Cartilage Characterization
Thus far, research in tissue engineering of condylar cartilage has exploited a variety of cell sources, bioactive signals, and shape-specific scaffolds. To-date shape-specific osteochondral condyle tissue replacements have been validated in vivo in small animal models.54–57 However, the future of condyle/ramus and osteochondral tissue replacements will require demonstrating long-term efficacy in large animal models. As reflected by the literature, validation of such engineered replacement tissues is based upon comparison with native biochemical and biomechanical tissue properties. The following section contains a review of native condyle anatomy, cell type, extracellular matrix (ECM) composition and biomechanical properties, followed by a synopsis of current condylar tissue engineering strategies.
From an anatomical standpoint, the condyle is longer mediolaterally than anteroposteriorly, forming an ellipse in the transverse plane. Fibrous connective tissue extends from the periphery of the disc, securing the disc to the condyle inferiorly and to the temporal bone superiorly. This arrangement of connective tissue forms a fluid-filled joint capsule with two discrete compartments. Anteriorly and posteriorly, the condyle connects to the TMJ disc via the capsular ligaments while mediolaterally, the condyle connects to the disc via the collateral ligaments. This arrangement ensures close contact between the disc and condyle during joint movement. The condyle is formed by the condylar process of the mandibular bone and is covered superiorly by a layer of zonal cartilage. The mandibular bone is comprised of cancellous bone and a layer of compact cortical bone. Generally speaking, the cartilage may be described by four distinct zones: fibrous, proliferative, mature, and hypertrophic. The proliferative zone separates the fibrocartilage of the fibrous zone from the hyaline cartilage of the mature and hypertrophic zones.58 Anteroposteriorly, the cartilage layer is thickest in the central superior region: 0.4–0.5 mm in the human.59 As the anatomical nature of this tissue is better characterized, engineering efforts may more successfully develop shape-specific, layered (osteochondral) implants.
Histological and biochemical evidence of cell type and ECM characteristics demonstrate the mandibular condyle is composed of a fibrocartilage, rich in type I collagen. The cellularity and biochemical content will now be described by zone, beginning most superiorly. This arrangement may be seen schematically in Fig. 5. The fibrous zone is cellularly composed primarily of low density fibrochondrocytes. The primary ECM component identified in rats60–62 and pigs63 is type I collagen, while type II collagen is minimally observed.60, 61, 63, 64 An anisotropic, anteroposterior fiber organization has been observed, similar to that of the disc.65, 66 Porcine67 and rat68 studies have identified the primary proteogylcan comprising this zone to be similar in nature to versican, consisting almost exclusively of chondroitin sulfate GAGs. Inferior to the fibrous zone is the proliferative zone. This zone acts as a cell reservoir containing mesenchymal chondrocyte precursor cells. To this effect, the proliferative zone is highly cellularized and the matrix is minimally developed. Type I collagen has been detected in this zone, observed most often as scattered fibers.62, 69 Similar to the fibrous zone, immunohistochemistry has identified versican-like chondroitin sulfate as the primary proteogylcan in the proliferative zone.67 The mature and hypertrophic zones are similar to one another in their cellularity and ECM composition. These two layers are cellularized by mature chondrocytes. Chondrocytes of the hypertrophic zone, however, are larger. The ECM in both zones is comprised primarily of type II collagen,60 yet type I and X have also been identified.64 Collagen organization in the mature and hypertrophic zones is isotropic, showing random bundle orientation.62, 70 Furthermore, aggrecan has been identified as the primary proteogylcan in these zones in porcine67 and rat64 models. Significantly, the articulating surface of the mandibular condyles is largely fibrous (rich in type I collagen), which is in contrast to the hyaline nature of other articulating surfaces, such as those found in the knee and hip.
Figure 5.
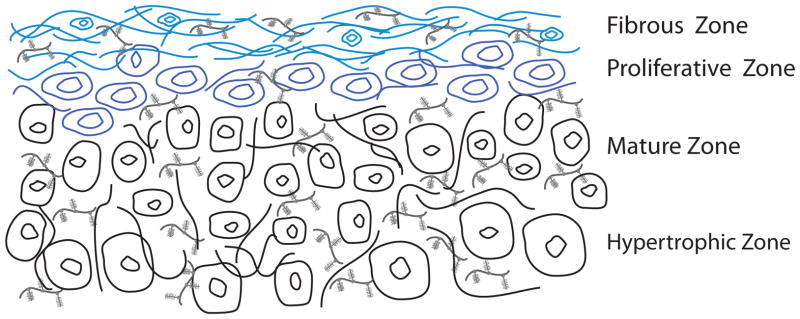
Zonal condylar cartilage schematic. Fibrous zone: anisotropic, anteroposterior fiber orientation (predominantly type I collagen and versican-like proteoglycans). Proliferative zone: predominantly cellular with minimal matrix. Mature and hypertrophic zones: isotropic, random fiber orientation (predominantly type II collagen and aggrecan proteoglycans).
Illustrating a structure-function relationship, biomechanical evidence suggests the condyle is stiffer under tension in the anteroposterior direction than in the mediolateral direction. In the porcine model, Young’s modulus has been measured as 9.0±1.7 MPa in the anteroposterior direction and 6.6±1.2 MPa in the mediolateral direction under axial tension to failure (n=8).71 This mechanical behavior agrees with Singh and Detamore’s66 work which identified anisotropic collagen alignment. This group also obtained moduli ranging from 22–29 MPa in the anteroposterior direction and 8–11 MPa in the mediolateral direction.66 Shear studies have likewise confirmed the anisotropy of mechanical behavior. Storage moduli in dynamic shear experiments at 2 Hz frequency range from 1.50–2.03 MPa in the anteroposterior direction, yet range from 0.33–0.55 MPa in the mediolateral direction (n=17).72 The anisotropic collagen orientation, tensile, and shear properties of the mandibular condyle suggest anteroposterior loading, matching the loading patterns observed during translation and rotation of the mandible in vivo.
Though compressive structure-function relationships have yet to be revealed for the condyle, regional variability has been established and likely contributes to specific condylar function. Compressive properties have been examined via atomic force microscopy (AFM), indentation testing, and unconfined compression. In one study of regional variability, rabbit condylar cartilage was divided into four regions and tested in compression using AFM.73 Young’s modulus and Poisson’s ratio were both revealed to decrease in magnitude as follows: greatest in the anteromedial region, followed by the anterolateral, then by the posteromedial, and finally lowest in posterolateral region. Notably, results suggest the condylar cartilage is stiffer medially than laterally.73 It has also been shown that porcine condylar cartilage deforms significantly less under intermittent compression than sustained compression,74 an expected result in light of the dynamic nature of the joint. In two other studies, aggregate moduli from in situ creep testing75 and equilibrium moduli from unconfined compression testing76 were reported. Creep testing demonstrated the greatest aggregate moduli in the central and medial positions, with the aggregate modulus of the medial position significantly greater than that of the lateral and anterior positions. Equilibrium moduli obtained during unconfined testing demonstrated the greatest stiffness in the posterior region and the greatest compliance in the anterior region.76 Although a consensus regarding the specific regional biomechanical variability remains to be established, these data suggest that the joint sustains significant load in the medial and posterior regions in vivo and more successfully resists cyclic, rather than sustained loading, a factor that may contribute to TMD progression.
Tissue Engineering Condylar Cartilage
Tissue engineering initiatives attempting to recapitulate the native condylar cartilage follow a three-part approach considering cell sourcing, biomaterials for construct scaffolding, and bioactive stimuli. Beginning first with cell sourcing, adult condylar cartilage cells have been explored in most detail in the literature. However, it is important to note the significant donor site morbidity and potential pathology in TMD patients associated with this cell source. As research progresses, it is expected that alternative primary and stem cells will receive more significant attention. Nonetheless, due to their appropriate phenotype, condylar chondrocytes offer an effective starting point for condylar cartilage engineering strategies. Among others, two distinct strategies have been established for acquiring primary condylar cartilage cells. The more common strategy for obtaining primary cells involves harvesting, mincing, and isolating condylar cells via a collagenase treatment.77 In contrast, a second procedure allows the cells to migrate out of the fibrous zone of condylar tissue onto surgical sponges yielding fibroblast-like cells upon isolation.78 Considering alternative cell sources, most recently, ankle hyaline cartilage cells have been determined to outperform condylar cartilage cells in terms of biosynthesis and cell proliferation when seeded in three dimensional non-woven polyglycolic acid (PGA) meshes,63 though the authors cited non-adherence of condylar cells as a possible factor in their relatively poor performance. The hyaline cartilage-seeded scaffolds yielded a more fibrocartilaginous tissue with both type I and II collagen. In contrast, condylar cartilage-seeded scaffolds yielded a more fibrous tissue which predominantly stained positive for type I collagen.63 This is not a surprising result considering the hyaline nature of the articulating cartilage of the ankle as compared to the fibrous nature of the cartilage of the TMJ condyle. Prior to this work, the same group explored human umbilical cord matrix stem cells (HUCMs). HUCM constructs were found to yield 55% and 200% higher cellularity at week 0 and 4 wks, respectively, as well as higher GAG content over condylar cartilage constructs.79 Due to donor site morbidity and tissue engineering challenges associated with condylar cartilage cell sourcing, it is apparent that researchers have begun to turn their attention toward alternative sources. More work is needed to exploit these potential sources, but promise exists in the arena of progenitor, mesenchymal, embryonic, and induced pluripotent stem cells.
