Abstract
Background
Our aim was to determine the incidence rates of head and neck cancer in Vietnamese Californians compared with other Asian and non-Asian Californians.
Methods
Age-adjusted incidence rates of head and neck cancer between 1988 and 2004 were computed for Vietnamese Californians compared with other racial/ethnic groups by time period, ethnicity, neighborhood-level socioeconomic status (SES), and sex using data from the population-based California Cancer Registry (CCR). Data by smoking and alcohol status were tabulated from the California Health Interview Survey.
Results
Vietnamese men had a higher incidence rate of head and neck cancer than other Asian men. Specifically, the laryngeal cancer rate was significantly higher for Vietnamese men (6.5/100,000; 95% confidence interval [CI], 5.0–8.2) than all other Asian men (range, 2.6–3.8/100,000), except Korean men (5.1/100,000; 95% CI, 3.9–6.4). Both Vietnamese and Korean men had the highest percentage of current smokers. Neighborhood SES was inversely related to head and neck cancer rates among Vietnamese men and women.
Conclusion
The higher incidence rate of head and neck cancer in Vietnamese men may correspond to the higher smoking prevalence in this group. Individual-level data are needed to establish the link of tobacco, alcohol, and other risk factors with head and neck cancer in these patients.
Keywords: head and neck cancer, Vietnamese, tobacco, socioeconomic status, immigrant status
Head and neck cancers account for 5% of all diagnosed cancers in the United States, but they are associated with considerable morbidity and mortality.1 Head and neck cancers are commonly found in the oral cavity, oropharynx, larynx, and hypopharynx and are most often of squamous histology. Although uncommon, they are severely debilitating because of their location. Often these tumors destroy the integrity of the surrounding organs that are crucial for swallowing and speech function. Moreover, treatments such as aggressive surgery, high-dose radiation, and chemotherapy also result in significant functional morbidities including dysphasia, dry mouth, hoarseness, and facial disfigurement. Because >75% of these cancers present at a late stage, the overall cure rate for head and neck cancers remains <50% and has not changed for decades.2
Established risk factors that have been associated with the development of head and neck cancers include tobacco consumption (both smoking and chewing), alcohol use, and the human papillomavirus (HPV).3 Smoking and alcohol are by far the strongest risk factors for development of head and neck cancer, with substantial molecular evidence linking tobacco-related carcinogens to these cancers.2 Moreover, mutations in P53 (TP53), a tumor suppressor protein, that have been commonly found in head and neck cancers, have also been linked to substantial tobacco and alcohol exposure.4 Heavy tobacco users have a 5-to 25-fold higher risk of developing head and neck cancer than nonsmokers, and alcohol can further increase this risk. A recent analysis of pooled data from 17 European and American case-control studies (11,221 cases and 16,168 controls) participating in the International Head and Neck Cancer Epidemiology Consortium revealed that the population attributable risk was 72% (95% confidence interval [CI], 61% to 79%) for head and neck cancer from tobacco or alcohol use, of which 4% was for alcohol alone, 33% was for tobacco alone, and 35% was for overlap between tobacco and alcohol.5
Although tobacco use has been declining in the overall U.S. population, it remains high among Vietnamese men. According to the 2007 California Health Interview Survey (CHIS), the prevalence of current smoking among Vietnamese men was 35.4% (95% CI, 22.6–48.2) which is significantly higher than that among non-Asian men (17.9%; 95% CI, 16.8–18.9). This high prevalence among Vietnamese men has remained relatively unchanged since 2001.6 Moreover, heavy alcohol use has been shown to be common in Vietnamese men living in the United States and associated with an increased likelihood of smoking in these men.7 We and others have previously found that the incidence rates of non-small cell lung cancer (NSCLC), another tobacco-related neoplasm, are higher in Vietnamese men than other Asian subgroups in California.8 However, little is known about the incidence rates of head and neck cancer in this population in comparison to other Asian and non-Asian ethnic populations. We hypothesized that the incidence rates of head and neck cancer are higher for Vietnamese men in California compared to other Asian populations in the same state. To address our hypothesis, we determined the incidence rate of head and neck cancer for the Vietnamese population in comparison to other Asian populations in California according to the primary tumor site, time period, sex, and neighborhood-level socioeconomic status (SES) using data from the CCR, the state-mandated population-based cancer registry. Because we lack reliable individual data on tobacco and alcohol use in the CCR, we used the CHIS database (available at http://www.chis.ucla.edu/) to gather the data on tobacco and alcohol consumption for the different ethnic groups.
