Abstract
Whole organ decellularization of complex organs, such as lungs, presents a unique opportunity for use of acellular scaffolds for ex vivo tissue engineering or for studying cell–extracellular matrix interactions ex vivo. A growing body of literature investigating decellularizing and recellularizing rodent lungs has provided important proof of concept models and rodent lungs are readily available for high throughput studies. In contrast, comparable progress in large animal and human lungs has been impeded owing to more limited availability and difficulties in handling larger tissue. While the use of smaller segments of acellular large animal or human lungs would maximize usage from a single lung, excision of small acellular segments compromises the integrity of the pleural layer, leaving the terminal ends of blood vessels and airways exposed. We have developed a novel pleural coating using non-toxic ionically crosslinked alginate or photocrosslinked methacrylated alginate which can be applied to excised acellular lung segments, permits inflation of small segments, and significantly enhances retention of cells inoculated through cannulated airways or blood vessels. Further, photocrosslinking methacrylated alginate, using eosin Y and triethanolamine at 530 nm wavelength, results in a mechanically stable pleural coating that permits effective cyclic 3-dimensional stretch, i.e., mechanical ventilation, of individual segments.
Electronic supplementary material
The online version of this article (doi:10.1007/s12195-014-0323-1) contains supplementary material, which is available to authorized users.
Keywords: Lung, Alginate, Decellularization, Ventilation, Artificial pleura
Introduction
There is rapidly growing interest in utilizing decellularized whole lungs as scaffolds for ex vivo lung engineering.1–3,5–7,10,17,20,22,23,26,27,30,31,33–36 Importantly, decellularized whole lung scaffolds provide a powerful tool with which to investigate lung biology including interaction of lung epithelial and other cells with the extracellular matrix (ECM) proteins in the scaffold and with which to study the effect of environmental influences such as cyclic mechanical stretch on lung cell behavior.
As rodent (mouse and rat) lungs are small, readily available, and easily handled, high-throughput approaches with whole acellular scaffolds can be utilized to assess the various factors which will likely be required for effective de- and recellularization such as different combinations of cell types, growth factors, and environmental factors such as oxygen tension and cyclic mechanical stretch.35 However, a more limited supply of larger animal and human lungs and the practical aspects of handling these impede comparable rapid and effective high throughput studies. In particular, using an entire lung or lobe to evaluate a single experimental condition slows effective progress. The development of novel and innovative techniques to excise multiple functional small lung segments (~1–3 cm3) from individual cadaveric or donor lungs unsuitable for transplant that retain 3-dimensional structure is vital for developing high-throughput studies of human and other large animal lungs. We have recently developed effective techniques for pig and human lungs where small acellular segments are excised that each contain identifiable bronchovascular bundles which can be cannulated and used for airway or vascular cell inoculations.33,34 Many small acellular segments can be produced from an individual lobe or lung thus significantly increasing the ability to perform high throughput studies on the same lung tissues.
However, decellularized lungs often leak, largely due to the removal of cells which form the pleural barrier. Excision of individual acellular lung segments further structurally damages the remaining pleura leaving vascular and airway branches exposed. Thus, the integrity of the segment used for cell inoculations and for mechanical ventilation (i.e., 3-dimensional cyclic mechanical stretch) is compromised. This has left researchers with two sub-optimal choices for high throughput studies: (1) recellularization of an entire acellular large animal or human lung or lobe, an impractical approach for high throughput mechanistic studies, or (2) recellularization of a leaky excised acellular segment. Prior approaches investigating recellularization of small acellular segments from large animal or human lungs have utilized non-specific monolayer cell seedings onto thin slices of acellular tissue or non-specific injections into thicker tissue segments.3,7,20,22 While these approaches can provide some information about cell behavior in the acellular scaffold, this is limited by the lack of specific cell inoculations into anatomically appropriate airway or vascular compartments and also by the lack of ability to have the inoculated segments undergo appropriate 3-dimensional mechanical stretch. The ideal material for coating excised segments would have sufficient mechanical stability to permit cellular inoculations and mechanical ventilation, is non-cytotoxic, and encourages cells to preferentially adhere to the acellular scaffold.
Alginate, a naturally occurring biocompatible polysaccharide extracted from brown seaweed, has several unique properties that have enabled its use as a matrix for the entrapment and/or delivery of a variety of biological agents.8,32 In addition, alginate inherently lacks the ability to induce mammalian cell adhesion, making it a natural choice for wound dressings and seals in vivo.14 Alginate is an anionic linear copolymer comprised of β-d-mannuronate and α-l-guluronate blocks and thus can be ionically crosslinked by the addition of divalent cations such as calcium in aqueous solution to form a hydrogel.15 This relatively mild and quick gelation process has enabled not only proteins but also cells to be safely incorporated into alginate matrices with retention of full biological activity.9 However, ionically crosslinked alginate hydrogels have a short life-span in the presence of calcium chelators. Therefore, researchers have investigated the chemical modification of alginate to enable covalent crosslinking for the purpose of increasing the residence life of the hydrogels in situ and increasing the durability of the materials.11,25,28
We reasoned that these biologic and mechanical properties would serve well as a pleural coating and thus describe the development of effective alginate-based artificial pleural coatings for use with lung regeneration studies involving small segments of acellular large animal and human lungs.
Methods
Decellularization of Human Lungs
Cadaveric human lung lobes were obtained from autopsy services at Fletcher Allen Hospital in Burlington, Vermont. Lungs were categorized as normal or diseased based on review of available clinical records, including known history of lung disease, smoking history, chest radiographs, and use of respiratory medications. No other patient-specific identifiers were utilized. Whole lungs or individual lobes were decellularized using a previously optimized method for lungs from large animals or humans based on modifications from approaches utilized in mouse lungs.1,2,6,30,33,34,36 In brief, lungs were exposed to sequential washes of Triton X-100, sodium deoxycholate (SDC), 1 M NaCl, and DNAse, with intermittent washes with deionized (DI) water and extensive rinsing with phosphate-buffered saline (PBS).