Research in scaffold selections primarily surrounds the idea of developing shape-specific scaffolds. For example, the Hollister group80 has demonstrated polycaprolactone (PCL), bioresorbable scaffolds may be constructed by solid free-form fabrication techniques based on CT (or alternatively MRI) imaging data to generate an anatomically-shaped mandibular condyle scaffold that attaches to the ramus via a collar. Seeded with bone morphogenetic protein-7 transformed fibroblasts, the group obtained compressive moduli and yield strengths in the lower range of reports for human trabecular bone.80 A second study from the same group demonstrated that biphasic PCL scaffolds may be differentially seeded with transformed fibroblasts and fully differentiated chondrocytes.57 This strategy yielded differential tissues with a mineralized interface when implanted subcutaneously.57 More recently, the presence of blood vessels, marrow stroma, and adipose tissue was demonstrated in the ceramic phase of these scaffolds, representing the region seeded with transformed fibroblasts.56 In an alternative strategy for developing shape-specific scaffolds, the Mao group54, 55 has demonstrated the potentials of sequential photopolymerization of poly(ethylene glycol) hydrogels. This strategy was used to obtain osteochondral constructs with shape and dimensions matching those of a human cadaveric mandibular condyle model.55 Importantly, this group has demonstrated the potentials of inducing differentiation of primary bone-marrow derived mesenchymal stem cells into chondrocyte and bone lineages for the development of stratified bone and cartilage layers.54, 55 As can be seen, there is a plethora of biomaterials that may be implemented for condylar tissue engineering, some offering patient-specific morphology.
In vitro culture techniques may include the application of biomechanical stimulation intended to mimic physiological loading conditions and therefore influence ECM architecture. Current efforts with bioreactors and direct stimulation have attempted to do so, specifically with the intention of encouraging cell growth and recreating the ECM architecture of healthy condylar cartilage. For example, mass transfer bioreactors can be used in culture toward obtaining a homogeneous cell distribution and improved nutrient and waste transport over static cultures. Rotating wall bioreactors stimulate cell proliferation and biosynthesis without causing cell damage, by exposing cells to a low shear force via laminar flow. Similarly, spinner flasks accelerate the exchange of oxygen and nutrients in the interior of scaffolds, improving cell proliferation and matrix synthesis. Hydrostatic and direct compression loading schemes may potentially be used to stimulate matrix deposition, improving mechanical properties of engineered condylar cartilage.81 With in vitro characterization identifying the tissue to deform significantly less under intermittent compression than sustained compression74 and in consideration of the native, dynamic loading patterns in the TMJ, Nicodemus et al.82 obtained surprising results in response to dynamic compressive strains. Bovine condylar chondrocytes were encapsulated in photopolymerized PEG hydrogels and constructs were exposed to dynamic loading at 0.3 Hz and 15% amplitude. Dynamic stimulation led to suppression in gene expression, cell proliferation and proteoglycan synthesis over unloaded controls.82 This work recognizes the need to further investigate the potential role of mechanical stimulation, via various loading schemes, in construct development.
Bioactive signals may also be used to encourage cell proliferation and biosynthesis with cellular responses depending on the specific signal or combination of signals. Addressing first the role of proliferative agents for condylar cartilage cells, bFGF has been found to have the greatest stimulatory effect on the proliferation of second passage human mandibular condylar chondrocytes over IGF-I and TGF-β1 treatments in monolayer culture.83 Studies exploring biosynthesis as well as proliferation have observed an inhibitory effect of bFGF on GAG and collagen synthesis84 in contrast to the enhancing effect of IGF-I on biosynthesis.85, 86 Specifically, an inhibition of GAG and collagen synthesis in rat condylar cartilage explants was observed in the presence of bFGF following 2 wks of culture.84 An increase in GAG and collagen synthesis, on the other hand, was observed in explants treated with IGF-I alone or in combination with bFGF, with bFGF downregulating IGF-I’s biosynthetic effects when used in combination. Considering next epidermal growth factor (EGF), Tsubai et al.,78 whose isolation technique was previously mentioned, explored EGF treatment in fibroblast-like condylar cells obtained from fetal rats. EGF was shown to bring cells into the s-phase of the cell cycle more quickly and to increase cell number over controls. Both measures indicate an increase in cellular proliferation.78 The authors also noted the role of EGF in matrix deposition, with tissue volume increasing toward the end of the culture period (21 days).78 As research moves toward alternative cell sources, our understanding of bioactive signals must be translatable. Notably, early work by Copray et al. 87 demonstrated that most of the factors enhancing proliferation explored in their study, including EGF, similarly enhanced proliferation in secondary mandibular condylar cartilage as well as primary costal chondrocytes. However, results must be validated in the specific culture system under review, considering not only the cell source but also the scaffold-type and mechanical stimulation.
Combined mechanical and bioactive stimulation has revealed interrelated roles of biochemical and biomechanical effectors. A study of rat condylar cartilage cells explored the effects of TGF-β1 and static tension-stress (5kPa) on cellular proliferation.88 It was demonstrated that TGF-β1 had a mitogenic effect at all concentrations under review (0.1, 1 and 10 ng/ml), but an additive effect was observed in the group treated with both TGF-β1 and static tension-stress. As various cell sources and culture systems are explored, this result illustrates the need for continued exploration of exogenous stimulation, both chemical and mechanical, throughout cell culture, toward developing shape-specific condylar replacements.
Glenoid Fossa and Articular Eminence
In attempts to repair or replace pathologic TMJ tissues, it is essential to continue with a discussion of the superior articulating surface of the joint, including the articular eminence and glenoid fossa. Together, the superior and inferior surfaces transmit loads experienced by the joint, through the TMJ disc. Important to note is the incongruence existing between the superior and inferior surfaces. The TMJ disc and the synovial fluid contained within the joint capsule fill this gap, ensuring smooth articulation. As previously mentioned, joint pathology, including OA and ID, can significantly affect this structure-function relationship.
Of the salient tissues in the joint, the glenoid fossa and articular eminence are the least characterized in terms of biochemical and biomechanical properties. The surface of the fossa has been described as a dense, fibrous tissue,89 though more specific characterization is still needed. As expected, the primary component of this fibrous tissue has been identified as collagen.90 Biomechanical evaluation of the glenoid fossa and articular eminence has demonstrated the aggregate moduli to be greater in the medial and posterior regions (42.6 and 58.9 kPa, respectively), and lower in the anterior, central, and lateral regions, all in the range of 35 kPa.89
With limited characterization information available, design criteria and validation metrics have yet to be established for engineering tissue replacements for the superior articulating surfaces. To our knowledge, tissue engineering efforts have not yet addressed this tissue. However, as research progresses toward the development of condylar and TMJ discal tissue replacements, the glenoid fossa and articular eminence must also be considered.
TMJ Disc Characterization
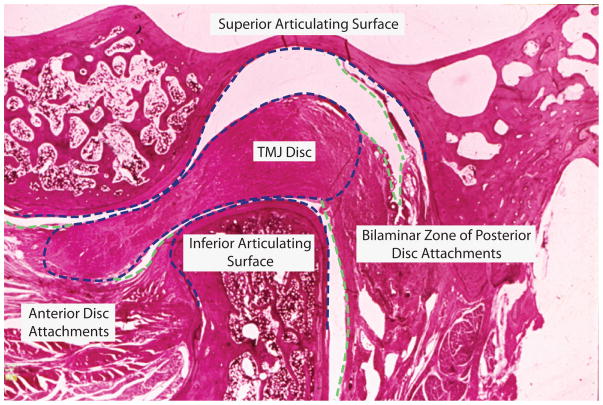
The following section briefly outlines the anatomy, structure, and function of the TMJ disc. More detailed reports by may be found in the literature.91–94 From a superior view, the human disc takes on a biconcave, elliptical shape and is longer mediolaterally (~23mm) than anteroposteriorly (~14mm),95 similar to the shape of the condyle. The disc may be divided into three zones: anterior band, intermediate zone, and posterior band.91 In the sagittal view of a human TMJ, seen in Fig. 6, the posterior band is thicker than the anterior band and the intermediate zone is the thinnest region. As described previously, the disc is attached along its periphery to the condyle and temporal bone via fibrous connective tissue. Anteriorly, the disc is attached to the articular eminence and to the condyle at the pterygoid fovea, via capsular ligaments. Posteriorly, the disc blends with the bilaminar zone, a network of fibro-elastic tissue, connecting superiorly to the glenoid fossa and inferiorly to the condyle. When the joint is in the neutral position, the disc is situated between the condyle and the glenoid fossa. With joint motion, less-tenuous superior attachments allow the superior surface of the disc to translate anteroposteriorly, and to a lesser extent mediolaterally, with respect to the fossa. The inferior surface of the disc, in contrast, remains in close proximity to the condyle. The shape and motion of the disc imparts its function: to separate the incongruent articulating surfaces and to transmit force between them.
Figure 6.
Sagittal TMJ histology. Articulating structures (blue) and discal attachments (green) of a non-pathological TMJ.
