Abstract
Background or Purpose
The extent to which ACOSOG Z0011 findings are applicable to patients undergoing breast-conserving therapy (BCT) is uncertain. We prospectively assessed how often axillary dissection (ALND) was avoided in an unselected, consecutive patient cohort meeting Z0011 eligibility criteria and whether subgroups requiring ALND could be identified preoperatively.
Methods
Patients with cT1,2cN0 breast cancer undergoing BCT were managed without ALND for metastases in <3 sentinel nodes (SNs) and no gross extracapsular extension (ECE). Patients with and without indications for ALND were compared using Fisher's exact and Wilcoxon rank sum tests.
Results
From 8/2010-11/2012, 2157 invasive cancer patients had BCT. 380 had histologic nodal metastasis; 93 did not meet Z0011 criteria. Of 287 with ≥1 H&E-positive SN (209 macrometastases), 242(84%) had indications for SN only. ALND was indicated in 45 for ≥3 positive SNs (n=29) or ECE (n=16). The median number of SNs removed in the SN group was 3 versus 5 in the ALND group (p<.0001). Age, hormone receptor and HER2 status, and grade did not differ between groups; tumors were larger in the ALND group (p<0.0001). 72% of ALND patients had additional positive nodes (median=1;1-19). No axillary recurrences have occurred (median follow-up, 13 months).
Conclusions
ALND was avoided in 84% of a consecutive series of patients having BCT, suggesting that most patients meeting ACOSOG Z0011 eligibility have a low axillary tumor burden. Age, ER, and HER2 status were not predictive of ALND, and the criteria used for ALND (≥3SNs, ECE) reliably identified patients at high risk for residual axillary disease.
Keywords: axillary dissection, positive sentinel node, ACOSOG Z0011, breast-conserving therapy, ALND
Introduction
The American College of Surgeons (ACOSOG) Z0011 trial demonstrated that in clinically node-negative women undergoing breast-conserving therapy (BCT) and found to have metastases to 1 or 2 sentinel nodes (SNs), sentinel lymph node biopsy (SLNB) alone resulted in rates of local control, disease-free, and overall survival equivalent to those seen after axillary dissection, but with significantly lower morbidity.1,2 The National Comprehensive Cancer Network3 now recommends considering no further surgery beyond SLNB for patients meeting ACOSOG Z0011 eligibility criteria. However, the appropriateness of applying these findings to the general population of women with breast cancer undergoing BCT has been questioned.4-6 One criticism is that the women included in ACOSOG Z0011 were highly selected and had a favorable prognosis; the majority were postmenopausal with estrogen receptor (ER) positive, T1 tumors, and many had micrometastatic disease in the SNs. There is concern that younger women and those with ER negative cancers were underrepresented, and that these characteristics might be associated with a greater axillary nodal tumor burden, or with disease less responsive to adjuvant therapy, making this group of women unsuitable for an approach which eliminates axillary dissection (ALND).
In 2010 we began to apply the ACOSOG Z0011 eligibility criteria1,2 to a consecutive cohort of patients and stopped ALND in those with < 3 involved SNs as routine practice. The purpose of this study was to determine how often an ALND was avoided in this unselected patient cohort meeting ACOSOG Z0011 eligibility criteria. Additionally, we examined patient and tumor characteristics to determine if subgroups meeting Z0011 criteria for ALND could be identified preoperatively.
Patients and Methods
In August 2010 we adopted the ACOSOG Z0011 findings and began managing the axilla in all women with T1 or T2, clinically node-negative invasive breast cancers undergoing BCT with positive SNs by routine hematoxylin and eosin (H&E) staining accordingly. It was expected that all patients would receive the adjuvant systemic therapy and whole breast irradiation that were part of the ACOSOG Z0011 eligibility requirements. ALND remained standard management for patients with SN metastases undergoing mastectomy, those with clinically positive nodes, and those receiving neoadjuvant therapy. Patients with nodal disease detected only by immunohistochemical staining are not included in this report, although their surgical management was similar. This study was approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board.
Intraoperative frozen sections of the SNs were not performed. Completion ALND was recommended for patients with ≥ 3 positive SNs, or for gross extranodal extension or matted nodal disease identified by palpation intraoperatively. Patients were advised that a second surgery for an ALND might be necessary at time of initial consultation.