MATERIALS AND METHODS
The population-based CCR, which is not a publicly available database, records information on all cancer diagnoses in the entire state of California. The CCR has agreements with 13 other states for case-sharing purposes and maintains high-quality data standards as part of the National Cancer Institute's (NCI) Surveillance, Epidemiology, and End Results (SEER) program. Reporting of new cancer diagnoses to the CCR has been mandated by California state law since 1985, and coverage is estimated to be 99% complete.9 Data from the CCR were obtained for incidence rates of head and neck cancer diagnosed between 1988 and 2004 in Asian ethnic populations and non-Asian racial/ethnic groups in California. The SEER*Stat software (available at http://seer.cancer.gov/seerstat/) was used to compute these rates (see below). Head and neck cancers were defined as those arising from the oral cavity, oropharynx, larynx, and hypopharynx, and were analyzed based on the primary tumor site, time period (1988–1993, 1994– 1999, 2000–2004), race/ethnicity (Vietnamese, Chinese, Japanese, Filipino, Korean, South Asian [including Asian Indians, Pakistanis, Sri Lankans, and Bangladeshis], non-Hispanic white, non-Hispanic black, and Hispanic), and sex (men, women). Tumors were classified histo-logically as squamous cell carcinomas (ICD-0-3 codes 8050–8089) or non-squamous tumors (ICD-0-3 histology codes others than those listed above for the individual primary site). The following ICD-0-3 site codes were used for head and neck subsite classification: C00-06 for oral cavity, C09-10 for oropharynx, C12-13 for hypopharynx and C14, and C32 for larynx.10
From the 1990 and 2000 Census Summary File 3 (SF-3), we obtained population counts by sex, race/ethnicity, immigrant status, and 5-year age group for the state of California. Age-adjusted rates (based on the 2000 U.S. population standard) were computed using SEER*Stat software and compared by calculating rate ratios with 95% CIs. In accordance with CCR policy, rates were not calculated based on fewer than 15 cases.
The CCR records each patient's residential address at the time of diagnosis. Using this address, we determined neighborhood-level SES for each patient using an index that combines census block-group averages of education, income, occupation, and cost of living, as described previously.11 This index was categorized into quintiles based on the state-wide distribution of neighborhood-level SES; the quintiles were then collapsed into 2 groups for ease of comparison (lower SES = quintiles 1, 2, and 3; higher SES = quintiles 4 and 5).
Because individual-level data on tobacco and alcohol use are not available in cancer registry data, we used data from CHIS (available at http://www.chis.ucla.edu/) to determine the population-level prevalence of tobacco and alcohol use for the Asian populations between in 2001 and 2005, an interval that fell within the larger time period that we used to determine the initial incidence rate of these cancers by race/ethnicity and sex. For cigarette use, we used the latest most completely updated dataset, which is the 2007 dataset. For the alcohol data, we pooled the available 2001 and 2003 CHIS data. In our assessment of alcohol usage, we looked at the number of drinks per day and the number of days at least 1 drink was consumed in the last month, among the Asian ethnicities. Data taken from CHIS provided a breakdown of the number of drinks per day into categories of 0, 1, 2, 3, 4, and 5 or more. For data reporting, we chose to add the percentages for 2 through 4 drinks per day. We believe that these groupings reflect light (0–1 drink/day), moderate (2–4 drinks/day) and heavy (>5 drinks/day) drinkers. Similarly, for the number of days at least 1 drink was consumed in the last month, CHIS provides an extensive breakdown into categories of 1, 2, 3, 4, 5–9, 10–20, and 21 or greater. Again for data reporting, we have summed the percentages for 1 through 9 days. We believe that grouping the numbers of day drank/month by every 10 days adequately distinguish the infrequent drinkers from the regular ones. This study was approved by the institutional review board for the Northern California Cancer Center.
RESULTS
Incidence of Head and Neck Cancers by Ethnicity and Subsite
Table 1 shows the estimated incidence rates, with 95% CI, and case numbers of site-specific head and neck cancer by race/ethnicity and sex. The incidence rates of all head and neck cancer subsites were highest for Vietnamese men when compared to other Asian men, except for the rate of oral cavity cancers, which was highest for South Asians. Specifically, Vietnamese men had the highest incidence rate of laryngeal cancer among all Asian men in California and the difference was statistically significant when compared to all other ethnic groups of Asian men, except Koreans.
Table 1.
Age-adjusted incidence rate (per 100,000 person-years, standardized to the 2000 U.S. population), with 95% CI, of site-specific head and neck cancers in men (upper) and women (lower) by race/ethnicity and primary tumor site in California, 1988–2004.