The TMJ disc is composed of a heterogeneous distribution of cells with characteristics of chondrocytes and fibroblasts, together termed TMJ disc cells. More specifically, the porcine disc has been be described by a non-uniform distribution of approximately 70% fibroblast-like cells and 30% chondrocyte-like cells.96 While both cell types are distributed throughout the disc, cells in the central portion of the intermediate zone tend to be more chondrocyte-like, while cells in the periphery of the disc tend to be more fibroblast-like.96–98 Across species, cellularity is higher in the anterior and posterior bands than in the intermediate zone. 96, 99 More specific variations in band cellularity appear to exist between species,95 and it has been reported that, with age, the disc becomes more fibrous100 and acellular.101
In terms of its biochemical composition, the disc is highly fibrous, illustrated by low GAG content and high type I collagen content. Water content has been reported in the range of 66–80% for bovine and porcine models.102–104 The primary ECM component is collagen, which comprises 30% of the disc by wet weight105 and 50% by volume.100, 106 The disc shows ring-like collagen alignment along the periphery and anteroposterior alignment through the central region. This anisotropy contributes to the structure-function relationship of the disc, with anteroposterior alignment supporting the tensile forces imposed on the disc during translation.107–109 With the condyle demonstrating a similar structure-function relationship, it is apparent that these two structures work closely together to distribute loads experienced by the joint. Unlike hyaline cartilages, which are composed primarily of type II collagen, the TMJ disc is composed primarily of type I collagen.110 Studies have also identified the presence of collage types III in trace amounts,111, 112 as well as collagen VI, IX, XIII in bovine113 and leporine models114. Cross-linked elastin fibers of relatively small diameter (0.5 μm)115 are also distributed throughout the disc and comprise 1–2% of the tissue by mass.112 There is a greater distribution of elastin in the superior surface than in the inferior surface116 and a significantly greater distribution in the peripheral bands than in the intermediate zone.110, 116, 117 Through its highly compliant nature, elastin likely plays a role in restoring the disc’s original shape following loading.97, 115, 118 GAGs, including chondroitin-6-sulfate, chondroitin-4-sulfate, dermatan-sulfate, keratin-sulfate and to a lesser extent hyaluronan, together comprise less than 5% of the disc.103, 104, 110, 119, 120 The proteoglycans identified throughout the tissue are chondroitin-sulfate proteoglycans (CSPG), likely aggrecan or versican, and dermatan-sulfate proteoglycans (DSPG), including decorin and biglycan.110 Overall, the low GAG content and high proportion of type I collagen in the disc exemplify fibrocartilage characteristics, closely resembling the superior articulating surface of the condyle.119
The mechanical properties of the TMJ disc show regional and interspecies variability, and can be best understood in light of the structure’s viscoelastic (time dependent) characteristics. In a study on the regional mechanical properties of the human TMJ disc, tissue behavior was shown to depend on the amplitude, rate, location, and time of deformation using a dynamic indentation apparatus.121 An overview of species and region-dependent tensile and compressive properties is presented in Table 1 and Table 2, respectively. Due to the rate- and history- dependence of the mechanical properties, careful attention should be paid to testing parameters as reported. Notably, an interspecies study by Kalpakci et al.95 aimed to quantify variability between species and to relate regional mechanical properties to biochemical content within the disc. The authors successfully associated the mechanical properties and biochemical content of the disc to loading schemes of herbivores (cow, goat, and rabbit- primarily translational motion) and omnivores (human and pig- both rotational and translational motion). Additionally, the authors concluded that the pig TMJ disc offers the best animal model for the human TMJ disc with the most statistical similarities in dimensions, collagen content, GAG content, and compressive properties.95 While GAG content has historically been correlated with compressive properties, and collagen content/organization has been correlated with tensile properties, evidence suggests collagen density and organization may be a primary determinant of both tensile and compressive properties.95 This is because a higher correlation with tensile properties has been found with collagen density and alignment, than with GAG distribution and density, in region-specific comparisons.95 However, GAGs, such as decorin, may play an indirect role, as they have been found to influence collagen alignment and orientation.122
Table 1.
Biomechanical properties of the TMJ disc under tension.
| Region | Species | Relaxed or Instantaneous | Modulus (MPa) (age where provided) | Stress/Strain Range (direction) | Reference |
|---|---|---|---|---|---|
| Lateral | Canine | Instantaneous | 39.5 | 0–1.5 MPa (AP) | Tanne et al.146 |
| Canine | Instantaneous | 83.7 | 1.5–4.0 MPa (AP) | Tanne et al.146 | |
| Bovine | Instantaneous | 22.5 (3 yr), 21.7 (7 yr), 24.0 (10 yr) | Tanaka et al.147 | ||
| Bovine | Relaxed, Creep | 12.4 (3 yr), 11.9 (7 yr), 10.2 (10 yr) | 1.5 MPa | Tanaka et al.147 | |
| Central | Canine | Instantaneous | 50.2 | 0–1.5 MPa (AP) | Tanne et al.146 |
| Canine | Instantaneous | 101.1 | 1.5–4.0 MPa (AP) | Tanne et al.146 | |
| Human | Instantaneous | 44.0 (53.3 for ID) | 0–2% strain | Tanaka et al.148 | |
| Human | Instantaneous | 95.7 (95.7 for ID) | 2–4% strain | Tanaka et al.148 | |
| Human | Relaxed, SR | 29.9 (41.0 for ID) | 0–2% strain | Tanaka et al.148 | |
| Human | Relaxed, SR | 61.2 (59.2 for ID) | 2–4% strain | Tanaka et al.148 | |
| Bovine | Instantaneous | 20.2 (3 yr), 21.0 (7 yr), 22.9 (10 yr) | Tanaka et al.147 | ||
| Bovine | Relaxed, Creep | 14.5 (3 yr), 12.9 (7 yr), 11.5 (10 yr) | 1.5 MPa | Tanaka et al.147 | |
| Porcine | Instantaneous | 76.4 | (AP) | Beatty et al.149 | |
| Porcine | Instantaneous | 3.2 | (ML) | Beatty et al.149 | |
| Medial | Canine | Instantaneous | 43.3 | 0–1.5 MPa (AP) | Tanne et al.146 |
| Canine | Instantaneous | 91.9 | 1.5–4.0 MPa (AP) | Tanne et al.146 | |
| Bovine | Instantaneous | 24.0 (3 yr), 24.3 (7 yr), 25.9 (10 yr) | Tanaka et al.147 | ||
| Bovine | Relaxed, Creep | 12.4 (3 yr), 10.4 (7 yr), 9.1 (10 yr) | 1.5 MPa | Tanaka et al.147 |
Table 2.
Biomechanical properties of the TMJ disc under compression.
| Region | Species | Relaxed or Instantaneous | Modulus (kPa) (surface where provided) | Strain Range | Authors |
|---|---|---|---|---|---|
| Lateral | Bovine | Instantaneous | 14.6E3 | del Pozo et al.150 | |
| Porcine | Relaxed, Creep | 16.3 (superior) | Kim et al.89 | ||
| Porcine | Relaxed, SR | 99.0 (inferior) | 15–30% | Allen et al.151 | |
| Porcine | Instantaneous | 371.0 (inferior) | 15–30% | Allen et al.151 | |
| Central | Bovine | Instantaneous | 15.5E3 | del Pozo et al.150 | |
| Porcine | Relaxed, Creep | 18.6 (superior) | Kim et al.89 | ||
| Canine | Instantaneous | 30.9E3 | Tanaka et al.152 | ||
| Porcine | Relaxed, SR | 121.0 (inferior) | 15–30% | Allen et al.151 | |
| Porcine | Instantaneous | 482.0 (inferior) | 15–30% | Allen et al.151 | |
| Medial | Porcine | Relaxed, Creep | 28.9 (superior) | Kim et al.89 | |
| Bovine | Instantaneous | 14.7E3 | del Pozo et al.150 | ||
| Porcine | Relaxed, SR | 173.0 (inferior) | 15–30% | Allen et al.151 | |
| Porcine | Instantaneous | 755.0 (inferior) | 15–30% | Allen et al.151 | |
| Anterior | Porcine | Relaxed, Creep | 18.8 (superior) | Kim et al.89 | |
| Bovine | Instantaneous | 17.3E3 | del Pozo et al.150 | ||
| Porcine | Relaxed, SR | 133.0 (inferior) | 15–30% | Allen et al.151 | |
| Porcine | Instantaneous | 1.42E3 (inferior) | 15–30% | Allen et al.151 | |
| Posterior | Bovine | Instantaneous | 15.5E3 | del Pozo et al.150 | |
| Porcine | Relaxed, Creep | 22.1 (superior) | Kim et al.89 | ||
| Porcine | Relaxed, SR | 169.0 (inferior) | 15–30% | Allen et al.151 | |
| Porcine | Instantaneous | 1.92E3 (inferior) | 15–30% | Allen et al.151 |
Tissue Engineering TMJ Disc
While early studies exploring tissue engineering of the TMJ disc have laid the foundation and demonstrated the potential for today’s efforts, early work lacked the characterization information needed for validation and progress in optimizing design criteria. Considering Fig. 4 and Tables 1 and 2, the TMJ disc shows biomechanical properties that may be matched more easily in tissue engineered constructs, this in contrast to other musculoskeletal soft tissues that are substantially stiffer and stronger.