Viscosity Measurements of Sodium Alginate (Na-AA)
Solutions of high molecular weight Na-AA (Manugel®, FMC Biopolymer, Philadelphia, PA) or low molecular weight Na-AA (Protanol®, FMC Biopolymer, Philadelphia, PA) were made at concentrations ranging from 0.5 to 5% (w/v) in deionized water. Solution viscosities were determined using a rheometer (AR2000, TA Instruments, New Castle, DE). Utilizing a 40 mm-diameter, 1° angled cone geometry, alginate solutions (0.5–2% (w/v)) were exposed to varying degrees of shear (1–140/s) at 1% radial shear strain.29
Synthesis of Calcium Alginate Hydrogels
Ionically crosslinked hydrogels were synthesized using varying concentrations of calcium chloride (CaCl2, 2–10% (w/v)) (Supplementary Fig. 1).13 Wet mass, as an indication of extent of crosslinking, was determined for different combinations of Manugel® and CaCl2 solutions. With the exception of the viscosity measurements, all subsequent references to Na-AA refer to Manugel® formulation.
Mass Swell Ratio Determination
The mass swelling ratio (Q mass) was determined by dividing the wet by dry mass of calcium alginate hydrogels synthesized with different initial concentrations of Na-AA. Following ionic crosslinking with 3% (w/v) CaCl2, all calcium alginate (Ca-AA) hydrogels were dried overnight in a desiccator to determine dry weight.11
Degradation of Calcium Alginate
200 μL of Manugel® solution at 2.5, 3, 4, and 5% (w/v) were ionically crosslinked with 200 μL of 4 or 10% (w/v) CaCl2 in a 48 well cell culture plate. Calcium alginate hydrogels were allowed to form and the excess liquid was aspirated off. Initial wet weights were taken for each hydrogel and taken at days 7 and 14. Degradation experiments were carried out in 0.05% (w/v) sodium azide in 1× PBS in a humidified incubator at 37 °C and 5% CO2.
Synthesis of Methacrylated Alginate (AA-MA)
Methacrylation of Manugel® was performed using an aqueous reaction with methacrylic anhydride.4,28 Briefly, a 2% (w/v) polymer solution was reacted with a 20 molar excess of methacrylic anhydride for 24 h at room temperature, adjusting pH to approximately 8.5 periodically with sodium hydroxide. The polymer was then precipitated with cold ethanol, re-hydrolyzed and dialyzed against deionized water extensively.11,28
Photocrosslinking of Methacrylated Alginate
Methacrylated alginate was dissolved in deionized water to make 2 or 3% (w/v) solutions with 0.00125% (w/v) eosin Y (photosensitizer), 125 mM triethanolamine (TEOA, initiator), and 19 mM 1-vinyl-2-pyrrolidinone (1VP, catalyst) and exposed to 530 nm green light using a custom light set-up comprised of four green LEDs soldered to 10 mm2 CoolBase (SR-05-M0070, Luxeon Star LEDs, LEDDynamics, Ontario, Canada) and mounted on a 40 mm2 × 10 mm Alpha Heat Sink (LPD40-10B, Luxeon Star LEDs, Ontario, Canada).4,12,24 The whole assembly is powered by 9 V DC. Photocrosslinked methacrylated alginate was made by injecting 2–3% (w/v) AA-MA solutions with eosin Y, TEOA, and 1VP between two glass coverslips and exposing to 530 nm light for 10 min (Supplementary Fig. 2). Discs of uniform size were then generated using a 6 mm biopsy punch.
Mechanical Characterization of Sodium and Methacrylated Alginate
One mL of a 3% (w/v) methacrylated alginate solution containing photoinitiator was placed between two parallel plates on an AR2000 Rheometer (TA Instruments) at room temperature (n = 3). Viscosity (Pa·s) of the solution was collected under 1% radial strain at strain rates between 1 and 140 per second over the course of 3 min. Following viscosity data collection, a green light system (520 nm) custom for rheometry collection was activated while collecting loss and storage moduli, and delta (the phase angle between the moduli) at 1% radial strain and 1 Hz over the course of 20 min. Gelation, or initiation of hydrogel photocrosslinking, was determined as the inflection point on the delta curve.19,37
Ionic or Photocrosslinking on Excised Segments of Acellular Lung
2.5% (w/v) Manugel® or 2–3% (w/v) AA-MA was applied to the excised lung segments and allowed to equilibrate prior to either ionic or photo-initiated crosslinking. Ionic crosslinking was achieved by exposing Manugel® coated acellular lung segments to a 3% (w/v) CaCl2 solution.33,34 AA-MA was covalently crosslinked by exposing coated acellular segments to 530 nm wavelengths in a custom made light box with four green LEDs (Shown in Fig. 1). The ability of excised segments of acellular lungs coated with calcium alginate hydrogels to retain injected solutions was assessed by instilling Trypan blue solution through a cannulated small airway.
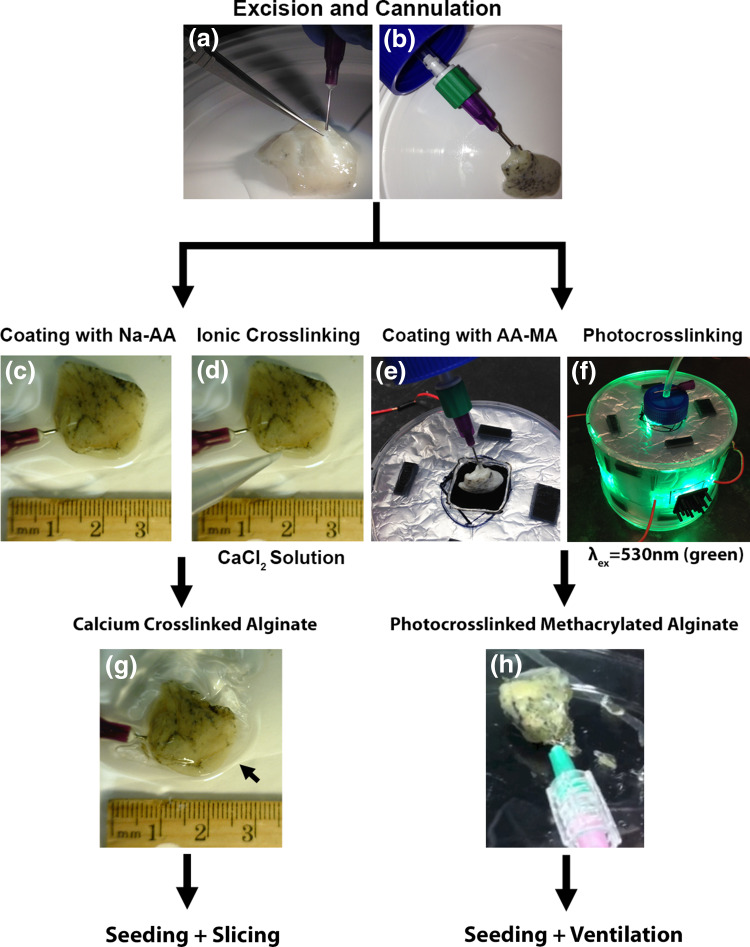
Figure 1.