| Larynx |
Oropharynx |
Hypopharynx |
Oral cavity |
All HNC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Race/Ethnicity | Rate (95% CI) | No. cases | Rate (95% CI) | No. cases | Rate (95% CI) | No. cases | Rate (95% CI) | No. cases | Rate (95% CI) | No. cases |
| Male | ||||||||||
| Vietnamese | 6.5 (5.0–8.2) | 89 | 1.9 (1.3–2.6) | 37 | 1.7 (1.0–2.6) | 23 | 4.3 (3.3–5.5) | 81 | 14.3 (12.2–16.6) | 230 |
| Chinese | 3.2* (2.2–3.7) | 188 | 0.8 (0.6–1.1)* | 53 | 0.8 (0.6–1.0) | 49 | 3.3 (2.9–3.8) | 203 | 8.1* (7.4–8.9) | 493 |
| Japanese | 2.6* (2.0–3.2) | 73 | 0.9 (0.6–1.4) | 26 | 0.7 (0.4–1.0) | 20 | 3.3 (2.7–4.1) | 93 | 7.5* (6.5–8.6) | 212 |
| Filipino | 3* (2.5–3.5) | 146 | 1.1 (0.9–1.4) | 60 | 0.4* (0.3–0.7) | 22 | 2.6* (2.2–3.1) | 130 | 7.1* (6.4–7.9) | 358 |
| Korean | 5.1 (3.9–6.4) | 82 | 1.7 (1.1–2.4) | 31 | 1.3 (0.7–2.0) | 19 | 3.8 (2.8–5.1) | 59 | 11.8 (10.1–13.8) | 191 |
| South Asian | 3.8* (2.6–5.4) | 40 | – | 7 | – | 10 | 5.3 (3.9–6.9) | 76 | 10.5* (8.5–12.8) | 133 |
| NH white | 7.1 (7.0–7.4) | 10,344 | 3* (2.9–3.1) | 4,436 | 1.5 (1.5–1.6) | 2,236 | 8.5* (8.3–8.6) | 12,323 | 20.1* (19.9–20.4) | 29,339 |
| NH black | 11.4* (10.7–12.0) | 1,337 | 3.8* (3.5–4.2) | 493 | 2.5 (2.2–2.8) | 293 | 9.6* (9.0–10.2) | 1,200 | 27.3* (26.3–28.4) | 3,323 |
| Hispanic | 5.4 (5.1–5.7) | 1,607 | 1.7 (1.6–1.9) | 618 | 1.2 (1.1–1.3) | 354 | 4.3 (4.0–4.5) | 1,438 | 12.5 (12.1–13.0) | 4,017 |
| Female | ||||||||||
| Vietnamese | – | 6 | 0.6 (0.3–1.0) | 17 | – | 2 | 2.8 (2.0–3.7) | 49 | 3.7 (2.4–4.8) | 74 |
| Chinese | 0.3 (0.2–0.4) | 21 | 0.2 (0.1–0.4) | 17 | – | 3 | 2.3 (1.9–2.6) | 167 | 2.8 (2.4–3.2) | 208 |
| Japanese | 0.3 (0.2–0.6) | 15 | – | 11 | – | 8 | 2.8 (2.2–3.4) | 106 | 3.6 (3.0–4.2) | 140 |
| Filipino | 0.4 (0.2–0.6) | 26 | 0.5 (0.4–0.6) | 32 | – | 6 | 2.7 (2.3–3.1) | 174 | 3.7 (3.2–4.2) | 238 |
| Korean | – | 13 | – | 6 | – | 0 | 1.3* (0.9–1.9) | 33 | 2.2 (1.6–2.8) | 52 |
| South Asian | – | 6 | – | 5 | – | 6 | 4.4 (3.2–5.9) | 55 | 6.1* (4.6–7.9) | 72 |
| NH white | 1.6 (1.6–1.7) | 2,803 | 1.0 (1.0–1.1) | 1,754 | 0.5 (0.4–0.5) | 832 | 4.4* (4.3–4.5) | 7,845 | 7.5* (7.4–7.6) | 13,234 |
| NH black | 2.3 (2.1–2.6) | 355 | 1.0 (0.9–1.2) | 155 | 0.4 (0.3–0.6) | 68 | 3.3 (3.1–3.6) | 520 | 7.1* (6.7–7.6) | 1,098 |
| Hispanic | 0.7 (0.6–0.8) | 291 | 0.4 (0.3–0.5) | 149 | 0.2 (0.1–0.2) | 59 | 2.3 (2.1–2.4) | 890 | 3.5 (3.3–3.7) | 1,389 |
Abbreviations: HNC, head and neck cancer; NH: non-Hispanic.
– Number too small for accurate rate calculation. In accordance with CCR policy, rates were not calculated when there were fewer than 15 cases.
The difference was statistically significant (p ≤ 0.05) when compared with the Vietnamese group.
Although most Asian ethnic groups had a lower incidence rate of head and neck cancer than non-Hispanic whites, non-Hispanic blacks, and Hispanics, the incidence rates of laryngeal, oropharyngeal, and hypopharyngeal cancers in Vietnamese men approached those of Hispanic and non-Hispanic white men (Table 1). The only other Asian subgroup with head and neck cancer incidence rates approaching that of Vietnamese men was Korean men. When incidence rates were stratified by histology, most of head and neck cancer cases in Vietnamese men were squamous cell carcinomas. There was no obvious difference in the incidence rate of head and neck cancer among women by Asian ethnic group (Table 1); however, the number of cases was too small to estimate rates in several groups, including Vietnamese women.
Because the examined period of 1988–2004 spanned more than a decade, during which incidence rates may have changed, we also examined laryngeal cancer incidence rates in 3 smaller time periods: 1988–1993, 1994–1999, and 2000–2004 (Table 2). The incidence of laryngeal cancer among Vietnamese men was significantly higher than that for men of other Asian ethnic groups, except Koreans, in all 3 periods.
Table 2.