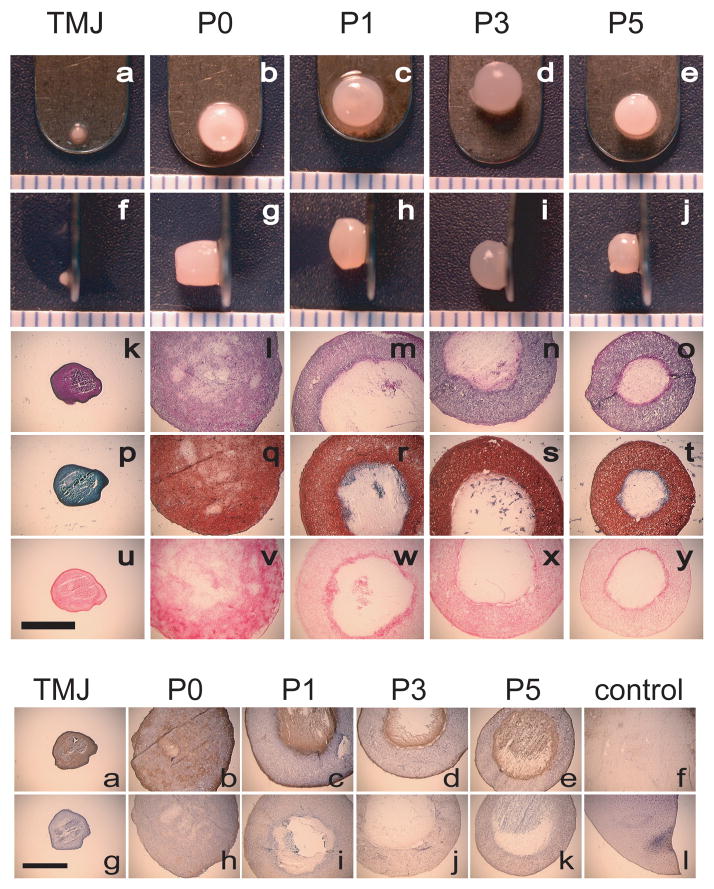
Considering first the exploration of cell sourcing, TMJ disc cells, articular chondrocytes, and, most recently, costal chondrocytes have been studied in detail with the latter showing clinical relevance and promise. Similar to the progression in cell source selection for condylar cartilage engineering, TMJ disc cells were first explored. In isolating and seeding second passage leporine disc cells on type I collagen scaffolds, it was observed that the constructs reduced significantly in size over 2 wks, from 16 mm to 12 mm.123 However, this early work demonstrated the ability to generate constructs possessing cells of a more chondrocytic phenotype, with rounded morphology and positive staining for proteoglycans, as compared to monolayer controls which showed a more fibroblastic phenotype.123 Considering possible variability between species and cell sources within the TMJ, second passage cells from human and porcine TMJ disc and articular eminence were explored with various scaffolds: polyamide, expanded polytetrafluorethylene (ePTFE), PGA, natural bone mineral blocks, and glass.124 Results demonstrated no significant differences between constructs seeded with human or porcine cells and cells from the disc or articular eminence. A predominantly chondrocyte-like cellularity was suggested by rounded cell morphology and the prevalence of type II collagen. Notably, in their conclusions, the authors pointed to functional loading and oxygen pressure as determinants of fibroblast or chondrocyte-like phenotypes. More recently, the Athanasiou group performed a series of studies aiming to refine construct development for a porcine disc cell source; selected results of this work will be addressed in the following sections. With regards to articular chondrocytes, cells obtained from the shoulder of newborn calves were seeded in TMJ disc shaped- polylactic acid (PLA)/PGA scaffolds and after 1 wk of scaffold incubation, the constructs were implanted subcutaneously in nude mice.125 Though the goal was to develop shape-specific replacement tissue for the TMJ disc, this technique yielded a shape-specific construct reminiscent of hyaline cartilage with positive sulfated GAG and type II collagen staining. In an alternative strategy for developing disc replacements, isolated mandibular chondro-progenitor cells from the condyle (unspecified zone of origin) of adult marmosets were suspended in unpolymerized type I collagen and fibrinogen, and seeded on type I collagen scaffolds.126 Biochemical analysis demonstrated that 3 to 9 days following initial culture, about 66% of the collagen was type I while the remaining 33% was type II. This time point represented the most disc-like properties.126 With further culture, the tissue began to take on more hyaline characteristics. At 21 days, collagen was identified as primarily type II and at 35 days, nearly 80% of the collagen was found to be type II. Most recently, it was demonstrated that costal chondrocytes (CCs) isolated from goat rib tissue show significant promise as a cell source.127–130 Notably, comparing primary and passaged CCs to primary and passaged disc-cells, it was demonstrated that CC scaffoldless constructs could be generated with cellularity and GAG content nearly an order of magnitude greater than the respective disc-cell constructs, see staining in Fig. 7 (top).127 Moreover, the CC constructs retained their size and shape. Primary CC constructs, stained positively for types I and II collagen while TMJ disc constructs stained positively for type I collagen exclusively, see staining in Fig. 7 (bottom).127 Though disc and articular chondrocytes have been explored in more detail historically, these results are particularly exciting due to the clinical relevance of the CC cell source, used by craniofacial surgeons in condylar rib grafts, as well as the lack of donor site morbidity.
Figure 7.
Primary and passaged costal chondrocytes in scaffoldless TMJ disc engineering. (top) Primary CC (P0) constructs stained uniformly for collagen, GAG, and cells. TMJ disc cell (TMJ) constructs did not stain for GAG, but stained uniformly for collagen and cells. Passaged CCs (P1, P3, P5) formed fluid-filled spheres, with only an outer ring staining for cells and ECM. (bottom) All constructs stained positive for type I collagen. Only CC constructs stained positive for type II collagen, the most intense staining around the outside of constructs and within fluid-filled centers. Controls: f- knee meniscus tissue and l- articular cartilage tissue. http://www.springerlink.com/content/gv8266l31307t815/.
Various scaffolds have been explored across cell sources for TMJ disc construct development. Synthetic scaffolds are advantageous for their ease of modification. Modifiable characteristics include shape, size, porosity, mechanical properties, degradation rate, and hydrophilicity. PLA and PGA are two biodegradable and biocompatible materials relevant for chondrocyte seeding. In attempts to optimize porcine disc cell culture, PGA nonwoven meshes were seeded using a spinner flask, orbital shaker, and novel pelleting seeding technique.131 Greatest type I collagen production was observed on PGA scaffolds seeded via spinner flask. In a subsequent study, poly-L-lactic acid (PLLA) was selected for exploration due to its slower degradation, the rationale being slower degradation would allow for greater matrix secretion and reduced construct contraction.132 Results, seen in Fig. 8, demonstrated PGA and PLLA constructs exhibit similar cell proliferation and matrix deposition at 4 wks, but PLLA constructs did not show the shrinking observed in PGA constructs.132 Considering native biomaterials, type I collagen is an extensively studied scaffold material for cartilage tissue engineering. Collagen may be used as a seeding vehicle either intact or following proteolytic digestion for gel encapsulation. Importantly, it has been demonstrated that collagen synthesis is enhanced in constructs seeded on collagen scaffolds.133 Electrospinning collagen scaffolds may potentially be used to encourage collagen synthesis and organization toward recapitulating aforementioned native tissue characteristics. In attempting to develop disc replacements, it is likely that a type I collagen sponge would yield constructs with morphology more similar to that of the disc, as compared to gel encapsulation. Gels, however, may be better suited for filling defects.
Figure 8.
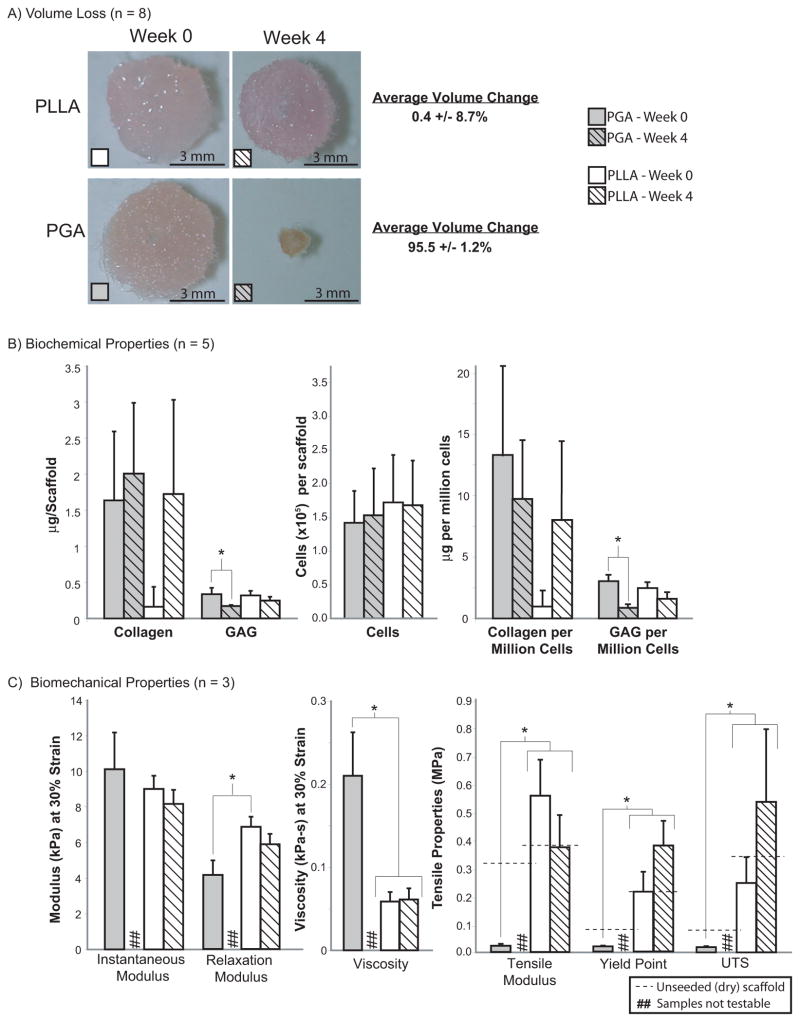
Comparisons of PLLA and PGA scaffolds for TMJ disc engineering. (A) PGA constructs experienced at least a 90% reduction in volume over 4 wks while PLLA constructs experienced negligible volume change. (B) Cellular, collagen, and GAG content of PLLA and PGA constructs were similar at 0 and 4 wks. (C) Under compression, PLLA constructs had larger relaxation moduli relative to PGA constructs at wk 0. PGA constructs at wk 0 had higher viscosity than PLLA constructs at 0 and 4 wks. Under tension, PLLA constructs at both time points were stiffer and stronger than PGA constructs at wk 0. http://jdr.sagepub.com/content/87/2/180.full.pdf+html.
Decellularized tissues present another scaffold option. For example, the porcine disc has been explored as a xenogeneic scaffold.134 Addressing the mechanical integrity of scaffolds following various decellularizing treatments, dodecyl sulfate treated tissues have been identified as potential seeding vehicles for TMJ disc engineering.134 Aside from their inherent potential immunogenicity, several disadvantages exist for decellularized tissues, including the inability to control scaffold size/shape and difficulty in reseeding the tissue.
A novel and promising method for tissue engineering the TMJ soft tissues involves self-assembly of constructs using a scaffoldless approach. It has been demonstrated that self-assembled articular cartilage constructs may be developed with aggregate moduli approaching that of native tissue with clinically relevant dimensions.135–137 Scaffoldless constructs eliminate the problem of scaffold-induced stress shielding, permitting important mechanotransductive events during tissue development and biosynthesis. Furthermore, self-assembled constructs circumvent disadvantages of scaffold use: hindrance of cell-to-cell communication, immunogenicity, and the potentially deleterious effects of byproducts of degradation. Thus, while numerous seeding vehicles have been explored, a scaffoldless technique holds significant potential for engineered TMJ disc replacements.