Routine preoperative axillary imaging was not used for screening. Patients having imaging prior to referral that showed possible nodal metastases were managed according to the number of abnormal nodes; if < 3 abnormal nodes were seen, no needle biopsy was performed. If ≥ 3 abnormal nodes were seen, fine needle aspiration (FNA) of the most suspicious node was undertaken. If metastatic disease was identified in the setting of ≥ 3 abnormal nodes on imaging, ALND was performed. Patients referred with biopsy-proven nodal metastases who did not have palpable adenopathy had a repeat ultrasound, and if < 3 abnormal nodes were visualized, were managed according to the protocol. The MSKCC nomogram estimating the risk of additional positive nodes after SLNB, was not used to guide treatment decisions.7
Data were prospectively collected for all eligible patients treated by 11 breast surgeons. ER and PR positivity was defined as the presence of staining in ≥ 1% of tumor cells. HER2 overexpression was defined as 3+ staining by immunohistochemistry or a FISH value of > 2. Reasons for deviations from the algorithm were documented.
Fisher's exact and the Wilcoxon tests were used to compare characteristics of the SN and ALND groups. All statistical analysis was performed with SAS 9.2 (SAS Institute, Cary, NC) and R 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria) statistical software. P-values were 2 sided, and values of < 0.05 were considered significant.
Results
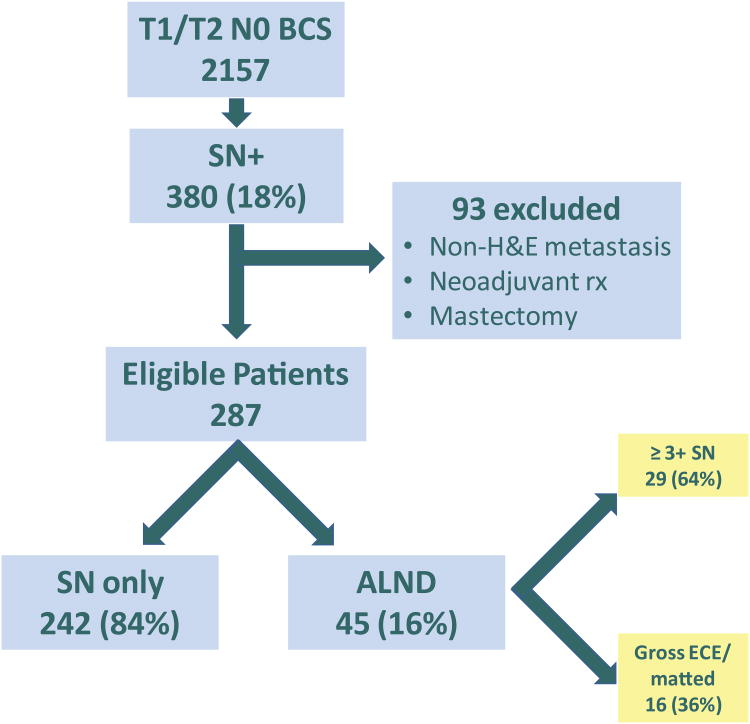
From 8/2010 to 11/2012, 2157 invasive breast cancer patients had breast-conserving surgery; 380 (18%) had a histologically positive SN. Ninety-three patients were ineligible due to SN metastasis detected by immunohistochemistry only (n = 58), conversion to mastectomy (n = 18), neoadjuvant chemotherapy (n = 11), or other miscellaneous reasons (n = 6). 287 patients, 75% of those with SN metastases, met ACOSOG Z0011 eligibility criteria; of these, 209 had macrometastases and 78 had micrometastases detected on routine H&E staining (Fig 1). The median patient age was 58 years (range, 28-92). The median tumor size was 1.7 cm, and 176 patients (61%) had T1 tumors. ER and/or PR were positive in 90% of patients, and 9% overexpressed HER2. Overall, a median of 3 SNs per patient were removed (range, 1-18), with a median of 1 positive SN. In 198 patients (69%), nodal metastases were limited to a single SN. Adjuvant systemic therapy was given to 276 (96%) patients; 3 patients are known not to have received treatment (2 patient refusals, 1 severe comorbidity), and decision making is ongoing in 8 patients. Follow-up at this time is insufficient to determine the number of patients receiving whole breast irradiation.
Fig 1.
Study Population.
BCS, breast-conserving surgery; SN, sentinel node; H&E, hematoxylin and eosin; ALND, axillary lymph node dissection; ECE, extracapsular extension
Of the 287 eligible patients, 242 (84%) met criteria for management with SLNB only. Completion ALND was indicated in 45 patients; however, only 39 received it. In 29 patients (64%), the indication was metastases in ≥ 3 SNs, and in 16 (36%), matted nodal disease or extracapsular extension of tumor (Fig 1).