Age-adjusted incidence rate (per 100,000 person-years, standardized to the 2000 U.S. population), with 95% CI, of laryngeal cancer in Asian men in California by ethnic group and time period.
| 1988–1993 |
1994–1999 |
2000–2004 |
||||
|---|---|---|---|---|---|---|
| Laryngeal cancer incidence | Rate (95% CI) | No. cases | Rate (95% CI) | No. cases | Rate (95% CI) | No. cases |
| Vietnamese | 10.5 (6.0–16.6) | 25 | 5.8 (3.6–8.6) | 29 | 5.5 (3.6–8.0) | 35 |
| Chinese | 3.9 (2.9–5.2)* | 58 | 3.2 (2.5–4.1)* | 68 | 2.6 (2–3.4)* | 62 |
| Japanese | 3.3 (2.1–5.0)* | 28 | 2.5 (1.6–3.8)* | 25 | 2.1 (1.3–3.3)* | 20 |
| Filipino | 3.4 (2.4–4.5)* | 44 | 2.9 (2.2–2.9)* | 50 | 2.8 (2.1–3.6)* | 52 |
| Korean | 7.3 (4.7–10.9) | 29 | 4.69 (2.89–7.12) | 26 | 4.1 (2.6–6.1) | 27 |
| South Asian | – | 10 | – | 11 | 3.8 (2.1–6.2) | 19 |
The difference was statistically significant (p ≤ .05) when compared with the Vietnamese group.
– Number too small for accurate rate calculation. In accordance with CCR policy, rates were not calculated when there were fewer than 15 cases.
Prevalence of Cigarette Smoking and Alcohol use in Asian Ethnic Groups
Table 3 shows the percent of never smokers (<100 cigarettes in the entire lifetime), former smokers (at least 100 cigarettes in the entire lifetime but currently not smoking), and current smokers (at least 100 cigarettes in the entire lifetime and currently smoking) in men and women for the different Asian ethnic groups in 2007. The percentage of current and past smokers was the highest in Vietnamese and Korean men. By contrast, the percentage of current and former smokers was lowest for Vietnamese women compared to other Asian women. These rates were tabulated for adults age >18. When we tried to evaluate the rates by age groups (eg, 18–49 vs 50 or greater), the numbers became too small and the rates were unstable. However, the trend is similar for all age groups and the percentages of current and past smokers were consistently highest in Vietnamese and Korean men. With regard to the relationship between smoking and alcohol use, the CHIS database only allowed us to determine the smoking status for non-binge drinkers and binge drinkers. For both categories, the Vietnamese men had the highest rate of current smokers; it was 55.3% (95% CI, 28.5– 82.1) and 30.7% (95% CI, 16.2–45.2) for binge and non-binge drinkers, respectively.
Table 3.
Percent of current smokers, former smokers, and nonsmokers among individuals age 18 and over from different Asian subgroups in California (2007 data from the California Health Interview Survey).10
| Race/ethnicity | Current (95% CI) | Former (95% CI) | Never (95% CI) |
|---|---|---|---|
| Male | |||
| Vietnamese | 35.4% (22.6–48.2) | 22.6% (15.5–29.6) | 42.0% (31.0–53.0) |
| Chinese | 14.5% (9.5–19.5) | 19.4% (14.0–24.8) | 66.1% (59.7–72.4) |
| Japanese | 12.8% (5.0–20.6) | 27.3% (18.4–36.3) | 59.9% (48.7–71.0) |
| Filipino | 19.4% (12.3–26.5) | 33.0% (24.3–41.7) | 47.6% (38.3–56.9) |
| Korean | 21.8% (13.8–29.8) | 26.0% (16.7–35.2) | 52.3% (41.5–63.1) |
| South Asian | 12.9% (7.2–18.6) | 12.7% (7.5–17.8) | 74.5% (67.0–81.9) |
| Other Asian | 16.5% (3.5–29.5) | 14.3% (5.4–23.2) | 69.2% (54.4–84.0) |
| All Asian | 18.7% (15.5–21.9) | 22.8% (19.8–25.9) | 58.5% (54.7–62.2) |
| Female | |||
| Vietnamese | 2.0% (0.2–3.8) | 1% (0.0–2.0) | 97.0% (94.9–99.1) |
| Chinese | 2.9% (0.0–5.7) | 5.3% (2.8–7.8) | 91.9% (88.2–95.5) |
| Japanese | 6.9% (3.3–10.4) | 18.3% (11.6–25.1) | 74.8% (67.6–82.1) |
| Filipino | 4.6% (2.2–6.9) | 9.2% (5.3–13.0) | 86.3% (81.9–90.7) |
| Korean | 9.5% (0.4–18.6) | 8.6% (0.2–17.0) | 81.9% (71.0–92.8) |
| South Asian | 4.3% (0–11.6) | 2.0% (0.3–3.6) | 93.7% (86.4–100) |
| Other Asian | 0.7% (0–1.8) | 16.4% (0–32.9) | 82.9% (66.4–99.3) |
| All Asian | 4.2% (2.7–5.8) | 7.4% (5.6–9.2) | 88.4% (86.1–90.7) |
Table 4 shows the distribution of alcohol consumption status by ethnicity from pooled CHIS data between 2001 and 2003. Current drinkers were defined as those who responded yes to alcohol consumption within the past month. The distribution of each ethnic subgroup for the number of days drank in the past month and the number of drinks per day is tabulated in Table 4. Although Korean men had the highest percentage of people who drink 5 or more drinks per day, the Japanese and Vietnamese men led the rank in terms of the percentage of people who drink 21 or more days in the past month. A similar trend was noted when these percentages were evaluated by age group (18–49 vs >50-years-old). When we tried to evaluate the alcohol consumption status for current and noncur-rent smokers, the percentages of heavy drinkers (those who drink more than 5 drinks/day or more 21 days in the past month) were unstable for all ethnic groups due to the small number of subjects. However, unlike the cigarette data, the relationship between head and neck cancer incidence rate and alcohol consumption in Asian Californians was less clear.