Considering bioactive signals, anabolic agents have been explored in greater detail, but catabolic treatments should also be noted as mediators in construct development. Anabolic growth factors explored toward the development of TMJ disc constructs include: TGF-β, platelet derived growth factor (PDGF), bFGF, and IGF-I. Beginning first with the exploration of TGF-β, it was observed that TGF-β enhanced proliferation in bovine disc cells by 250% in monolayer culture.113 More recently, in a study exploring PDGF, bFGF, and IGF-I treatments to TMJ disc cells on a 2D surface, bFGF was found to be the most beneficial mediator of proliferation, GAG synthesis, and collagen synthesis.138 Additionally, PDGF and bFGF were found to be the most potent upregulators of GAG synthesis, while IGF-I was most successful in upregulating collagen production 4.5x over the control.138 In a second study, the response of TMJ disc cells seeded on PGA scaffolds to TGF-β1, IGF-I, and bFGF was compared. 139 While all growth factors improved mechanical properties over controls, IGF-I and TGF-β1 were most effective in promoting collagen synthesis. Catabolic treatments such as chondroitinase-ABC may also be used to control matrix modification. By temporarily depleting GAG side chains and thereafter encouraging development of newly synthesized, organized ECM, chondroitinase-ABC has been shown to increase tensile properties in self-assembled articular cartilage constructs.140 Thus, bioactive signals, both catabolic and anabolic, may be used for various purposes in TMJ disc engineering.
Though not considered an anabolic or catabolic agent, intercellular signaling has also been explored as a mediator of construct development. Seeding density is one means by which intercellular signaling is indirectly affected in tissue engineering. For example, seeding density has been shown to affect morphology, collagen and GAG content, and permeability in PGA scaffolds seeded with TMJ disc cells.141 Results have suggested a maximum seeding density of 75 million cells/mL scaffold volume.141 Likewise, in the self-assembly process it has been shown that a minimum seeding density of 2 million cells/construct yields constructs possessing morphological, biochemical, and biomechanical properties approaching those of native tissue.142 With properties improving as density increases toward upward limit, an optimal seeding density of 3.75 million cells/construct has been identified, based on morphological, histological, biochemical and biomechanical results.142 Thus, controlling the initial cell seeding density is a powerful modulator of the tissue engineering process.
Mechanical stimulation is of particular relevance for tissue engineering avascular cartilage, as loading facilitates nutrient delivery, waste removal, and biosynthesis in vivo. TMJ disc engineering efforts have thus far explored the application of hydrostatic pressure and low shear forces in a rotating wall bioreactor. Both stimuli implement loading schemes reminiscent of loading patterns experienced in vivo. It is important to note that while the development of synovial fluid pressure has been observed in vivo during operator-induced mandibular motion of the pig TMJ,143 hydrostatic loading, implemented for the purpose of tissue engineering, may exceed the magnitude and frequency of that experienced by the disc in vivo.144 Despite this fact, engineering efforts have demonstrated that static hydrostatic pressure increases collagen content over unloaded controls, improving the mechanical integrity of constructs.144 Specifically, in exploring the role of hydrostatic pressure in monolayer culture and on 3D PGA scaffolds seeded with porcine TMJ disc cells, static loading at 10 MPa for 4 hrs was found to be most beneficial in promoting biosynthesis. In monolayer culture, and similarly on 3D scaffolds, the static loading group yielded the highest amount of collagen, and specifically, more type I collagen than type II compared to control and cyclic loading groups.144 In light of the biochemical content of the native disc, this result demonstrates static loading may be a suitable regimen.
Considering shear stimulation, shear stress is experienced in vivo by the disc during joint rotation and translation and may be recapitulated in culture via a rotating wall bioreactor. Toward this end, TMJ disc cells were seeded in a spinner flask on nonwoven PGA scaffolds and constructs were cultured either statically or in a low-shear rotating bioreactor.145 Scaffolds cultured in the bioreactor contracted earlier, yielding a denser matrix with higher collagen II content over static controls. Overall, however, the authors found no notable benefit to using bioreactor culture, as no significant differences were observed in matrix composition and construct stiffness compared to static culture. Though counterintuitive, these results seem to corroborate the results obtained by Nicodemus et al.82 demonstrating the beneficial application of static over dynamic compressive loading for condylar tissue engineering. Further investigation is needed to elucidate the potential independent benefit of mechanical stimulation and the interrelated benefits of mechanical and biochemical stimuli for both discal and condylar cartilage engineering. With further comprehension of the in vivo loading environment in healthy joints, bioreactors may potentially be designed to more accurately recapitulate the native mechanical environment experienced during tissue development.
Conclusions
To address the mechanically demanding and biochemically active environment of the TMJ, tissue engineering is emerging as a suitable option for replacing diseased, displaced, or degenerated tissues. Characterizing the biochemical and biomechanical properties of the joint structures, including the condyle, TMJ disc, superior articulating surface, and disc attachments, in both healthy and diseased cases, continues to facilitate the development and validation of tissue engineering strategies. Simultaneously, characterization efforts are aiding researchers and clinicians in developing their understanding of TMD etiology and progression. Thus far, native tissue characterization studies have identified distinct differences between the biochemical and biomechanical properties of the TMJ disc and condyle, thus calling for concurrent, yet independent, tissue engineering strategies. With refined design objectives and validation metrics, and with a growing awareness of TMD as a pathology in need of clinical action, it can be expected that tissue engineering for both the mandibular condyle and TMJ disc will progress significantly over the next decade.
Acknowledgments
This work was supported by grant R01DE019666 from the National Institute of Health.
Contributor Information
Meghan K. Murphy, Email: mkmurphy@ucdavis.edu.
Regina F. MacBarb, Email: rfmacbarb@ucdavis.edu.
Mark E. Wong, Email: mark.e.wong@uth.tmc.edu.
References
- 1.Zarb GA, Carlsson GE. Temporomandibular disorders: osteoarthritis. J Orofac Pain. 1999;13:295–306. [PubMed] [Google Scholar]
- 2.Laskin DM, Greenfield W, Gale E. The President’s Conference on the Examination, Diagnosis, and Management of Temporomandibular Disorders. Chicago: American Dental Association; 1983. [Google Scholar]
- 3.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 4.Solberg WK, Woo MW, Houston JB. Prevalence of mandibular dysfunction in young adults. J Am Dent Assoc. 1979;98:25–34. doi: 10.14219/jada.archive.1979.0008. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson GE, LeResche L. Epidemiology of temporomandibular disorders. In: Sessle BJ, Bryant P, Dionne R, editors. Temporomandibular disorders and related pain conditions. Seattle: IASP Press; 1995. pp. 497–506. [Google Scholar]
- 6.Carlsson GE. Epidemiology and treatment need for temporomandibular disorders. J Orofac Pain. 1999;13:232–7. [PubMed] [Google Scholar]
- 7.Martins-Junior RL, Palma AJ, Marquardt EJ, Gondin TM, de Kerber FC. Temporomandibular disorders: a report of 124 patients. J Contemp Dent Pract. 2010;11:071–8. [PubMed] [Google Scholar]
- 8.Goncalves DA, Dal Fabbro AL, Campos JA, Bigal ME, Speciali JG. Symptoms of temporomandibular disorders in the population: an epidemiological study. J Orofac Pain. 2010;24:270–8. [PubMed] [Google Scholar]
- 9.Wilkes CH. Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg. 1989;115:469–77. doi: 10.1001/archotol.1989.01860280067019. [DOI] [PubMed] [Google Scholar]
- 10.Warren MP, Fried JL. Temporomandibular disorders and hormones in women. Cells Tissues Organs. 2001;169:187–92. doi: 10.1159/000047881. [DOI] [PubMed] [Google Scholar]
- 11.van Loon JP, de Bont LG, Stegenga B, Spijkervet FK, Verkerke GJ. Groningen temporomandibular joint prosthesis. Development and first clinical application. Int J Oral Maxillofac Surg. 2002;31:44–52. doi: 10.1054/ijom.2001.0175. [DOI] [PubMed] [Google Scholar]
- 12.Farrar WB, McCarty WL., Jr The TMJ dilemma. J Ala Dent Assoc. 1979;63:19–26. [PubMed] [Google Scholar]
- 13.Bertram S, Rudisch A, Innerhofer K, Pumpel E, Grubwieser G, Emshoff R. Diagnosing TMJ internal derangement and osteoarthritis with magnetic resonance imaging. J Am Dent Assoc. 2001;132:753–61. doi: 10.14219/jada.archive.2001.0272. [DOI] [PubMed] [Google Scholar]
- 14.Brooks SL, Westesson PL, Eriksson L, Hansson LG, Barsotti JB. Prevalence of osseous changes in the temporomandibular joint of asymptomatic persons without internal derangement. Oral Surg Oral Med Oral Pathol. 1992;73:118–22. doi: 10.1016/0030-4220(92)90168-p. [DOI] [PubMed] [Google Scholar]