Schematic of generating small segments of acellular lung and coating with calcium alginate or methacrylated alginate hydrogels for use in high throughput studies. (a) Small segments of acellular lung can be excised from whole human lungs and small airways or vessels can be identified and cannulated. (b) Cannulas inserted into small airways or vessels can be secured with surgical clips for future cellular inoculations (c, d, g) or for studies with mechanical ventilation (e, f, h). (c) Excised segments can be coated with 2.5% Na-AA solution and (d) crosslinked with 3% calcium chloride solution. (e) Excised segments can be coated with a 2–3% solution of methacrylated alginate (AA-MA) containing eosin Y, TEOA, and 1VP and (f) photocrosslinked using exposure to green light (λ = 530 nm). (g) Calcium alginate coated segments can be seeded through cannulated small airways or vessels and further sliced for high throughput studies or left as whole segments. (h) Photocrosslinked methacrylated alginate coated segments can be seeded through cannulated small airways and further exposed to ventilation for high throughput studies in whole segments
Cell Culture and Cellular Inoculations
Human lung epithelial carcinoma cells, A549 (ATCC) were cultured in DMEM/F12 basal medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, and 1% l-glutamine. Human bronchiolar epithelial cells, HBEs (courtesy of Albert van der Vliet, University of Vermont, originally from Drs. J. Yankaskas and R. Wu)39 were cultured in DMEM/F12 basal medium supplemented with cholera toxin (10 ng/mL), epidermal growth factor (10 ng/mL), insulin (5 μg/mL), transferrin (5 μg/mL), dexamethasone (0.1 μM), bovine pituitary extract (15 μg/mL), bovine serum albumin (0.5 mg/mL), and 1% penicillin–streptomycin. Human endothelial progenitor cell line, CBF (courtesy of Mervin Yoder (IUPUI)) were cultured on collagen coated tissue culture plastic in EGM-2 complete medium (Lonza) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Human lung fibroblasts were cultured in DMEM basal medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, 1% l-glutamine. All cell lines were cultured at 37 °C and 5% CO2. Cells were seeded and cultured at specified confluence and passaged and maintained accordingly. For all cell seedings, cells were washed with PBS 3×, lifted with TrypLE Express (Life Technologies), washed with FBS containing medium and re-suspended at the appropriate concentration in their respective complete medium.
For recellularization studies, endothelial cells were seeded into vascular spaces of excised acellular human lung segments through cannulated small vessels and epithelial cells were seeded through cannulated small airways. The excised segments were either coated with calcium alginate hydrogels or left uncoated. Inoculation efficiency was assessed by seeding approximately same sized segments which had either been coated with calcium alginate hydrogels or left as excised with 2 × 106 HLFs. Cells which had leaked out of the segments were counted using a hemocytometer. For long term culture studies, calcium alginate coated segments were seeded with either HBEs, or CBFs through cannulated small airways or vessels, respectively. Cells were allowed to adhere and then the segments were sliced into 2–3 mm thick slices and cultured for 28 days.33,34 Slices were harvested at 1, 3, 7, 14, 21, and 28 days.
Cytotoxicity of Degradation Products
HLFs were seeded at 10,000 cells/well in 48 well tissue culture plates and allowed to adhere for 24 h. Cytotoxic effects of degradation products were evaluated by incubating Ca-AA or AA-MA hydrogels made from a 6 mm biopsy punch in 500 μL of HLF complete media for 24 h. The supernatants were collected and applied to HLFs and incubated an additional 24 h. Cytotoxicity was assessed using Cytotox96 (Promega) according to the manufacturer’s direction. HLFs cultured on tissue culture plastic in complete media were used as a control. Maximum LDH release was assessed in HLFs similarly cultured for 48 h and completely lysed with 9% Triton X-100.
Mechanical Ventilation of Methacrylated Alginate Coated Segments
Segments coated with methacrylated alginate were mechanically ventilated (HugoSachs MiniVent Type 845, Harvard Apparatus, Holliston, MA) at 200–300 μL tidal volumes, 1–3 Hz frequency, and 2 cm H2O positive end expiratory pressure (PEEP). Mechanical stability of the coating and the ability of the segment to be inflated without rupturing the pleural coating were evaluated by visual inspection. Retention of air in the segments was confirmed by the presence of air escaping through the PEEP trap.
Lung Histology
Acellular human lung segments, segments coated with calcium alginate or photocrosslinked methacrylated alginate, and recellularized slices of acellular tissue were fixed for 10 min in 4% paraformaldehyde at room temperature. After embedding in paraffin, 5 μm tissue sections were mounted on glass slides. Following deparaffinization, sections were stained with hematoxylin & eosin (H&E) and assessed by standard light microscopy.1,6,30,33,34,36 All light microscopy images were taken with an EVOS™ Digital Color Fluorescence Microscope (Advanced Microscope Group, Bothell, WA).
Statistics
Differences in initial wet mass were determined using two-way ANOVA, with Tukey’s multiple comparison’s test. Multiplicity p-values are reported for each comparison. Cytotoxicity (LDH) analysis of degradation products and differences between qmass was performed using one-way ANOVA. All cytotoxicity data are represented as percent of maximum LDH release control (cells cultured in tissue culture plastic for 48 h and lysed with 9% Triton X-100) ± standard deviation. Cytotoxicity and qmass data are presented as mean ± standard deviation. Post-analysis multiple comparisons were conducted using the Tukey test with a 95% confidence level and p < 0.05 considered statistically significant.40 Cell retention data was analyzed using Student’s t test. All statistical analyses were performed using GraphPad Prism 6.