Table 4.
Alcohol consumption by Asian ethnic group (pooled 2001 and 2003 data from the California Health Interview Survey).
| No. of drinks per day (2001, 2003 pooled) |
No. of days drank in past month (2001, 2003 pooled) |
||||||
|---|---|---|---|---|---|---|---|
| Race/ethnicity | 0 (CI) | 1 (CI) | 2–4 (CI) | ≥5 (CI) | 1–9 (CI) | 10–20 (CI) | ≥21 (CI) |
| Male | |||||||
| Vietnamese | 0.6* (0.1–1.2) | 59.4 (52.9–66.5) | 33.4 (26.5–40.4) | 6.5 (3.4–9.9) | 77.6 (71.2–84.0) | 11.3 (5.4–17.2) | 11.2 (7.2–15.1) |
| Chinese | 0.6* (0–1.3) | 70.2 (65.2–75.2) | 25.5 (20.7–30.3) | 3.8 (1.6–5.9) | 82.6 (78.5–86.6) | 10.3 (7.2–13.4) | 7.1 (4.1–10.1) |
| Japanese | – | 54.1 (45.8–62.4) | 39.2 (31.1–47.3) | 6.7* (2.6–10.8) | 68.6 (60.9–76.2) | 17.9 (11.7–24.0) | 13.6 (7.9–19.3) |
| Filipino | – | 35.1 (28.6–41.6) | 51.5 (44.6–58.4) | 12.7 (7.6–17.8) | 84.6 (79.5–89.7) | 12.7 (7.8–17.7) | 2.6* (0.9–4.4) |
| Korean | – | 37.7 (30.6–44.9) | 47.8 (40.3–55.3) | 14.5 (9.3–19.7) | 81.8 (76.5–87.1) | 12.5 (7.7–17.2) | 5.7 (3.0–8.5) |
| South Asian | – | 45.6 (37.7–53.4) | 52.7 (44.9–60.6) | 1.7* (0–3.7) | 84.7 (78.9–90.8) | 8.2 (4.1–12.3) | 7.0* (2.3–11.7) |
| Other Asian | – | 44.6 (33.1–56.0) | 39.1 (27.8–50.4) | 12.6* (3.4–21.8) | 79.7 (69.2–90.2) | 11.7* (3.8–19.5) | 8.7* (0.5–16.8) |
| All Asian | 0.6 (0.1–1.2) | 50.9 (48.1–53.8) | 40.5 (37.6–43.3) | 8.0 (6.2–9.7) | 81.4 (79.1–83.7) | 11.6 (9.7–13.5) | 7.0 (5.6–8.4) |
| Female | |||||||
| Vietnamese | 6.2* (0.9–11.5) | 78.6 (67.9–89.2) | 14.6* (4.7–24.4) | – | 97 (94.3–99.6) | 1.7* (0–3.5) | 1.3* (0.0–3.2) |
| Chinese | 1.1* (0.0–2.2) | 79.3 (74.0–84.7) | 19.0 (13.7–24.3) | – | 87.7 (83.9–94.1) | 10.3 (6.7–13.9) | 2.0* (0.6–3.4) |
| Japanese | – | 73.2 (65.1–81.3 | 25.9 (17.9–34.0) | – | 81.4 (75.1–87.7) | 13.7 (8.3–19.2) | 4.9* (1.3–8.4) |
| Filipino | – | 62.5 (55.5–69.5) | 33.9 (26.9–40.8) | 3.2* (0.4–6.0) | 91.8 (87.7–95.8) | 5.6* (2.2–9.1) | 2.6* (0.3–4.9) |
| Korean | – | 57.3 (49.3–65.3) | 41.0 (33.0–49.0) | – | 92.7 (89.6–98.8) | 5.3 (2.8–7.8) | 2.0* (0.1–3.9) |
| South Asian | – | 72.5 (62.9–82.1) | 26.6 (17.1–36.1) | – | 86.9 (79.7–94.2) | 9.7* (3.2–16.2) | – |
| Other Asian | – | 63.5 (50.6–76.5) | 34.3 (21.6–47.1) | – | 93.1 (87.6–98.5) | 5.5* (0.3–10.7) | – |
| All Asian | 1.0 (0.4–1.5) | 69.6 (66.4–72.8) | 28 (24.9–31.2) | 1.4 (0.6–2.3) | 89.4 (87.5–91.3) | 8.1 (6.4–9.8) | 2.5 (1.6–3.5) |
Statistically unstable due to the small number of subjects in the category.
– The number of subjects in the category was too small for percentage calculation.