- 15.de Bont LG, Boering G, Liem RS, Eulderink F, Westesson PL. Osteoarthritis and internal derangement of the temporomandibular joint: a light microscopic study. J Oral Maxillofac Surg. 1986;44:634–43. doi: 10.1016/s0278-2391(86)80075-1. [DOI] [PubMed] [Google Scholar]
- 16.Moffett BC, Jr, Johnson LC, McCabe JB, Askew HC. Articular Remodeling in the Adult Human Temporomandibular Joint. Am J Anat. 1964;115:119–41. doi: 10.1002/aja.1001150108. [DOI] [PubMed] [Google Scholar]
- 17.Arnett GW, Milam SB, Gottesman L. Progressive mandibular retrusion-idiopathic condylar resorption. Part II. Am J Orthod Dentofacial Orthop. 1996;110:117–27. doi: 10.1016/s0889-5406(96)70099-9. [DOI] [PubMed] [Google Scholar]
- 18.Arnett GW, Milam SB, Gottesman L. Progressive mandibular retrusion--idiopathic condylar resorption. Part I. Am J Orthod Dentofacial Orthop. 1996;110:8–15. doi: 10.1016/s0889-5406(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 19.Kai S, Kai H, Tabata O, Shiratsuchi Y, Ohishi M. Long-term outcomes of nonsurgical treatment in nonreducing anteriorly displaced disk of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:258–67. doi: 10.1016/s1079-2104(98)90005-1. [DOI] [PubMed] [Google Scholar]
- 20.Ogus H. The mandibular joint: internal rearrangement. Br J Oral Maxillofac Surg. 1987;25:218–26. doi: 10.1016/s0266-4356(87)80022-0. [DOI] [PubMed] [Google Scholar]
- 21.Sharawy M, Ali AM, Choi WS. Experimental induction of anterior disk displacement of the rabbit craniomandibular joint: an immuno-electron microscopic study of collagen and proteoglycan occurrence in the condylar cartilage. J Oral Pathol Med. 2003;32:176–84. doi: 10.1034/j.1600-0714.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 22.Imai H, Sakamoto I, Yoda T, Yamashita Y. A model for internal derangement and osteoarthritis of the temporomandibular joint with experimental traction of the mandibular ramus in rabbit. Oral Dis. 2001;7:185–91. [PubMed] [Google Scholar]
- 23.Stegenga B, de Bont LG, Boering G. Osteoarthrosis as the cause of craniomandibular pain and dysfunction: a unifying concept. J Oral Maxillofac Surg. 1989;47:249–56. doi: 10.1016/0278-2391(89)90227-9. [DOI] [PubMed] [Google Scholar]
- 24.Nitzan DW. The process of lubrication impairment and its involvement in temporomandibular joint disc displacement: a theoretical concept. J Oral Maxillofac Surg. 2001;59:36–45. doi: 10.1053/joms.2001.19278. [DOI] [PubMed] [Google Scholar]
- 25.Petersson A. What you can and cannot see in TMJ imaging - an overview related to the RDC/TMD diagnostic system. J Oral Rehabil. 2010;37:771–778. doi: 10.1111/j.1365-2842.2010.02108.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–60. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeely ML, Armijo Olivo S, Magee DJ. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys Ther. 2006;86:710–25. [PubMed] [Google Scholar]
- 28.Gray RJ, Quayle AA, Hall CA, Schofield MA. Physiotherapy in the treatment of temporomandibular joint disorders: a comparative study of four treatment methods. Br Dent J. 1994;176:257–61. doi: 10.1038/sj.bdj.4808429. [DOI] [PubMed] [Google Scholar]
- 29.Treacy K. Awareness/relaxation training and transcutaneous electrical neural stimulation in the treatment of bruxism. J Oral Rehabil. 1999;26:280–7. doi: 10.1046/j.1365-2842.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- 30.Rocabado M. The importance of soft tissue mechanics in stability and instability of the cervical spine: a functional diagnosis for treatment planning. Cranio. 1987;5:130–8. doi: 10.1080/08869634.1987.11678183. [DOI] [PubMed] [Google Scholar]
- 31.Fricton JR. Management of masticatory myofascial pain. Semin Orthod. 1995;1:229–43. doi: 10.1016/s1073-8746(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 32.Ingawale S, Goswami T. Temporomandibular joint: disorders, treatments, and biomechanics. Ann Biomed Eng. 2009;37:976–96. doi: 10.1007/s10439-009-9659-4. [DOI] [PubMed] [Google Scholar]
- 33.Manjanna KM, Shivakumar B, Pramod Kumar TM. Microencapsulation: an acclaimed novel drug-delivery system for NSAIDs in arthritis. Crit Rev Ther Drug Carrier Syst. 2010;27:509–45. doi: 10.1615/critrevtherdrugcarriersyst.v27.i6.20. [DOI] [PubMed] [Google Scholar]
- 34.Dionne RA. Pharmacologic treatments for temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:134–142. doi: 10.1016/s1079-2104(97)90104-9. [DOI] [PubMed] [Google Scholar]
- 35.Dimitroulis G, Gremillion HA, Dolwick MF, Walter JH. Temporomandibular disorders. 2. Non-surgical treatment. Aust Dent J. 1995;40:372–6. doi: 10.1111/j.1834-7819.1995.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 36.Mountziaris PM, Kramer PR, Mikos AG. Emerging intra-articular drug delivery systems for the temporomandibular joint. Methods. 2009;47:134–40. doi: 10.1016/j.ymeth.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitzan DW, Price A. The use of arthrocentesis for the treatment of osteoarthritic temporomandibular joints. J Oral Maxillofac Surg. 2001;59:1154–9. doi: 10.1053/joms.2001.26716. discussion 1160. [DOI] [PubMed] [Google Scholar]
- 38.Laskin DM. Surgical Management of Internal Derangements. In: Laskin DM, Greene CS, Hylander WL, editors. TMDs an evidenc-based approach to diagnosis and treatment. Hanover Park: Quintessence Publishing Co; 2006. p. 476. [Google Scholar]
- 39.Holmlund A, Hellsing G, Bang G. Arthroscopy of the rabbit temporomandibular joint. Int J Oral Maxillofac Surg. 1986;15:170–5. doi: 10.1016/s0300-9785(86)80137-5. [DOI] [PubMed] [Google Scholar]
- 40.Holmlund A. Diagnostic TMJ arthroscopy. Oral Surg Oral Diagn. 1992;3:13–8. [PubMed] [Google Scholar]
- 41.Dolwick MF, Dimitroulis G. Is there a role for temporomandibular joint surgery? Br J Oral Maxillofac Surg. 1994;32:307–13. doi: 10.1016/0266-4356(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 42.Dolwick MF. The role of temporomandibular joint surgery in the treatment of patients with internal derangement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:150–5. doi: 10.1016/s1079-2104(97)90106-2. [DOI] [PubMed] [Google Scholar]
- 43.Bjornland T, Larheim TA. Discectomy of the temporomandibular joint: 3-year follow-up as a predictor of the 10-year outcome. J Oral Maxillofac Surg. 2003;61:55–60. doi: 10.1053/joms.2003.50010. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson L, Westesson PL. Discectomy as an effective treatment for painful temporomandibular joint internal derangement: a 5-year clinical and radiographic follow-up. J Oral Maxillofac Surg. 2001;59:750–8. doi: 10.1053/joms.2001.24288. discussion 758–9. [DOI] [PubMed] [Google Scholar]
- 45.Takaku S, Toyoda T. Long-term evaluation of discectomy of the temporomandibular joint. J Oral Maxillofac Surg. 1994;52:722–6. doi: 10.1016/0278-2391(94)90486-3. discussion 727–8. [DOI] [PubMed] [Google Scholar]
- 46.Henry CH, Wolford LM. Treatment outcomes for temporomandibular joint reconstruction after Proplast-Teflon implant failure. J Oral Maxillofac Surg. 1993;51:352–8. doi: 10.1016/s0278-2391(10)80343-x. discussion 359–60. [DOI] [PubMed] [Google Scholar]
- 47.Wolford LM. Factors to consider in joint prosthesis systems. Proc (Bayl Univ Med Cent) 2006;19:232–8. doi: 10.1080/08998280.2006.11928170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimitroulis G. Condylar morphology after temporomandibular joint discectomy with interpositional abdominal dermis-fat graft. J Oral Maxillofac Surg. 2011;69:439–46. doi: 10.1016/j.joms.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 49.McLeod NM, Saeed NR, Hensher R. Internal derangement of the temporomandibular joint treated by discectomy and hemi-arthroplasty with a Christensen fossa-eminence prosthesis. Br J Oral Maxillofac Surg. 2001;39:63–6. doi: 10.1054/bjom.2000.0562. [DOI] [PubMed] [Google Scholar]
- 50.Troulis MJ, Tayebaty FT, Papadaki M, Williams WB, Kaban LB. Condylectomy and costochondral graft reconstruction for treatment of active idiopathic condylar resorption. J Oral Maxillofac Surg. 2008;66:65–72. doi: 10.1016/j.joms.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 51.Qiu YT, Yang C, Chen MJ. Endoscopically assisted reconstruction of the mandibular condyle with a costochondral graft through a modified preauricular approach. Br J Oral Maxillofac Surg. 2010;48:443–7. doi: 10.1016/j.bjoms.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Wang YL, Yang C, Qiu YT, Chen MJ, Zhang SY. The clinical application of arthroscope-assisted reconstruction of the mandibular condyle with costochondral graft. Hua Xi Kou Qiang Yi Xue Za Zhi. 2008;26:534–6. 540. [PubMed] [Google Scholar]
- 53.Wong ME, Allen KD, Athanasiou KA. Tissue Engineering of theTemporomandibular Joint. In: Brozino JD, editor. Tissue Engineering and Artificial Organs. Boca Raton: CRC Press; 2006. [Google Scholar]
- 54.Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–23. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 55.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–44. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 56.Schek RM, Taboas JM, Hollister SJ, Krebsbach PH. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod Craniofac Res. 2005;8:313–9. doi: 10.1111/j.1601-6343.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 57.Schek RM, Taboas JM, Segvich SJ, Hollister SJ, Krebsbach PH. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 2004;10:1376–85. doi: 10.1089/ten.2004.10.1376. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Detamore MS. Tissue engineering the mandibular condyle. Tissue Eng. 2007;13:1955–71. doi: 10.1089/ten.2006.0152. [DOI] [PubMed] [Google Scholar]
- 59.Hansson T, Oberg T, Carlsson GE, Kopp S. Thickness of the soft tissue layers and the articular disk in the temporomandibular joint. Acta Odontol Scand. 1977;35:77–83. doi: 10.3109/00016357709055993. [DOI] [PubMed] [Google Scholar]