There were 10 algorithm deviations, summarized in Table 1. Six cases met criteria for completion ALND, but the procedure was not performed. These included 2 patients who had extensive nodal sampling (9 and 12 nodes) as part of their SN procedure, and 3 patients who refused further surgery. Four patients had an un-indicated ALND, including 1 estimated to have a 76% likelihood of additional positive nodes by the MSKCC nomogram. Twelve of the 45 patients with indications for ALND also required margin re-excision; a second operation solely for completion ALND was indicated in 33 (11.5%) of the 287 patients in this study.
Table 1.
Summary of algorithm deviations (n = 10; 3%).
| Un-indicated ALND (n = 4) | Indicated ALND not completed (n = 6) | ||
|---|---|---|---|
| Violation | N | Violation | N |
| MD decision | 1 | Extensive nodal sampling at SLN | 2 |
| Nomogram | 1 | Multiple comorbidities | 1 |
| Grossly positive LN | 1 | Patient decision | 3 |
| Patient decision | 1 | ||
ALND, axillary lymph node dissection; SLN, sentinel lymph node; LN, lymph node
Patient and disease characteristics of the study groups are compared in Table 2. The 10 algorithm-deviation patients are analyzed according to the treatment they should have received. There was no significant difference in median patient age (58 versus 60 years; p = 0.35), nuclear grade (p = 0.56), or ER, PR, and HER2 status (p = 0.71) between groups. Patients requiring ALND had tumors that were slightly larger than those in patients having SLNB only, both by initial imaging evaluation (p < 0.01) and pathologic measurement (p < 0.001). The use of systemic therapy also did not vary between groups.
Table 2.
Patient and disease characteristics of the SN and ALND groups.
| SN (242) | ALND (45) | ||||
|---|---|---|---|---|---|
| median | min, max | median | min, max | p-value | |
| Age (years) | 58 | 28, 92 | 60 | 35, 83 | 0.35 |
| T size (cm) | |||||
| Imaging | 1.7 | .08, 6.0 | 2 | .90, 5.8 | 0.0045 |
| Pathologic | 1.6 | 0.25, 5.0 | 2.2 | 0.4, 4.5 | 0.0001 |
| Subtype | n | % | n | % | |
| HR+/HER2- | 201 | 83% | 37 | 82% | |
| HR+/HER2+ | 16 | 7% | 2 | 4% | |
| HR- | 23 | 10% | 6 | 13% | 0.71 |
| Nuclear grade* | |||||
| 1 (includes lobular) | 22 | 9% | 6 | 14% | |
| 2 | 136 | 57% | 22 | 50% | |
| 3 | 80 | 34% | 16 | 36% | 0.56 |
| Adjuvant therapy | |||||
| Any | 232 | 96% | 44 | 98% | 0.14 |
| Chemotherapy and endocrine therapy | 145 | 60% | 27 | 60% | |
| Endocrine therapy alone | 47 | 19% | 6 | 13% | |
| Chemotherapy alone | 17 | 7% | 7 | 16% | |
| Unknown | 7 | 3% | 1 | 2% | |
| None | 3 | 1% | 0 | 0 | |
SN, sentinel node; ALND, axillary lymph node dissection; HR, hormone receptor
missing 5 patients' data
The median number of SNs removed in the SN group was 3, compared to 5 in the ALND group (p < 0.0001), likely relating to the finding of grossly abnormal nodes intraoperatively and the need to document metastases in ≥ 3 nodes to perform ALND. The median number of positive SNs was 1 in the SN group and 3 in the ALND group (p < .0001). In the 39 patients who underwent the indicated ALND, 28 (72%) had additional positive nodes (median = 1; range, 1-19) (Fig 2). Residual tumor burden was small in half of the patients, with only 1 or 2 additional involved nodes, but 6 patients had ≥ 10 additional positive nodes. Using the MSKCC nomogram7, the median likelihood of additional positive nodes in the SN group was 34% (range, 5-81%) versus 57% (range, 6-94%) in the ALND group (p < 0.0001).
Fig 2.
Number of additional positive nodes in patients undergoing axillary lymph node dissection.
At a median follow-up of 13 months, no axillary recurrences have occurred. There has been 1 in-breast recurrence in the SN-only group and 3 distant recurrences in the ALND group.