Incidence of Head and Neck Cancers in Vietnamese by Socioeconomic Status
We evaluated the incidence rate of head and neck cancer in relation to neighborhood-level SES in all Asian subgroups (Table 5). As shown in this table, lower-SES Vietnamese, Filipinos, and Korean men had a significantly higher incidence rate of head and neck cancer than their higher-SES counterparts. Although the overall incidence rate for head and neck cancer was relatively low in Vietnamese women, it was twice as high in the lower-SES group as in the higher-SES group. In general, incidence rates of subtypespecific head and neck cancers were also higher in the lower-SES group and the difference was statistically significant for laryngeal cancers (Figure 1).
Table 5.
Age-adjusted incidence rate (per 100,000 person-years, standardized to the 2000 U.S. population), with 95% CI, of head and neck cancers in men and women by Asian ethnicity and neighborhood socioeconomic status in California, 1988–2004.
| Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower SES† |
Higher SES |
Lower SES |
Higher SES |
|||||||
| Ethnicity | Rate | (CI) | Rate | (CI) | p value | Rate | (CI) | Rate | (CI) | p value |
| Vietnamese | 9.6 | (7.8–11.5) | 4.7 | (3.6–6.0) | <.01* | 2.5 | (1.8–3.3) | 1.2 | (0.7–1.9) | .01* |
| Chinese | 3.9 | (3.4–4.5) | 4.2 | (3.7–4.7) | .52 | 1.1 | (0.9–1.4) | 1.7 | (1.4–2.0) | .01* |
| Japanese | 3.8 | (3.1–4.6) | 3.7 | (3.1–4.5) | 1.00 | 1.7 | (1.3–2.2) | 1.8 | (1.4–2.3) | .81 |
| Filipino | 4.4 | (3.8–5.0) | 2.7 | (2.3–3.2) | <.01* | 2.1 | (1.8–2.5) | 1.6 | (1.3–1.9) | .04* |
| Korean | 7.4 | (5.9–9.0) | 4.5 | (3.5–5.6) | <.01* | 1.1 | (0.7–1.6) | 1.1 | (0.7–1.6) | 1.00 |
| South Asian | 4.0 | (2.8–5.4) | 6.5 | (4.9–8.4) | .02* | 2.5 | (1.6–3.5) | 3.6 | (2.4–5.2) | .17 |
Abbreviations: SES, socioeconomic status; NH, non-Hispanic.
Rate for higher SES is significantly different than rate for lower SES (p < .05).
See methods for definition of SES.
FIGURE 1.
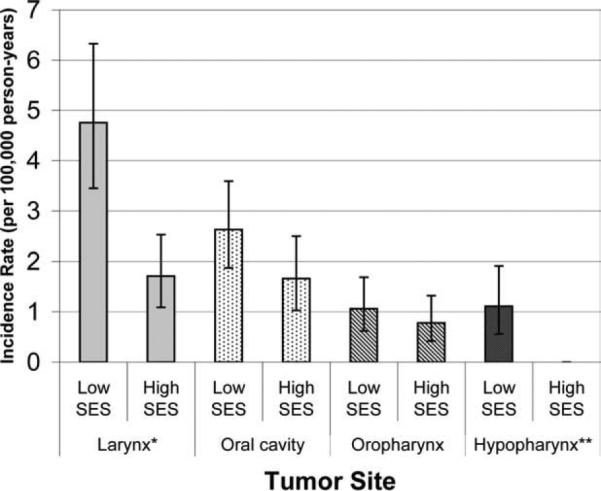
Age-adjusted incidence rate (standardized to the 2000 U.S. population) for different head and neck cancer sub-sites per 100,000 person-years in Vietnamese men by socioeconomic status (* the difference between the incidence rates of larynx cancer for the low and high SES group was statistically significant with p < .01. ** The incidence rate of hypopharynx cancer for the high SES group was not calculatable due to the very small case count).
DISCUSSION
In our population-based analysis, we noted that the incidence rate of head and neck cancer was generally higher in Vietnamese men than other Asian men. Specifically, the incidence rate of laryngeal cancer was significantly higher for Vietnamese men than for those of other Asian ethnicities, except Korean men, and it was as high as that for Hispanic and non-Hispanic white men. These data seem to be consistent with the high prevalence of smoking in Vietnamese men. Although the CCR lacks individual patient-level smoking data, CHIS surveys for Asians in the same state showed that Vietnamese and Korean men were more likely than other Asian men to be current or past smokers, followed by Filipino men. Cigarette use most likely contributed to the higher incidence rate of head and neck cancer noted among Vietnamese and Korean men. Our group has previously performed a population-based study of the incidence rates of NSCLC in California Asians between 1998 and 2003 and found that the Vietnamese had the highest rate of these tumors (39.2 per 100,000 person-years) among the Asian populations.8 These data for NSCLC are also consistent with the high smoking prevalence among the male Vietnamese American population. The relationship between smoking and head and neck cancer may also explain the low incidence rate of these cancers in Vietnamese women because this group has the lowest percentage of current smoker and the highest percentage of former smokers among the Asian subgroups in California.