Results
Characterization of Calcium Alginate Hydrogel Formation
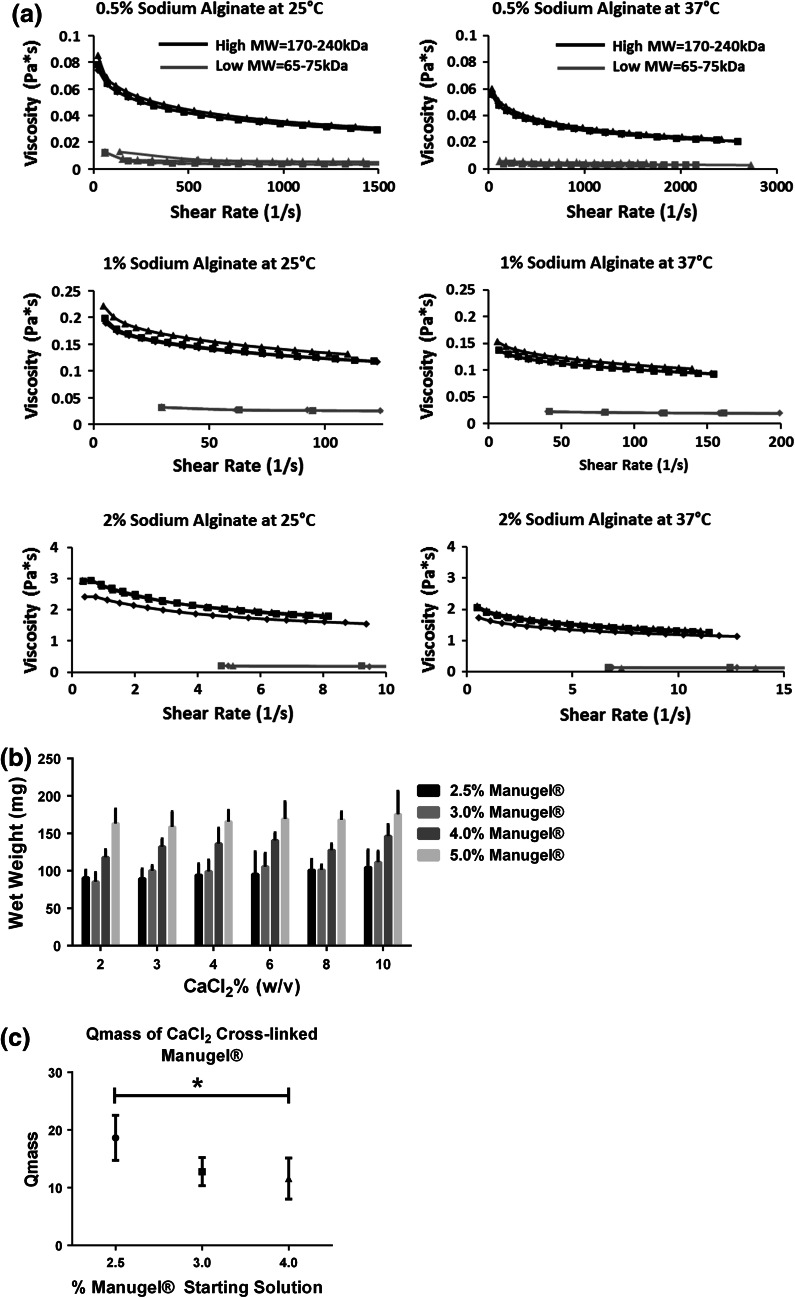
Ionically crosslinked alginate hydrogels form rapidly when Na-AA solutions are mixed with divalent cations, such as calcium. Calcium alginate hydrogels were formed by mixing different concentrations of Na-AA with calcium chloride. The initial solution viscosity for both high (Manugel®) and low (Protanol®) molecular weight Na-AA in deionized water were characterized using rheometry. At the same concentrations (w/v) and temperature, high molecular weight Na-AA had higher viscosity values than lower molecular weight Na-AA (Fig. 2a). Temperature was found to decrease solution viscosity, while increasing the concentration increased solution viscosity for both high and low molecular weight Na-AA solutions (Fig. 2a).
Figure 2.
Solution viscosity and extent of hydrogel crosslinking is dependent on Na-AA solution concentration. (a) High molecular weight Na-AA, Manugel® GMB (high MW = 170–240 kDa) exhibits increased viscosity as compared to lower molecular weight, Protanol® LF 10/60FT (low MW = 65–75 kDa) Na-AA at the same concentration and temperature. Increasing Na-AA concentration increases viscosity for both high and low MW alginate, respectively. Increasing temperature from 25 to 37 °C decreases viscosity for both high and low MW alginate, respectively. (n = 3, individual plots). (b) Initial Na-AA concentration and not CaCl2 concentration effects initial wet weight of calcium alginate hydrogels. See Supplementary Table 1 for p values. (c) Starting Manugel® concentration significantly effects the wet to dry mass ratio (Q mass) (n = 4). All data represented as means ± standard deviations. *Statistically significant differences at p < 0.05
The wet weight of final hydrogels and the mass swelling ratio (Q mass) are indicators of the extent of ionic crosslinking. Using the high molecular weight formulation of alginate, we characterized the extent of crosslinking using different initial solution concentrations and calcium chloride solutions. There were no significant differences between calcium alginate hydrogels formed from 2.5 and 3% (w/v) Manugel® solutions at any CaCl2 concentration, but significant differences existed between all other combinations of 2.5, 3, 4, and 5% Manugel® concentrations for all CaCl2 concentrations. No significant differences existed between CaCl2 solution concentrations at the same Na-AA concentration. Thus, we found that the initial Manugel® solution concentration, and not the CaCl2 concentration, significantly affected the wet weight and therefore the extent of crosslinking in calcium alginate hydrogels (Fig. 2b; Supplementary Table 1).
We next characterized the swelling behavior of calcium alginate hydrogels by obtaining the wet to dry mass ratio (Q mass) for 2.5, 3 and 5% (w/v) initial Manugel® concentrations. We found that calcium alginate hydrogels synthesized from 2.5% (w/v) initial Manugel® concentrations had the highest initial Q mass (~18) and was significantly greater than those synthesized from 4% (w/v) Manugel® solutions (Fig. 2c). Therefore, calcium alginate hydrogels synthesized from Manugel® solutions at lower concentrations swell more and hold more water.