Discussion
Breast cancers are being detected at an earlier stage due to widespread acceptance of breast cancer screening, resulting in patients diagnosed with smaller primary tumors and lower axillary tumor burden. Currently, systemic treatment decisions rely primarily on tumor biology rather than the number of axillary nodes containing metastases. Additionally, systemic therapy is now recognized as an important contributor to local control.8 In this setting, ACOSOG Z0011 supported eliminating ALND for a group of patients unlikely to have significant residual axillary disease1,2, but concerns remain about the ability to generalize these findings.
When we applied Z0011 selection criteria to a consecutive series of 287 SN positive patients undergoing BCT, extensive axillary nodal disease, evidenced by involvement of ≥ 3 SNs, was uncommon. Eighty-four percent of patients met criteria for SLNB alone and were spared the morbidity of ALND. To our knowledge, no other studies have prospectively addressed this question, but in a retrospective report of 42 patients for whom ALND was performed according to surgeon preference in patients meeting ACOSOG Z0011 eligibility criteria, 32 (76%) were spared ALND.9 In contrast, in a retrospective study of 449 T1 and T2, clinically node-negative patients undergoing BCT between 1994 and 2009 and found to have sentinel node metastases, ALND was performed in 328 (79%).10 At our own institution in 2006, 99% of patients found to have SN metastases by frozen section and 77% with disease detected by routine H&E staining underwent ALND.11
Of patients selected for ALND on the basis of involvement of ≥ 3 SNs, gross extracapsular extension, or matted nodes, 72% had additional nodal disease at the time of completion ALND, suggesting that these criteria identify patients at high risk of residual nodal disease. We did not, however, find that patient age, ER status, HER2 overexpression, and tumor grade predicted the need for ALND, suggesting that these variables are not sufficient selection criteria for ALND preoperatively.
Although the MSKCC nomogram7 predicted a significantly higher risk of residual axillary disease in patients in the ALND group compared to the SN group, the predictions were highly variable. The application of the ACOSOG Z0011 approach to axillary management necessitates acceptance of the fact that low-volume residual disease is left behind in the axilla in some patients, and will be adequately treated with tangent field whole-breast irradiation and systemic therapy. This concept is supported by several lines of evidence. The use of neoadjuvant chemotherapy in patients with histologically proven nodal metastases completely eradicates nodal disease in approximately 25% of cases12,13, and the use of tangent-field irradiation and endocrine therapy in patients having no axillary surgery results in axillary first-failure rates of < 4%14-16 in spite of 20-30% of these patients having nodal metastases. In ACOSOG Z0011, regional recurrence after SLNB alone was < 1%, despite the fact that 27% of patients randomized to the ALND arm of the study had additional metastases identified.1,2 In the IBCSG 2301 trial randomizing patients with sentinel node micrometastases to ALND or no further surgery, 13% of those in the ALND arm had additional nodal disease, but the axillary first failure rate in the sentinel node arm was only 1%, and no survival differences were noted.17
We chose not to routinely image the axilla with ultrasound or MRI because the preoperative finding of metastases in 1 or 2 nodes would not change patient management. Identification of extensive nodal disease preoperatively would be an indication for needle biopsy to allow immediate ALND, but we thought such extensive disease would be uncommon in this population. This supposition is supported by our finding that only 45 of 287 patients required ALND, and in 12 of these, re-operation was also indicated for margin re-excision. Axillary ultrasound relies upon abnormalities in nodal size or architecture to identify metastatic disease, and sensitivity and specificity for the identification of any nodal metastases range from 49-87% and 56-97%, respectively18-20, so it is unlikely that its use would have identified all cases requiring ALND for extracapsular extension or > 2 nodal metastases.
Our study also provides information on the degree to which the ACOSOG Z0011 population is representative of patients undergoing primary BCT for clinical T1 and T2, node-negative breast cancers. In our unselected patient cohort, patient and tumor characteristics were remarkably similar to those of women enrolled in ACOSOG Z0011 (Table 3).1,2 Using the current definition of hormone-receptor positivity of any staining for ER and PR21, only 10% of patients in our study were ER negative compared to 17% of patients in each Z0011 arm. ACOSOG Z0011 did not centrally specify the level of ER defining receptor positivity, and patient recruitment took place during a time when the definition of ER positivity was not standardized, potentially accounting for the lower number of ER negative cases seen in our study. In ACOSOG Z0011, as in our study, median patient age was in the mid-to-late 50s, reflecting the fact that breast cancer incidence increases with age. Patients in our series had relatively small tumors and low axillary disease burden; 176 (61%) had T1 tumors compared to 70% of patients in ACOSOG Z0011, and 198 (69%) had only 1 positive SLN, compared to 71% in ACOSOG Z0011. However, 73% of patients in our study had SN macrometastases compared to 59% in ACOSOG Z0011, likely representing our use of this approach for all clinically node-negative women with T1 and T2 tumors undergoing breast conservation with whole breast RT as opposed to the ability to randomize patients into ACOSOG Z0011 after SLNB, a process which could have selected for lower volume metastases not identified on frozen section.