Because alcohol use is another known major risk factor for head and neck cancer development, we also investigated the CHIS database for information on alcohol use for the different Asian ethnic groups in California. The relationship between head and neck cancer incidence and alcohol consumption in these groups is not as clear as that for cigarette use. Although the Koreans had the highest percentage of men drinking 5 or more drinks per day and the Vietnamese had the second highest percentage of men drinking more than 20 days per month, other ethnic groups such as Japanese, Filipinos, and other Asian men were not far behind. The lack of a clear association between alcohol use and the incidence rate of head and neck cancer could be explained in part by the confounding effect of tobacco smoking, which is a stronger risk factor for head and neck cancer than alcohol use, and other unknown confounding dietary and lifestyle risk factors. Presently, the mechanism by which ethanol enhances head and neck cancer risk is unclear. Although ethanol may increase head and neck cancer risk directly through some topical carcinogenic effects and/or indirectly by enhancing the effects of other carcinogens such as tobacco, it has not been found to readily induce head and neck cancer in animal models by itself.12 While prior association studies have shown that cigarette smoking was a strong risk factor for head and neck cancer among never drinkers, alcohol was only a weak risk factor and apparent only at high doses, in the absence of tobacco use.13 In a large consortium study, only 4% of head and neck cancer cases were thought to be due to alcohol alone, whereas 33% were due to tobacco alone, and 35% were due to tobacco and alcohol combined.5 Moreover, different types of alcoholic beverage conferred different relative risk for head and neck cancer; while beer and liquor consumption had comparable estimates of head and neck cancer relative risk, wine consumption was associated with a weaker risk.14 All these confounders may contribute to the lack of a clear relationship between alcohol consumption and head and neck cancer risk in Vietnamese men.
Tobacco use has also been found to be more common in Asian groups with lower education levels and socioeconomic status.15,16 Likewise, other risk factors for head and neck cancer, specifically oral cavity, and oropharyngeal carcinomas, include high alcohol consumption,2,17,18 poor dental hygiene,18 low fruit and vegetable consumption,3,19 and HPV infection.18,20 Because these risk factors are commonly associated with low SES,21–23 we hypothesized that the incidence rate of head and neck cancer in California Asians, specifically in Vietnamese men, would be higher in those of lower SES. Although the CCR does not collect individual patient-level data on SES, we were able to assess neighborhood-level SES based on address of residence and found that, indeed, Vietnamese men and women living in lower-SES neighborhoods had double the incidence rate of head and neck cancer compared to those living in higher-SES neighborhoods. The SES disparity was the largest for laryngeal cancers. Significantly higher incidence of head and neck cancer in the lower-SES group was also noted for Koreans and Filipinos, but not Chinese, Japanese, and South Asians. These findings are consistent with those from a previous report for South Asians in England, which noted a significantly higher incidence of oral cavity and pharyngeal cancer in those living in lower-SES neighborhoods.24 A recent meta-analysis of 41 studies 15,344 cases and 33,852 controls found that the odds ratio for developing oral cavity cancer was 1.85 for those with lower education levels, 1.84 for those with lower occupational/social class, and 2.41 for those with lower income, when compared to those in the highest SES strata.25 The association between oral cancer risk and SES was universal across the world, in both high- and low-income countries, and remained significant after adjusting for other potential confounders. These results and the results of the present study suggest that the link between low SES and head and neck cancer development is independent of sex and ethnicity and should be explicitly recognized in future effort for prevention and early detection of head and neck cancer.
Although our study sample size was incomparably large for our population and highly enriched for Asian populations, our study has several major limitations, particularly the lack of individual-level data on tobacco and alcohol use. In addition, the relative rarity of head and neck cancer resulted in small numbers of cases and hence some unstable incidence rates, especially among women and for specific head and neck tumor sites. Although we did have individual data on neighborhood-level SES based on residential address at diagnosis, we did not have information on individual-level measures of SES, such as education and income. While neighborhood-level and individual-level SES are correlated,26 the 2 groups of measures capture different types of exposures that are independently associated with health outcomes.27 Therefore, our findings in relation to SES must be interpreted cautiously, as neighborhood-level measures of SES capture both aspects of individuals within the neighborhood as well as characteristics of the neighborhood itself.28 Nevertheless, our study does suggest a high prevalence of cigarette use in Vietnamese men and a higher risk of developing cigarette-related head and neck cancer in this population, especially those living in lower-SES neighborhoods. These results suggest that prevention strategies for these cancers in the Vietnamese community will need to start with smoking cessation efforts dedicated to lower-SES Vietnamese men. Such efforts can build on previous studies of smoking cessation and attitudes toward smoking in the Vietnamese community.29–33
In particular, it may be useful to evaluate whether raising public awareness of the high rates of head and neck cancer among Vietnamese men is an effective message to help prevent smoking initiation or facilitate smoking cessation in the Vietnamese community.
In summary, we have found a higher incidence rate of head and neck cancer, specifically laryngeal carcinomas, in Vietnamese and Korean men when compared to other Asian ethnic groups in California. The incidence rate was higher in those with lower neighborhood-level SES. Future smoking and alcohol cessation efforts and education on head and neck cancer risks should be targeted to lower-SES Vietnamese men to prevent the development of these debilitating cancers.
Acknowledgment
This research was also supported by the Stanford Cancer Center Seed grant for population based study. We would like to thank Kari Fish for her assistance in some data analysis and Simon Wu for his administrative assistance.