Degradation Characteristics of Calcium Alginate Hydrogels
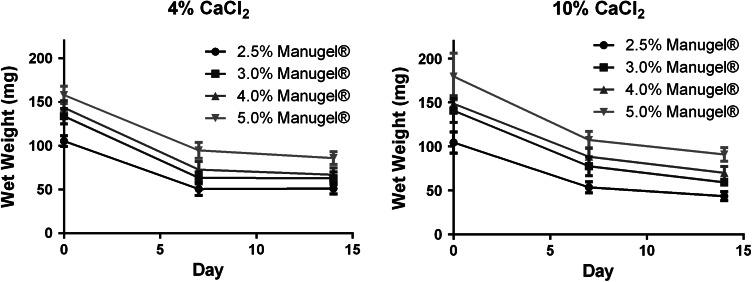
The hydrolytic degradation behavior of calcium alginate hydrogels was evaluated over 14 days in PBS and 37 °C. The initial wet weight loss at 7 days is reflective of the removal of non-crosslinked Na-AA fragments, as solutions were not changed between the initial synthesis and day 7 (Fig. 3a). There is minimal weight loss from day 7 to 14 in calcium alginate hydrogels made from 2.5 to 5% (w/v) Na-AA and crosslinked with either 4 or 10% CaCl2, indicating the stability of the final calcium alginate hydrogels (Fig. 3).
Figure 3.
Calcium alginate does not significantly degrade over 14 days and is non-cytotoxic. Noncrosslinked alginate is removed between days 0 and 7. Little further degradation is observed between 7 and 14 days, indicating the stability of the hydrogels up to 14 days. n = 6 for each time point and Manugel® concentration. Bars represent the mean ± standard deviation
Calcium Alginate as a Pleural Seal
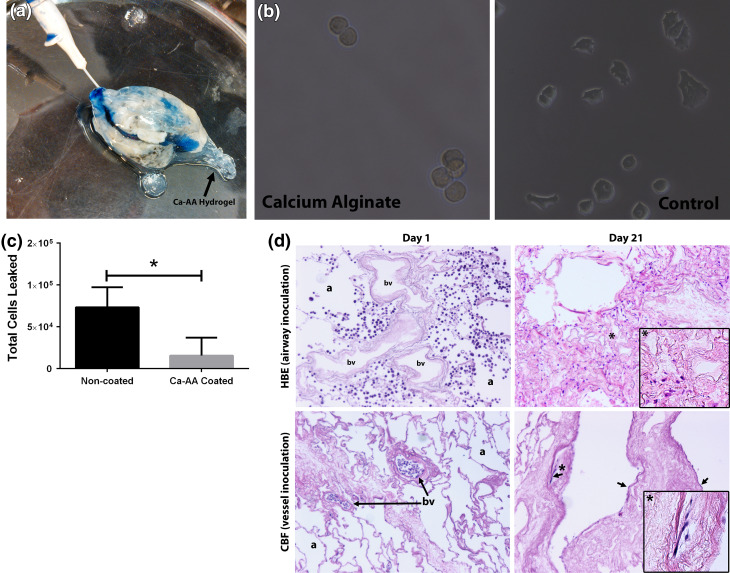
The ability of calcium alginate hydrogels to be used as a pleural seal on excised segments of acellular lung was initially assessed by instilling Trypan blue through cannulated small airways and vessels. Lungs which were not coated with calcium alginate visibly leaked Trypan blue (data not shown) while lungs coated with calcium alginate retained the Trypan blue solution, indicating preservation of the airway or vasculature branching and that the calcium alginate was effective at permitting inflation and retaining solutions (Fig. 4a). The Trypan blue can be seen to be heterogeneously distributed throughout the excised acellular segment, indicating that inoculation through a single cannulated small vessel or airway does not permit coverage of the entire segment. Rather solutions only flow through the downstream network accessible by that entry point.
Figure 4.
Calcium alginate permits selective cellular seeding through small airways or vessels into excised segments of acellular human lungs. (a) Trypan blue solution is retained in calcium alginate coated segments of acellular lung following instillation through a cannulated small airway. (b) Calcium alginate hydrogel is nonadherent to A549 cells 4 h after cell seeding, whereas cells can be seen adopting an adherent phenotype on tissue culture plastic at the same time point. (c) Calcium alginate coated segments significantly enhance cellular retention of HLFs in excised acellular lung segments. (d) H&E assessment demonstrates that calcium alginate coated segments allow selected seeding of HBEs or CBFs through small airways and small vessels, respectively. HBEs are retained in airspaces (a) and are not present in blood vessels (bv) on day one of slice culture. CBFs, a human endothelial progenitor cell line, are retained in blood vessels on day one and no cells are found in airspaces. Both HBEs and CBFs can be found on day 21 and have adopted adherent phenotypes in airspaces or blood vessels, respectively, in slice culture in normal human lung. CBFs can be seen to have adopted characteristic flattened morphology (black arrows) on day 21 in large vessels. Original magnification of images is 100×; insets for day 21 indicated by the * taken at 200×
We next tested the ability of calcium alginate to prevent cellular adhesion. In previous attempts of developing a pleural coating, we found that cells preferentially adhered to the material (e.g., agar) and did not adhere to the scaffold. Calcium alginate is known to be non-adherent to cells owing to its high hydrophilicity. We confirmed that calcium alginate hydrogels are nonadherent to cells by studying the adhesion time course of a test cell population, A549 cells, on calcium alginate hydrogels and compared this behavior to those seeded on traditional cell culture plastic. After 4 h, the A549 cells can be seen to be adopting an adherent phenotype on tissue culture plastic, while they remain rounded and non-adherent on calcium alginate hydrogels (Fig. 4b).
We then examined the ability of calcium alginate hydrogels to enhance cellular retention and to permit biologically relevant seeding through small airways and vessels. Using another test cell population, human lung fibroblasts (HLFs), we inoculated cells through cannulated small airways in calcium alginate and non-coated segments excised from acellular human lungs. We found that the use of calcium alginate as a synthetic pleura material significantly enhanced retention of cells in inoculated segments (Fig. 4c). HBEs or human CBFs inoculated through cannulated small airways or small vessels, respectively, can be seen to be exclusively in their inoculated components on day 1 when segments are coated with calcium alginate and sliced for high throughput studies (Fig. 4d). Further, we are able to culture cells in these thin slices for up to 21 days (Fig. 4d), which indicates that the scaffold, calcium alginate material and their degradation products are non cytotoxic, over 21 days. However, due to loss of surfactant and natural contraction of the acellular tissue with time, some regions appear atelectatic. In rodents, we previously found that these airspaces and vessels which had collapsed with time could be reopened with subsequent inflation.1 This further points to the likely necessity of mechanical ventilation and perfusion for lung tissue regeneration strategies to prevent collapse of tissue and loss of oxygen and nutrient supply.