- 60.Mizoguchi I, Takahashi I, Nakamura M, Sasano Y, Sato S, Kagayama M, et al. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch Oral Biol. 1996;41:863–9. doi: 10.1016/s0003-9969(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 61.Delatte M, Von den Hoff JW, van Rheden RE, Kuijpers-Jagtman AM. Primary and secondary cartilages of the neonatal rat: the femoral head and the mandibular condyle. Eur J Oral Sci. 2004;112:156–62. doi: 10.1111/j.0909-8836.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 62.Shibata S, Baba O, Ohsako M, Suzuki S, Yamashita Y, Ichijo T. Ultrastructural observation on matrix fibers in the condylar cartilage of the adult rat mandible. Bull Tokyo Med Dent Univ. 1991;38:53–61. [PubMed] [Google Scholar]
- 63.Wang L, Lazebnik M, Detamore MS. Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthritis Cartilage. 2009;17:346–53. doi: 10.1016/j.joca.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Teramoto M, Kaneko S, Shibata S, Yanagishita M, Soma K. Effect of compressive forces on extracellular matrix in rat mandibular condylar cartilage. J Bone Miner Metab. 2003;21:276–86. doi: 10.1007/s00774-003-0421-y. [DOI] [PubMed] [Google Scholar]
- 65.Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 2003;125:558–65. doi: 10.1115/1.1589778. [DOI] [PubMed] [Google Scholar]
- 66.Singh M, Detamore MS. Tensile properties of the mandibular condylar cartilage. J Biomech Eng. 2008;130:011009. doi: 10.1115/1.2838062. [DOI] [PubMed] [Google Scholar]
- 67.Roth S, Muller K, Fischer DC, Dannhauer KH. Specific properties of the extracellular chondroitin sulphate proteoglycans in the mandibular condylar growth centre in pigs. Arch Oral Biol. 1997;42:63–76. doi: 10.1016/s0003-9969(97)83718-1. [DOI] [PubMed] [Google Scholar]
- 68.Mao JJ, Rahemtulla F, Scott PG. Proteoglycan expression in the rat temporomandibular joint in response to unilateral bite raise. J Dent Res. 1998;77:1520–8. doi: 10.1177/00220345980770070701. [DOI] [PubMed] [Google Scholar]
- 69.Klinge RF. The structure of the mandibular condyle in the monkey (Macaca mulatta) Micron. 1996;27:381–7. doi: 10.1016/s0968-4328(96)00038-8. [DOI] [PubMed] [Google Scholar]
- 70.de Bont LG, Boering G, Havinga P, Liem RS. Spatial arrangement of collagen fibrils in the articular cartilage of the mandibular condyle: a light microscopic and scanning electron microscopic study. J Oral Maxillofac Surg. 1984;42:306–13. doi: 10.1016/0278-2391(84)90110-1. [DOI] [PubMed] [Google Scholar]
- 71.Kang H, Bao G, Dong Y, Yi X, Chao Y, Chen M. Tensile mechanics of mandibular condylar cartilage. Hua Xi Kou Qiang Yi Xue Za Zhi. 2000;18:85–7. [PubMed] [Google Scholar]
- 72.Tanaka E, Iwabuchi Y, Rego EB, Koolstra JH, Yamano E, Hasegawa T, et al. Dynamic shear behavior of mandibular condylar cartilage is dependent on testing direction. J Biomech. 2008;41:1119–23. doi: 10.1016/j.jbiomech.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Hu K, Radhakrishnan P, Patel RV, Mao JJ. Regional structural and viscoelastic properties of fibrocartilage upon dynamic nanoindentation of the articular condyle. J Struct Biol. 2001;136:46–52. doi: 10.1006/jsbi.2001.4417. [DOI] [PubMed] [Google Scholar]
- 74.Kuboki T, Shinoda M, Orsini MG, Yamashita A. Viscoelastic properties of the pig temporomandibular joint articular soft tissues of the condyle and disc. J Dent Res. 1997;76:1760–9. doi: 10.1177/00220345970760110701. [DOI] [PubMed] [Google Scholar]
- 75.Lu XL, Mow VC, Guo XE. Proteoglycans and mechanical behavior of condylar cartilage. J Dent Res. 2009;88:244–8. doi: 10.1177/0022034508330432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh M, Detamore MS. Stress relaxation behavior of mandibular condylar cartilage under high-strain compression. J Biomech Eng. 2009;131:061008. doi: 10.1115/1.3118776. [DOI] [PubMed] [Google Scholar]
- 77.Takigawa M, Okada M, Takano T, Ohmae H, Sakuda M, Suzuki F. Studies on chondrocytes from mandibular condylar cartilage, nasal septal cartilage, and spheno-occipital synchondrosis in culture. I. Morphology, growth, glycosaminoglycan synthesis, and responsiveness to bovine parathyroid hormone (1–34) J Dent Res. 1984;63:19–22. doi: 10.1177/00220345840630010201. [DOI] [PubMed] [Google Scholar]
- 78.Tsubai T, Higashi Y, Scott JE. The effect of epidermal growth factor on the fetal rabbit mandibular condyle and isolated condylar fibroblasts. Arch Oral Biol. 2000;45:507–15. doi: 10.1016/s0003-9969(00)00012-1. [DOI] [PubMed] [Google Scholar]
- 79.Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003–10. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 80.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–27. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 81.Darling EM, Athanasiou KA. Articular cartilage bioreactors and bioprocesses. Tissue Eng. 2003;9:9–26. doi: 10.1089/107632703762687492. [DOI] [PubMed] [Google Scholar]
- 82.Nicodemus GD, Villanueva I, Bryant SJ. Mechanical stimulation of TMJ condylar chondrocytes encapsulated in PEG hydrogels. J Biomed Mater Res A. 2007;83:323–31. doi: 10.1002/jbm.a.31251. [DOI] [PubMed] [Google Scholar]
- 83.Jiao Y, Wang D, Han WL. Effects of various growth factors on human mandibular condylar cartilage cell proliferation. Zhonghua Kou Qiang Yi Xue Za Zhi. 2000;35:346–9. [PubMed] [Google Scholar]
- 84.Delatte ML, Von den Hoff JW, Nottet SJ, De Clerck HJ, Kuijpers-Jagtman AM. Growth regulation of the rat mandibular condyle and femoral head by transforming growth factor-{beta}1, fibroblast growth factor-2 and insulin-like growth factor-I. Eur J Orthod. 2005;27:17–26. doi: 10.1093/ejo/cjh068. [DOI] [PubMed] [Google Scholar]
- 85.Delatte M, Von den Hoff JW, Maltha JC, Kuijpers-Jagtman AM. Growth stimulation of mandibular condyles and femoral heads of newborn rats by IGF-I. Arch Oral Biol. 2004;49:165–75. doi: 10.1016/j.archoralbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 86.Maor G, Hochberg Z, Silbermann M. Insulin-like growth factor I accelerates proliferation and differentiation of cartilage progenitor cells in cultures of neonatal mandibular condyles. Acta Endocrinol (Copenh) 1993;128:56–64. doi: 10.1530/acta.0.1280056. [DOI] [PubMed] [Google Scholar]
- 87.Copray JC, Brouwer N, Prins AP, Jansen HW. Effects of polypeptide growth factors on mandibular condylar cartilage of the rat in vitro. J Biol Buccale. 1988;16:109–17. [PubMed] [Google Scholar]
- 88.Song J, Luo S, Fan Y. Effects of static tension-stress and TGF-beta 1 on proliferation of mandibular condylar chondrocytes. Hua Xi Kou Qiang Yi Xue Za Zhi. 2003;21:61–3. 73. [PubMed] [Google Scholar]
- 89.Kim KW, Wong ME, Helfrick JF, Thomas JB, Athanasiou KA. Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann Biomed Eng. 2003;31:924–30. doi: 10.1114/1.1591190. [DOI] [PubMed] [Google Scholar]
- 90.Berkovitz BK. Collagen crimping in the intra-articular disc and articular surfaces of the human temporomandibular joint. Arch Oral Biol. 2000;45:749–56. doi: 10.1016/s0003-9969(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 91.Rees L. The structure and function of the mandibular joint. Br Dent J. 1954;96 [Google Scholar]
- 92.Dolwick M. The temporomandibular joint: Normal and abnormal anatomy. In: Helms CAKR, Dolwick MF, editors. Internal Derangements of the Temporomandibular Joint. San Francisco: Radiology Research and Education Foundation; 1983. pp. 1–14. [Google Scholar]
- 93.Piette E. Anatomy of the human temporomandibular joint. An updated comprehensive review. Acta Stomatol Belg. 1993;90:103–27. [PubMed] [Google Scholar]
- 94.Werner JA, Tillmann B, Schleicher A. Functional anatomy of the temporomandibular joint. A morphologic study on human autopsy material. Anat Embryol (Berl) 1991;183:89–95. doi: 10.1007/BF00185839. [DOI] [PubMed] [Google Scholar]
- 95.Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. An interspecies comparison of the temporomandibular joint disc. J Dent Res. 2011;90:193–8. doi: 10.1177/0022034510381501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, et al. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2006;64:243–8. doi: 10.1016/j.joms.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mills DK, Fiandaca DJ, Scapino RP. Morphologic, microscopic, and immunohistochemical investigation into the function of the primate TMJ disc. J Orofac Pain. 1994;8 [PubMed] [Google Scholar]
- 98.Milam SB, Klebe RJ, Triplett RG, Herbert D. Characterization of the extracellular matrix of the primate temporomandibular joint. J Oral Maxillofac Surg. 1991;49:381–91. doi: 10.1016/0278-2391(91)90376-w. [DOI] [PubMed] [Google Scholar]
- 99.Mah J. Histochemistry of the foetal human temporomandibular joint articular disc. Eur J Orthod. 2004;26:359–65. doi: 10.1093/ejo/26.4.359. [DOI] [PubMed] [Google Scholar]
- 100.Berkovitz BK, Pacy J. Ultrastructure of the human intra-articular disc of the temporomandibular joint. Eur J Orthod. 2002;24:151–8. doi: 10.1093/ejo/24.2.151. [DOI] [PubMed] [Google Scholar]
- 101.Minarelli AM, Liberti EA. A microscopic survey of the human temporomandibular joint disc. J Oral Rehabil. 1997;24:835–40. doi: 10.1046/j.1365-2842.1997.00595.x. [DOI] [PubMed] [Google Scholar]
- 102.Sindelar BJ, Evanko SP, Alonzo T, Herring SW, Wight T. Effects of intraoral splint wear on proteoglycans in the temporomandibular joint disc. Arch Biochem Biophys. 2000;379:64–70. doi: 10.1006/abbi.2000.1855. [DOI] [PubMed] [Google Scholar]
- 103.Nakano T, Scott PG. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch Oral Biol. 1989;34:749–57. doi: 10.1016/0003-9969(89)90082-4. [DOI] [PubMed] [Google Scholar]