Table 3.
Comparison of patient and tumor characteristics in current study to those of women enrolled in ACOSOG Z0011.
| MSKCC (n = 287) | ACOSOG Z0011 (n = 856) | |||
|---|---|---|---|---|
|
| ||||
| SN only | ALND | SN only | ALND | |
|
| ||||
| Age (median) | 58 | 60 | 54 | 56 |
|
| ||||
| T size (median) (cm) | 1.6 | 2.2 | 1.6 | 1.7 |
|
| ||||
| ER+ | 91% | 86% | 83% | 83% |
|
| ||||
| Nuclear grade* | ||||
| 1(includes lobular) | 9% | 14% | 26% | 22% |
| 2 | 57% | 50% | 47% | 49% |
| 3 | 34% | 36% | 27% | 29% |
|
| ||||
| Additional positive nodes | – | 72% | – | 27% |
MSKCC, Memorial Sloan-Kettering Cancer Center; SN, sentinel node; ALND, axillary lymph node dissection; ER, estrogen receptor
Bloom Richardson grade used in Z0011
The question of whether ACOSOG Z0011 results are applicable to women who are premenopausal and those with ER negative tumors is not fully addressed in our study due to the relatively small numbers of ER negative women and the lack of long-term follow-up. However, the finding that these women are not more likely to require ALND for a heavy nodal disease burden suggests that they should not a priori routinely undergo ALND. Patients with ER negative, HER2 negative tumors are not more likely to have extensive nodal disease burdens after adjusting for tumor size and grade, in comparison to ER positive patients. Several studies suggest that these patients are actually less likely to have ≥ 4 positive axillary nodes22-24 in spite of their poor prognosis. Additionally, 2 large studies examining nodal recurrence in patients undergoing ALND have failed to identify ER status or age as predictors of an increased risk of nodal recurrence.25,26
The median follow-up of 13 months is clearly too brief to draw any conclusions about the incidence of nodal recurrences, but none have been observed to date. In spite of the short duration of follow-up, 1 patient has had an in-breast recurrence, and 3 have developed distant metastases, suggesting that regional recurrence is unlikely to be the greatest problem facing women in this patient population.
Conclusions
In summary, in this prospective study to determine how often ALND can be avoided in a consecutive series of patients meeting ACOSOG Z0011 eligibility criteria, 84% of patients not selected on the basis of age, tumor characteristics, axillary imaging, or nomogram predictions were found to have metastases in ≤ 2 axillary nodes, thus avoiding the morbidity and financial cost of ALND.27 While it is possible that this is a reflection of our institutional patient population, we used standard selection criteria for BCT3,28, suggesting that similar outcomes can be expected in other practice settings. Longer follow-up is needed to determine the rate of nodal recurrence in this patient population, but our finding of a heavy residual nodal disease burden in patients with ≥ 3 involved SNs, matted nodes, and gross extracapsular extension sounds a note of caution to those attempting to extrapolate ACOSOG Z0011 results beyond the eligibility criteria of the trial.
Synopsis.
Here we examine the applicability of ACOSOG Z0011 to an unselected population meeting trial eligibility criteria. Axillary dissection and its associated morbidity was avoided in 84% of patients.
Acknowledgments
This study was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of Interest None
Presented at the plenary session of the 2013 Society of Surgical Oncology Annual Cancer Symposium, March 8, 2013.