Characterization of Photocrosslinked Methacrylated Alginate
While calcium alginate can be used for cellular inoculations, it does not have sufficient durability for ventilation studies. Therefore, we sought to modify the Na-AA backbone to include methacrylate groups, permitting covalent crosslinking with an appropriate chemical or photoinitiated crosslinking system. Using a well characterized aqueous chemistry, we generated methacrylated alginate (AA-MA) from the high molecular weight (Manugel®) formulation. The viscosity of a 3% (w/v) methacrylated alginate decreased when tested at the same temperature and shear rate as compared to 2% (w/v) Manugel® solutions, due to the exposure of methacrylic acid during chemical modification and subsequent chain scission. Therefore, the reduced viscosity of approximately 50% was taken into consideration when formulating solutions for coating the small acellular lung segments. However, it should be noted that the viscosity of the methacrylated alginate solutions were less affected by the increasing shear rate (1/s) as compared to the Na-AA solutions (Figs. 2a and 5a).
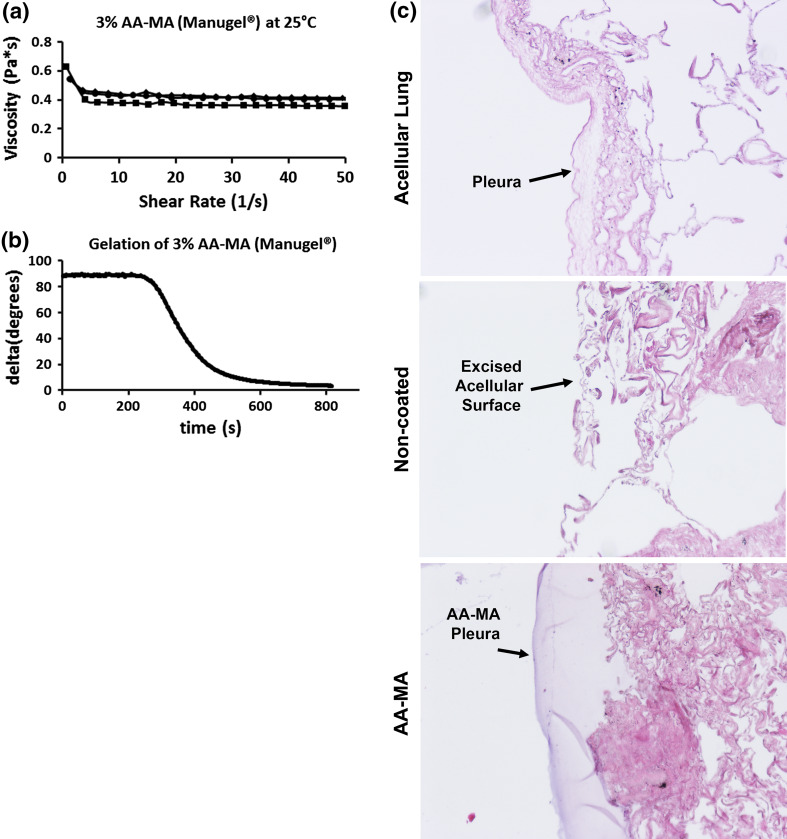
Figure 5.
Methacrylated alginate can be photocrosslinked using eosin Y, TEOA, and 1VP with 530 nm (green) excitation on excised segments of acellular human lung. (a) AA-MA viscosity is shown for a 3% (w/v) solution at room temperature and does not significantly change over the range of shear rates tested. (b) AA-MA gelation initiates at the inflection point of the delta (°) curve of the negative slope. Initiation of gelation occurs at approximately 280 s and is completed by 800 s. (c) Histologic assessment of excised segments of acellular human lungs coated with AA-MA shows the formation of a continuous pleural barrier which is adherent and intercalates with the contours of the acellular lung
The addition of methacrylate groups was confirmed by testing the ability of the 3% AA-MA solution to be covalently crosslinked using a photocrosslinking system. The eosin Y, TEOA, and 1VP system has been previously used in other photo-initiated crosslinking systems.12,18,24 The main benefits of this system are that it is generally regarded as having extremely low cytotoxicity as compared to UV based photocrosslinking systems. This is attributed to the small amounts of photoinitiators and catalysts required for crosslinking. Further, the maximum absorbance for eosin Y is 510 nm, so photocrosslinking occurs in this system using visible green light. Green light is well regarded to be more cytocompatible as compared to UV excitation, which is often used in other photocrosslinking systems. We found that exposure to green light induced covalent crosslinking of methacrylated alginate (Supplementary Fig. 2, Fig. 5b). The gelation time, or initiation of crosslinking and hydrogel formation, was determined from the delta (°) vs. time (s) curve, shown in (Fig. 5b). Delta is the phase angle between the loss and storage moduli, which represents the viscous and elastic response, respectively, of a polymer solution. As a polymer hydrogel begins to form, delta drops from 90° to 0° signifying complete gelation. The onset of gelation occurs at the inflection point, or the point at which the curve begins to drop significantly. Therefore, gelation was determined to initiate around 280 s and crosslinking was complete at 800 s. Thus, we successfully generated methacrylated alginate and were able to generate covalently crosslinked hydrogels using an eosin Y, TEOA, and 1VP system with green light excitation.
Photocrosslinked Methacrylated Alginate as a Pleural Seal
The ability of methacrylated alginate to be photocrosslinked onto acellular lung segments using green light was assessed. We found that methacrylated alginate solutions with photoinitiator crosslinked onto acellular lungs and formed a synthetic pleural layer (Fig. 1). Acellular segments contain regions devoid of a naïve pleural barrier and are thus leaky. The methacrylated alginate solution intercalated with the acellular lung and formed an intact synthetic pleural barrier after photocrosslinking (Fig. 5c). Finally, we assessed the ability of photocrosslinked methacrylated alginate to serve as a functional synthetic pleura, permitting mechanical ventilation. Excised acellular segments cannulated through small airways which were not coated with alginate are unable to retain air and inflate. However, excised acellular segments which had been coated with methacrylated alginate solutions and photocrosslinked could be ventilated (Video 1). Thus, photocrosslinked methacrylated alginate can be used as a synthetic pleural barrier to permit mechanical ventilation.