- 104.Nakano T, Scott PG. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch Oral Biol. 1996;41:845–53. doi: 10.1016/s0003-9969(96)00040-4. [DOI] [PubMed] [Google Scholar]
- 105.Gage JP, Shaw RM, Moloney FB. Collagen type in dysfunctional temporomandibular joint disks. J Prosthet Dent. 1995;74:517–20. doi: 10.1016/s0022-3913(05)80355-5. [DOI] [PubMed] [Google Scholar]
- 106.Almarza AJ, Athanasiou KA. Design characteristics for the tissue engineering of cartilaginous tissues. Ann Biomed Eng. 2004;32:2–17. doi: 10.1023/b:abme.0000007786.37957.65. [DOI] [PubMed] [Google Scholar]
- 107.Scapino RP, Obrez A, Greising D. Organization and function of the collagen fiber system in the human temporomandibular joint disk and its attachments. Cells Tissues Organs. 2006;182:201–25. doi: 10.1159/000093969. [DOI] [PubMed] [Google Scholar]
- 108.Scapino RP, Canham PB, Finlay HM, Mills DK. The behaviour of collagen fibres in stress relaxation and stress distribution in the jaw-joint disc of rabbits. Arch Oral Biol. 1996;41:1039–52. doi: 10.1016/s0003-9969(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 109.de Bont LG, Liem RS, Havinga P, Boering G. Fibrous component of the temporomandibular joint disk. Cranio. 1985;3:368–73. doi: 10.1080/08869634.1985.11678119. [DOI] [PubMed] [Google Scholar]
- 110.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24:45–57. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirschmann PN, Shuttleworth CA. The collagen composition of the mandibular joint of the foetal calf. Arch Oral Biol. 1976;21:771–3. doi: 10.1016/0003-9969(76)90069-8. [DOI] [PubMed] [Google Scholar]
- 112.Carvalho RS, Yen EH, Suga DM. The effect of growth on collagen and glycosaminoglycans in the articular disc of the rat temporomandibular joint. Arch Oral Biol. 1993;38:457–66. doi: 10.1016/0003-9969(93)90181-k. [DOI] [PubMed] [Google Scholar]
- 113.Landesberg R, Takeuchi E, Puzas JE. Cellular, biochemical and molecular characterization of the bovine temporomandibular joint disc. Arch Oral Biol. 1996;41:761–7. doi: 10.1016/s0003-9969(96)00068-4. [DOI] [PubMed] [Google Scholar]
- 114.Ali AM, Sharawy MM. An immunohistochemical study of collagen types III, VI and IX in rabbit craniomandibular joint tissues following surgical induction of anterior disk displacement. J Oral Pathol Med. 1996;25:78–85. doi: 10.1111/j.1600-0714.1996.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 115.Keith DA. Elastin in the bovine mandibular joint. Arch Oral Biol. 1979;24:211–5. doi: 10.1016/0003-9969(79)90142-0. [DOI] [PubMed] [Google Scholar]
- 116.Gross A, Bumann A, Hoffmeister B. Elastic fibers in the human temporo-mandibular joint disc. Int J Oral Maxillofac Surg. 1999;28:464–8. [PubMed] [Google Scholar]
- 117.O’Dell NL, Starcher BC, Wilson JT, Pennington CB, Jones GA. Morphological and biochemical evidence for elastic fibres in the Syrian hamster temporomandibular joint disc. Arch Oral Biol. 1990;35:807–11. doi: 10.1016/0003-9969(90)90005-u. [DOI] [PubMed] [Google Scholar]
- 118.Christensen L. Elastic tissue in the temporomandibular disc of miniature swine. J Oral Rehabil. 1975;2:373–377. doi: 10.1111/j.1365-2842.1975.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 119.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical analysis of the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2006;44:124–8. doi: 10.1016/j.bjoms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 120.Axelsson S, Holmlund A, Hjerpe A. Glycosaminoglycans in normal and osteoarthrotic human temporomandibular joint disks. Acta Odontol Scand. 1992;50:113–9. doi: 10.3109/00016359209012753. [DOI] [PubMed] [Google Scholar]
- 121.Beek M, Aarnts MP, Koolstra JH, Feilzer AJ, van Eijden TM. Dynamic properties of the human temporomandibular joint disc. J Dent Res. 2001;80:876–80. doi: 10.1177/00220345010800030601. [DOI] [PubMed] [Google Scholar]
- 122.Scott JE, Orford CR, Hughes EW. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J. 1981;195:573–81. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas M, Grande D, Haug RH. Development of an in vitro temporomandibular joint cartilage analog. J Oral Maxillofac Surg. 1991;49:854–6. doi: 10.1016/0278-2391(91)90015-e. discussion 857. [DOI] [PubMed] [Google Scholar]
- 124.Springer IN, Fleiner B, Jepsen S, Acil Y. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials. 2001;22:2569–77. doi: 10.1016/s0142-9612(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 125.Puelacher WC, Wisser J, Vacanti CA, Ferraro NF, Jaramillo D, Vacanti JP. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J Oral Maxillofac Surg. 1994;52:1172–7. doi: 10.1016/0278-2391(94)90538-x. discussion 1177–8. [DOI] [PubMed] [Google Scholar]
- 126.Girdler NM. In vitro synthesis and characterization of a cartilaginous meniscus grown from isolated temporomandibular chondroprogenitor cells. Scand J Rheumatol. 1998;27:446–53. doi: 10.1080/030097498442280. [DOI] [PubMed] [Google Scholar]
- 127.Anderson DE, Athanasiou KA. Passaged goat costal chondrocytes provide a feasible cell source for temporomandibular joint tissue engineering. Ann Biomed Eng. 2008;36:1992–2001. doi: 10.1007/s10439-008-9572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anderson DE, Athanasiou KA. A comparison of primary and passaged chondrocytes for use in engineering the temporomandibular joint. Arch Oral Biol. 2009;54:138–45. doi: 10.1016/j.archoralbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johns DE, Athanasiou KA. Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell Tissue Res. 2008;333:439–47. doi: 10.1007/s00441-008-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johns DE, Wong ME, Athanasiou KA. Clinically relevant cell sources for TMJ disc engineering. J Dent Res. 2008;87:548–52. doi: 10.1177/154405910808700609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10:1787–95. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 132.Allen KD, Athanasiou KA. Scaffold and growth factor selection in temporomandibular joint disc engineering. J Dent Res. 2008;87:180–5. doi: 10.1177/154405910808700205. [DOI] [PubMed] [Google Scholar]
- 133.Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–20. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 134.Lumpkins SB, Pierre N, McFetridge PS. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4:808–16. doi: 10.1016/j.actbio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 135.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–79. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 136.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114–23. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Detamore MS, Athanasiou KA. Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol. 2004;49:577–83. doi: 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 139.Detamore MS, Athanasiou KA. Evaluation of three growth factors for TMJ disc tissue engineering. Ann Biomed Eng. 2005;33:383–90. doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J Orthop Res. 2009;27:949–56. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Almarza AJ, Athanasiou KA. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomed Eng. 2005;33:943–50. doi: 10.1007/s10439-005-3311-8. [DOI] [PubMed] [Google Scholar]
- 142.Revell CM, Reynolds CE, Athanasiou KA. Effects of initial cell seeding in self assembly of articular cartilage. Ann Biomed Eng. 2008;36:1441–8. doi: 10.1007/s10439-008-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roth TE, Goldberg JS, Behrents RG. Synovial fluid pressure determination in the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1984;57:583–8. doi: 10.1016/0030-4220(84)90276-7. [DOI] [PubMed] [Google Scholar]
- 144.Almarza AJ, Athanasiou KA. Effects of hydrostatic pressure on TMJ disc cells. Tissue Eng. 2006;12:1285–94. doi: 10.1089/ten.2006.12.1285. [DOI] [PubMed] [Google Scholar]
- 145.Detamore MS, Athanasiou KA. Use of a Rotating Bioreactor toward Tissue Engineering the Temporomandibular Joint Disc. Tissue Eng. 2005;11:1188–1197. doi: 10.1089/ten.2005.11.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanne K, Tanaka E, Sakuda M. The elastic modulus of the temporomandibular joint disc from adult dogs. J Dent Res. 1991;70:1545–8. doi: 10.1177/00220345910700121401. [DOI] [PubMed] [Google Scholar]
- 147.Tanaka E, Tanaka M, Hattori Y, Aoyama J, Watanabe M, Sasaki A, et al. Biomechanical behaviour of bovine temporomandibular articular discs with age. Arch Oral Biol. 2001;46:997–1003. doi: 10.1016/s0003-9969(01)00072-3. [DOI] [PubMed] [Google Scholar]
- 148.Tanaka E, Shibaguchi T, Tanaka M, Tanne K. Viscoelastic properties of the human temporomandibular joint disc in patients with internal derangement. J Oral Maxillofac Surg. 2000;58:997–1002. doi: 10.1053/joms.2000.8743. [DOI] [PubMed] [Google Scholar]
- 149.Beatty MW, Bruno MJ, Iwasaki LR, Nickel JC. Strain rate dependent orthotropic properties of pristine and impulsively loaded porcine temporomandibular joint disk. J Biomed Mater Res. 2001;57:25–34. doi: 10.1002/1097-4636(200110)57:1<25::aid-jbm1137>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 150.del Pozo R, Tanaka E, Tanaka M, Okazaki M, Tanne K. The regional difference of viscoelastic property of bovine temporomandibular joint disc in compressive stress-relaxation. Med Eng Phys. 2002;24:165–71. doi: 10.1016/s1350-4533(01)00127-8. [DOI] [PubMed] [Google Scholar]
- 151.Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2006;39:312–22. doi: 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 152.Tanaka E, Tanaka M, Miyawaki Y, Tanne K. Viscoelastic properties of canine temporomandibular joint disc in compressive load-relaxation. Arch Oral Biol. 1999;44:1021–6. doi: 10.1016/s0003-9969(99)00097-7. [DOI] [PubMed] [Google Scholar]