Contract grant sponsor: The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/ CCR921930-02 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended, nor should be inferred.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 3.Curado MP, Hashibe M. Recent changes in the epidemiology of head and neck cancer. Curr Opin Oncol. 2009;21:194–200. doi: 10.1097/CCO.0b013e32832a68ca. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZF, Morgenstern H, Spitz MR, et al. Environmental tobacco smoking, mutagen sensitivity, and head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2000;9:1043–1049. [PubMed] [Google Scholar]
- 5.Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ResearchUCfHP. California Health Interview Survey . CHIS 2005 Methodology Series. UCLA Center for Health Policy Research; Los Angeles: 2005. [Google Scholar]
- 7.Makimoto K. Drinking patterns and drinking problems among Asian-Americans and Pacific Islanders. Alcohol Health Res World. 1998;22:270–275. [PMC free article] [PubMed] [Google Scholar]
- 8.Raz DJ, Gomez SL, Chang ET, et al. Epidemiology of non-small cell lung cancer in Asian Americans: incidence patterns among six subgroups by nativity. J Thorac Oncol. 2008;3:1391–1397. doi: 10.1097/JTO.0b013e31818ddff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofer BM, Kwong SL, M. A, Bates JH, McCusker ME, Snipes KP. Cancer in California, 2007. Sacramento, CA: 2007. [Google Scholar]
- 10.Conway DI, Hashibe M, Boffetta P, et al. Enhancing epidemiologic research on head and neck cancer: INHANCE - The international head and neck cancer epidemiology consortium. Oral Oncol. 2009;45:743–746. doi: 10.1016/j.oraloncology.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 12.IARC Monogr Eval Carcinog Risks Hum. 1988;44:1–378. [No authors listed] Alcohol drinking. IARC Working Group, Lyon, 13–20 October 1987. [PMC free article] [PubMed] [Google Scholar]
- 13.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 14.Purdue MP, Hashibe M, Berthiller J, et al. Type of alcoholic beverage and risk of head and neck cancer—a pooled analysis within the INHANCE Consortium. Am J Epidemiol. 2009;169:132–142. doi: 10.1093/aje/kwn306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H, Shimizu R, Chen MS., Jr English language proficiency and smoking prevalence among California's Asian Americans. Cancer. 2005;104(12 Suppl):2982–2988. doi: 10.1002/cncr.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An N, Cochran SD, Mays VM, McCarthy WJ. Influence of American acculturation on cigarette smoking behaviors among Asian American subpopulations in California. Nicotine Tob Res. 2008;10:579–587. doi: 10.1080/14622200801979126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Gillison ML, D'souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 19.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83:1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 20.Gillison ML, Shah KV. Human papillomavirus-associated head and neck squamous cell carcinoma: mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol. 2001;13:183–188. doi: 10.1097/00001622-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Davis K. The intersection of socioeconomic variables, oral health, and systemic disease: all health care is cultural. Compend Contin Educ Dent Suppl. 2000;30:40–48. quiz 66. [PubMed] [Google Scholar]
- 22.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87:1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 23.Minh HV, Huong DL, Giang KB. Self-reported chronic diseases and associated sociodemographic status and lifestyle risk factors among rural Vietnamese adults. Scand J Public Health. 2008;36:629–634. doi: 10.1177/1403494807086977. [DOI] [PubMed] [Google Scholar]
- 24.Moles DR, Fedele S, Speight PM, Porter SR, dos Santos Silva I. Oral and pharyngeal cancer in South Asians and non-South Asians in relation to socioeconomic deprivation in South East England. Br J Cancer. 2008;98:633–635. doi: 10.1038/sj.bjc.6604191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conway DI, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LM. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int J Cancer. 2008;122:2811–2819. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- 26.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55:111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen IH, Kaplan GA. Neighborhood social environment and risk of death: multilevel evidence from the Alameda County Study. Am J Epidemiol. 1999;149:898–907. doi: 10.1093/oxfordjournals.aje.a009733. [DOI] [PubMed] [Google Scholar]
- 29.Spigner C, Yip MP, Huang B, Tu SP. Chinese and Vietnamese adult male smokers’ perspectives regarding facilitators of tobacco cessation behavior. Asian Pac J Cancer Prev. 2007;8:429–435. [PubMed] [Google Scholar]
- 30.Nguyen BH, Nguyen KP, McPhee SJ, Nguyen AT, Tran DQ, Jenkins CN. Promoting cancer prevention activities among Vietnamese physicians in California. J Cancer Educ. 2000;15:82–85. doi: 10.1080/08858190009528662. [DOI] [PubMed] [Google Scholar]
- 31.Lai KQ, McPhee SJ, Jenkins CN, Wong C. Applying the quit & win contest model in the Vietnamese community in Santa Clara county. Tob Control. 2000;9(Suppl 2):II56–II59. doi: 10.1136/tc.9.suppl_2.ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiecha JM, Lee V, Hodgkins J. Patterns of smoking, risk factors for smoking, and smoking cessation among Vietnamese men in Massachusetts (United States). Tob Control. 1998;7:27–34. doi: 10.1136/tc.7.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MS, Jr, Guthrie R, Moeschberger M, et al. Lessons learned and baseline data from initiating smoking cessation research with southeast Asian adults. Asian Am Pac Isl J Health. 1993;1:194–214. [PubMed] [Google Scholar]