Cytotoxicity of Calcium Alginate or Photocrosslinked Methacrylated Alginate
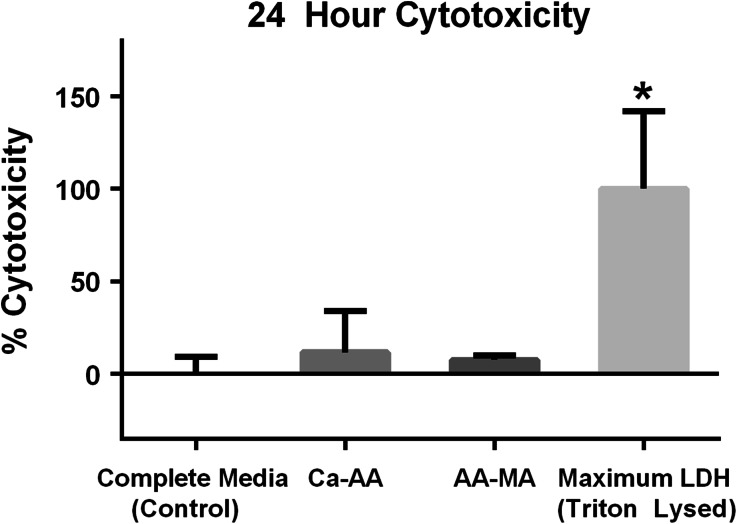
The cytotoxicity of calcium alginate and photocrosslinked methacrylated alginate degradation products was assessed by culturing HLF cells in supernatants from hydrogels incubated for 24 h at 37 °C and 5% CO2 in complete medium. Neither the calcium alginate hydrogel or photocrosslinked methacrylated alginate degradation products induced significant cytotoxicity as compared to control HLF cells cultured on tissue culture plastic with complete medium (Fig. 6).
Figure 6.
The degradation products of calcium alginate and photocrosslinked methacrylated alginate are non cytotoxic after 24 h of culture. Calcium alginate hydrogel degradation products (Ca-AA) (n = 3) and photocrosslinked methacrylated alginate (AA-MA) (n = 3) degradation products did not elicit a cytotoxic response, as compared to control HLF cells cultured in complete media on tissue culture plastic (n = 6). Maximum LDH release represent the LDH content detected after lysing cells after 48 h of culture (n = 3). Data are represented as mean ± standard deviation. *Statistically significant differences as compared to control with p < 0.05 considered significant
Discussion
We have developed a novel and innovative approach utilizing crosslinked alginate hydrogels as a functional interim artificial pleural coating. We have further developed a formulation comprised of methacrylated alginate which can be crosslinked on the acellular lung using either CaCl2 or photo-polymerization to form an effective non-toxic, flexible coating for decellularized lung segments. The physical properties of the cross-linked alginates provide both tensile strength and in the case of photocrosslinked methacrylated alginate appropriate flexibility to allow 3-dimensional stretch, i.e., mechanical ventilation, of the coated small acellular lung segments. Importantly, the coatings do not rapidly degrade which will allow long term study of coated small lung segments as recellularization occurs, including reconstitution of a native pleural coating. Further, degradation products from the crosslinked alginate coatings are non-toxic to inoculated cells.
This is a simple and elegant novel approach for generating an effective artificial pleural coating for use in studying recellularization of decellularized human lung segments and, in particular, effects of cyclic mechanical stretch on cells inoculated into the coated segments. A single literature report describes use of non-crosslinked alginate clinically to close pleural defects in vivo.21 Non-crosslinked alginate can also activate macrophages, an effect not observed with the use of crosslinked alginates.16,38 However, to our knowledge, this approach has not been utilized elsewhere and other available potential coatings such as agar, latex, and silicone are either ineffective or toxic to inoculated cells (data not shown). Neither crosslinked calcium alginate nor methacrylated alginate have been previously evaluated or utilized as an artificial pleural coating in any context.
The use of the crosslinked alginate as interim pleura is required for physiologic inoculation through small airways and vessels into acellular lung segments to permit high throughput studies from a single acellular large animal or human lung. The addition of methacrylate groups onto the alginate backbone permits covalent crosslinking by free radical polymerization and yields a final material with improved mechanical stability over ionically crosslinked alginate. Covalently crosslinked alginate can be used for studies of 3-dimensional cyclic mechanical stretch, without which, we found that studies of 3-dimensional cyclic mechanical ventilation are not possible. In future studies and in further optimization of methacrylated alginate formulations, it will be important to fully characterize the mechanical properties and modes of failure (i.e., maximal burst pressure, hydrolytic degradation, cyclic fatigue, etc.). Further, it will be important to fully characterize the mode of adhesion (mechanical vs. chemical) of the methacrylated alginate formulations on acellular segments of human lungs. The use and optimization of alginate as a pleural barrier is an important methodological advancement for maximizing the use of acellular lungs from large animals and humans.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 Video 1 Excised segments of human lungs coated with photocrosslinked methacrylated alginate can be mechanically ventilated (WMV 7015 kb)
Supplemental Fig. 1 Calcium alginate hydrogels can be generated by mixing 2.5% (w/v) Manugel® and 3% (w/v) CaCl2. At t0, a 2.5% (w/v) Manugel® is applied to a material or surface and allowed to equilibrate. A 3% (w/v) CaCl2 solution is added and the gel is ionically crosslinked (TIFF 46174 kb)
Supplemental Fig. 2. Methacrylated alginate hydrogels can be photocrosslinked by exposing AA-MA solutions with eosin Y, TEOA, and 1VP to 530 nm (green) excitation. Solutions of AA-MA with eosin Y, TEOA, and 1VP are poured between two glass coverslips and exposed to 530 nm green excitation light for 10 min to complete photocrosslinking (TIFF 31678 kb)
Acknowledgments
The authors wish to thank Joseph Platz, Charles Parsons, Dino Sokocevic for decellularization, imaging, and experimental assistance; Alex Trick and Michael Bula for designing and constructing the initial LED light box; Elice Brooks for cell culture; Benjamin Cares for alginate synthesis and Marc H. Soldini for rheometry characterizations; Joseph Consiglio, PhD, and Al Correira (Harvard Apparatus, Holliston, MA) for technical assistance and assistance with the HugoSachs Minivents and DINOlite imaging; Mervin Yoder MD, Indiana University, for the CBF cells; Albert van der Vliet, PhD for the HBE cells; and FMC Biopolymer for Manugel® and Protanol® samples. These studies were supported by NIH ARRA RC4HL106625 (DJW), NHLBI R21HL094611 (DJW), NHLBI R21HL108689 (DJW), and the UVM Lung Biology Training grant T32 HL076122 from the NHLBI.
Conflict of interest
D.E. Wagner, S.L. Fenn, N.R. Bonenfant, E.R. Marks, Z.D. Borg, P.E. Saunders, R.A. Floreani, and D.J. Weiss have no conflicts of interest to declare.