References
- 1.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–32. doi: 10.1097/SLA.0b013e3181f08f32. discussion 32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sneige N, Wang J, Baker BA, et al. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: a report of 24 cases. Mod Pathol. 2002;15(10):1044–50. doi: 10.1097/01.MP.0000027624.08159.19. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AE, Morrow M, Duggal S, et al. Should ACOSOG Z0011 change practice with respect to axillary lymph node dissection for a positive sentinel lymph node biopsy in breast cancer? Clin Exp Metastasis. 2012;29(7):687–92. doi: 10.1007/s10585-012-9515-z. [DOI] [PubMed] [Google Scholar]
- 5.Shah-Khan M, Boughey JC. Evolution of axillary nodal staging in breast cancer: clinical implications of the ACOSOG Z0011 trial. Cancer Control. 2012;19(4):267–76. doi: 10.1177/107327481201900403. [DOI] [PubMed] [Google Scholar]
- 6.Guth U, Myrick ME, Viehl CT, et al. The post ACOSOG Z0011 era: does our new understanding of breast cancer really change clinical practice? Eur J Surg Oncol. 2012;38(8):645–50. doi: 10.1016/j.ejso.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer--bigger is not better. N Engl J Med. 2012;367(1):79–82. doi: 10.1056/NEJMsb1202521. [DOI] [PubMed] [Google Scholar]
- 9.Caudle AS, Hunt KK, Tucker SL, et al. American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol. 2012;19(10):3144–51. doi: 10.1245/s10434-012-2531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi M, Kuerer HM, Mittendorf EA, et al. Impact of the American College of Surgeons Oncology Group (ACOSOG) Z0011 criteria applied to a contemporary patient population. J Am Coll Surg. 2013;216(1):105–13. doi: 10.1016/j.jamcollsurg.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber WP, Barry M, Stempel MM, et al. A 10-year trend analysis of sentinel lymph node frozen section and completion axillary dissection for breast cancer: are these procedures becoming obsolete? Ann Surg Oncol. 2012;19(1):225–32. doi: 10.1245/s10434-011-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26(5):814–9. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 13.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 14.Martelli G, Miceli R, Daidone MG, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol. 2011;18(1):125–33. doi: 10.1245/s10434-010-1217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudenstam CM, Zahrieh D, Forbes JF, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10-93. J Clin Oncol. 2006;24(3):337–44. doi: 10.1200/JCO.2005.01.5784. [DOI] [PubMed] [Google Scholar]
- 16.Veronesi U, Orecchia R, Zurrida S, et al. Avoiding axillary dissection in breast cancer surgery: a randomized trial to assess the role of axillary radiotherapy. Ann Oncol. 2005;16(3):383–8. doi: 10.1093/annonc/mdi089. [DOI] [PubMed] [Google Scholar]
- 17.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez S, Anorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186(5):1342–8. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita M, Hovanessian-Larsen L, Sener SF. The role of axillary ultrasound in the detection of metastases from primary breast cancers. Am J Surg. 2013;205:242–5. doi: 10.1016/j.amjsurg.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Yang WT, Ahuja A, Tang A, et al. High resolution sonographic detection of axillary lymph node metastases in breast cancer. J Ultrasound Med. 1996;15(3):241–6. doi: 10.7863/jum.1996.15.3.241. [DOI] [PubMed] [Google Scholar]
- 21.Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. 2001;93(13):979–89. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 22.Crabb SJ, Bajdik CD, Leung S, et al. Can clinically relevant prognostic subsets of breast cancer patients with four or more involved axillary lymph nodes be identified through immunohistochemical biomarkers? A tissue microarray feasibility study Breast Cancer Res. 2008;10(1):R6. doi: 10.1186/bcr1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foulkes WD, Grainge MJ, Rakha EA, et al. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res Treat. 2009;117(1):199–204. doi: 10.1007/s10549-008-0102-6. [DOI] [PubMed] [Google Scholar]
- 24.Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol. 2009;16(10):2705–10. doi: 10.1245/s10434-009-0606-2. [DOI] [PubMed] [Google Scholar]
- 25.Grills IS, Kestin LL, Goldstein N, et al. Risk factors for regional nodal failure after breast-conserving therapy: regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys. 2003;56(3):658–70. doi: 10.1016/s0360-3016(03)00017-8. [DOI] [PubMed] [Google Scholar]
- 26.Yates L, Kirby A, Crichton S, et al. Risk factors for regional nodal relapse in breast cancer patients with one to three positive axillary nodes. Int J Radiat Oncol Biol Phys. 2012;82(5):2093–103. doi: 10.1016/j.ijrobp.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 27.Camp MS, Greenup RA, Taghian A, et al. Application of ACOSOG Z0011 Criteria Reduces Perioperative Costs. Ann Surg Oncol. 2013;20:836–41. doi: 10.1245/s10434-012-2664-0. [DOI] [PubMed] [Google Scholar]
- 28.Morrow M, Harris JR. Practice guideline for the breast conservation therapy in the management of invasive breast carcinoma. J Am Coll Surg. 2007;205(2):362–76. doi: 10.1016/j.jamcollsurg.2007.02.057. [DOI] [PubMed] [Google Scholar]