Ethical Standards
All human subjects research was carried out in accordance with institutional guidelines. No animal studies were carried out by the authors for this article.
Footnotes
This article has been designated as a 2013 BMES Outstanding Contribution.
Contributor Information
Darcy E. Wagner, Email: Darcy.wagner@med.uvm.edu
Spencer L. Fenn, Email: sfenn@uvm.edu
Nicholas R. Bonenfant, Email: Nicholas.Bonenfant@med.uvm.edu
Elliot R. Marks, Email: ermarks@uvm.edu
Zachary Borg, Email: zborg@uvm.edu.
Patrick Saunders, Email: psaunders@uvm.edu.
Rachael A. Floreani, Email: floreani@uvm.edu
Daniel J. Weiss, Email: Daniel.weiss@uvm.edu
References
- 1.Bonenfant NR, Sokocevic D, Wagner DE, et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials. 2013;34:3231–3245. doi: 10.1016/j.biomaterials.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonvillain RW, Danchuk S, Sullivan DE, et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng. Part A. 2012;18:2437–2452. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth AJ, Hadley R, Cornett AM, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2004;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortiella J, Niles J, Cantu A, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. Part A. 2010;16:2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 6.Daly AB, Wallis JM, Borg ZD, et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng. Part A. 2012;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilpin, S. E., J. P. Guyette, G. Gonzalez, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J. Heart Lung Transpl. 2013. doi:10.1016/j.healun.2013.10.030 [DOI] [PubMed]
- 8.Gombotz WR, Wee SF. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998;31:267–285. doi: 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 9.Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J. Controlled Release. 2009;134:26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen T, Roszell B, Zang F, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng. Part C. 2012;18:632–646. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Park Y, Tae G, et al. Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. J. Biomed. Mater. Res. A. 2009;88A:967–975. doi: 10.1002/jbm.a.31947. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/S0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemoine D, Wauters F, Bouchend’homme S, Préat V. Preparation and characterization of alginate microspheres containing a model antigen. Int. J. Pharm. 1998;176:9–19. doi: 10.1016/S0378-5173(98)00303-2. [DOI] [Google Scholar]
- 16.Liu WF, Ma M, Bratlie KM, Dang TT, Langer R, Anderson DG. Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials. 2011;32:1796–1801. doi: 10.1016/j.biomaterials.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longmire TA, Ikonomou L, Hawkins F, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macchiarini P, Wain J, Almy S, Dartevelle P. Experimental and clinical evaluation of a new synthetic, absorbable sealant to reduce air leaks in thoracic operations. J. Thorac. Cardiovasc. Surg. 1999;117:751–758. doi: 10.1016/S0022-5223(99)70296-5. [DOI] [PubMed] [Google Scholar]
- 19.Muller R, Gerard E, Dugand P, Rempp P, Gnanou Y. Rheological characterization of the gel point: a new interpretation. Macromolecules. 1991;24:1321–1326. doi: 10.1021/ma00006a017. [DOI] [Google Scholar]
- 20.Nichols JE, Niles J, Riddle M, et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng. Part A. 2013;19:2045–2062. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta S, Hirose M, Ishibashi H. Pleural covering method of polyglycolic acid felt with sodium alginate water solution for prevention of postoperative pulmonary fistula. Kyobu Geka. 2008;61:561–563. [PubMed] [Google Scholar]
- 22.O’Neill JD, Anfang R, Anandappa A, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. 2013;96:1046–1056. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 24.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/S0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 25.Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials. 2012;33:3279–3305. doi: 10.1016/j.biomaterials.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng. Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smeds KA, Pfister-Serres A, Miki D, et al. Photocrosslinkable polysaccharides for in situ hydrogel formation. J. Biomed. Mater. Res. 2001;55:254–255. doi: 10.1002/1097-4636(200105)55:2<254::AID-JBM1012>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Smidsrød O. Solution properties of alginate. Carbohydr. Res. 1970;13:359–372. doi: 10.1016/S0008-6215(00)80593-5. [DOI] [Google Scholar]
- 30.Sokocevic D, Bonenfant NR, Wagner DE, et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 2013;34:3256–3269. doi: 10.1016/j.biomaterials.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song JJ, Kim SS, Liu Z, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann. Thorac. Surg. 2011;92:998–1006. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002;28:621–630. doi: 10.1081/DDC-120003853. [DOI] [PubMed] [Google Scholar]
- 33.Wagner DE, Bonenfant NR, Parsons CS, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35:3281–3297. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner DE, Bonenfant NR, Sokocevic D, et al. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 2014;35(9):2664–2679. doi: 10.1016/j.biomaterials.2013.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner DE, Bonvillain RW, Jensen T, et al. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology. 2013;18:895–911. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis JM, Borg ZD, Daly AB, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng. Part C. 2012;18:420–432. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter HH. Can the gel point of a cross-linking polymer be detected by the G′–G″ crossover? Polym. Eng. Sci. 1987;27:1698–1702. doi: 10.1002/pen.760272209. [DOI] [Google Scholar]
- 38.Yang D, Jones KS. Effect of alginate on innate immune activation of macrophages. J. Biomed. Mater. Res. A. 2009;90A:411–418. doi: 10.1002/jbm.a.32096. [DOI] [PubMed] [Google Scholar]
- 39.Yankaskas JR, Haizlip JE, Conrad M, et al. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am. J. Physiol. 1993;264:C1219–C1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 40.Zar J. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 2 Video 1 Excised segments of human lungs coated with photocrosslinked methacrylated alginate can be mechanically ventilated (WMV 7015 kb)
Supplemental Fig. 1 Calcium alginate hydrogels can be generated by mixing 2.5% (w/v) Manugel® and 3% (w/v) CaCl2. At t0, a 2.5% (w/v) Manugel® is applied to a material or surface and allowed to equilibrate. A 3% (w/v) CaCl2 solution is added and the gel is ionically crosslinked (TIFF 46174 kb)
Supplemental Fig. 2. Methacrylated alginate hydrogels can be photocrosslinked by exposing AA-MA solutions with eosin Y, TEOA, and 1VP to 530 nm (green) excitation. Solutions of AA-MA with eosin Y, TEOA, and 1VP are poured between two glass coverslips and exposed to 530 nm green excitation light for 10 min to complete photocrosslinking (TIFF 31678 kb)