Abstract
Objectives
1) to investigate the feasibility of combining transcranial direct current stimulation (tDCS) to the lower extremity (LE) motor cortex with novel locomotor training to facilitate gait in subjects with chronic stroke and low ambulatory status, and 2) to obtain insight from study subjects and their caregivers to inform future trial design.
Methods
Double-blind, randomized controlled study with additional qualitative exploratory descriptive design. One-month follow-up.10 subjects with stroke were recruited and randomized to active tDCS or sham tDCS for 12 sessions. Both groups participated in identical locomotor training with a robotic gait orthosis (RGO) following each tDCS session. RGO training protocol was designed to harness cortical neuroplasticity. Data analysis included assessment of functional and participation outcome measures and qualitative thematic analysis.
Results
Eight subjects completed the study. Both groups demonstrated trends toward improvement, but the active tDCS group showed greater improvement than the sham group. Qualitative analyses indicate beneficial effects of this combined intervention.
Conclusions
It is feasible to combine tDCS targeting the LE motor cortex with our novel locomotor training. It appears that tDCS has the potential to enhance the effectiveness of gait training in chronic stroke. Insights from participants provide additional guidance in designing future trials.
Keywords: Neuronal Plasticity, Electric Stimulation, Robotics, Lower Extremity
1. Introduction
A common theme in the motor recovery literature is that specific and intensive motor training induces central nervous system reorganization to a degree formerly thought possible only during the early post-natal period. This neuroplastic change plays a crucial role in learning and memory processes, as well as recovery of function, after neurological injuries (Dancause et al., 2005; Johansson, 2000; Kopp et al., 1999; Nudo, Plautz, & Frost, 2001). The fact that it is possible to modulate neuroplastic change has influenced modern concepts of physical therapy and research in rehabilitation.
Extensive research shows that transcranial direct current stimulation (tDCS) is a promising intervention to up-modulate neuroplasticity and improve upper extremity motor performance in neurological populations (Fregni et al., 2005; Fregni & Pascual-Leone, 2007; Fujiwara et al.; Hummel & Cohen, 2005; Nitsche et al., 2007; Pascual-Leone, Tarazona, & Catala, 1999; Schlaug, Renga, & Nair, 2008). Studies in humans over the last decade indicate that low intensity tDCS (1–2 mA) has the capacity to alter the excitability of cortical neurons (Been, Ngo, Miller, & Fitzgerald, 2007; Boggio, Zaghi, & Fregni, 2009; Boros, Poreisz, Munchau, Paulus, & Nitsche, 2008; Fregni & Pascual-Leone, 2007; Gandiga, Hummel, & Cohen, 2006; Lang et al., 2007; Nitsche et al., 2003; Paulus, 2003; Vines, Cerruti, & Schlaug, 2008). The precise mechanism of tDCS is unknown but is believed to involve either manipulation of ion channels or influence on the balance of ions inside and outside the neural membrane, which changes the threshold for neural firing (Nitsche, et al., 2003; Schlaug & Renga, 2008). Neuromodulation occurs in a polarity-dependent manner (Fregni & Pascual-Leone, 2007; Schlaug & Renga, 2008): anodal stimulation increases cortical excitability, whereas cathodal stimulation diminishes it.
While research has shown that tDCS has the potential to improve upper extremity motor recovery following stroke when paired with intensive motor training, only a few studies have examined the effects of tDCS on lower extremity motor function. One study investigating the effects of anodal tDCS in subjects with mild motor impairment after stroke demonstrated improvements in paretic ankle control (Madhavan, Weber, & Stinear, 2011). Another study of the effects of anodal tDCS on toe pinch force in healthy subjects showed transient improvement in torque (Tanaka, Hanakawa, Honda, & Watanabe, 2009). On the other hand, one study evaluating the effects of active tDCS paired with locomotor training after stroke did not yield superior results when compared to sham tDCS and identical training (Geroin et al., 2011).
Evidence to clarify and optimize the role of tDCS in locomotor restoration after stroke is needed. To this end, we designed a novel protocol for locomotor training with a robotic gait orthosis (LT-RGO) to follow the application of tDCS. Our novel approach to LT-RGO aligns most closely and comprehensively with basic principles of neuroplasticity (ie, intensive and repetitive practice; task-specificity; gradual increase in demand; motivation; attention to task; active engagement; feedback). The primary objective of this study was to investigate the feasibility of combining tDCS to the lower extremity motor cortex with novel LT-RGO to facilitate gait in subjects with chronic stroke and low ambulatory status. The central hypothesis was that application of active anodal tDCS to the cortical motor area that controls the lower extremity, paired with our novel LT-RGO protocol, would enhance gait more than a control condition (ie, sham tDCS paired with identical training). A secondary objective was to obtain insight from study subjects and their caregivers to inform future trial design.
2. Methods
The study design included both a double-blind, sham-controlled, randomized quantitative design and an exploratory descriptive qualitative design. The ethics boards of the local university and rehabilitation hospital in which the study was conducted approved the protocol. All subjects provided informed consent prior to participation. We enrolled 10 subjects as a convenience sample. Eligible subjects had impaired gait following a single stroke sustained at least 12 months prior to enrollment. Radiographic studies and medical histories were obtained to confirm diagnosis, site, and type of lesion. Exclusion criteria (eg, history of seizure; ferromagnetic material in the cranium; cardiac, neural, or medication implants; severe spasticity and/or decubitus ulcer(s) interfering with the LT-RGO harness; cognitive deficit severe enough to preclude informed consent) were largely structured to ensure subjects’ safety in all aspects of participation.
Subjects were paired based on similar baseline ambulatory characteristics at eligibility screening and then randomly allocated to either the active anodal tDCS group or the control group (sham tDCS). Immediately following the tDCS, each subject participated in LT-RGO under the guidance of a physical therapist certified in use of the Lokomat (Lokomat, Hocoma Inc, Zurich, Switzerland). The intervention was repeated 3 times per week for 4 weeks. Subjects, the 2 physical therapists who administered LT-RGO, and the outcomes assessors were blinded to the tDCS condition.
2.1. Transcranial direct current stimulation
For active tDCS, stimulation current was set to 2 mA and applied for 20 minutes. The intensity and duration were selected based on previous studies demonstrating these parameters to be safe (Boggio et al., 2007; Jo et al., 2009; Kim et al., 2010) and effective to induce persistent increases in cortical excitability as measured by transcranial magnetic stimulation motor evoked potentials in the tibialis anterior muscle (Jeffery, Norton, Roy, & Gorassini, 2007). The control group received sham tDCS for 20 minutes with current set to ramp up and then down over the first 75 seconds of the session (Gandiga et al., 2006). This sham protocol produces the same sensation as active tDCS without having a measurable effect on cortical excitability; it is a widely used technique to preserve subject blinding in investigations using tDCS (Gandiga, et al., 2006). For both groups, a 25 cm2 anode was positioned over the cortical motor area controlling the leg; and a 35 cm2 cathode was positioned supraorbitally. We used a larger cathode than anode in order to minimize potential unintended effects on cortical areas underlying the cathode. See Figure 1 for a depiction of the tDCS set-up.
Fig. 1.
This is a mock depiction of the tDCS delivery. The salmon-colored electrode is the anode, or excitatory electrode; it was placed over the cortical motor area controlling the lower extremity. The blue electrode is the cathode, or inhibitory electrode; it was placed supraorbitally.
2.2. LT-RGO
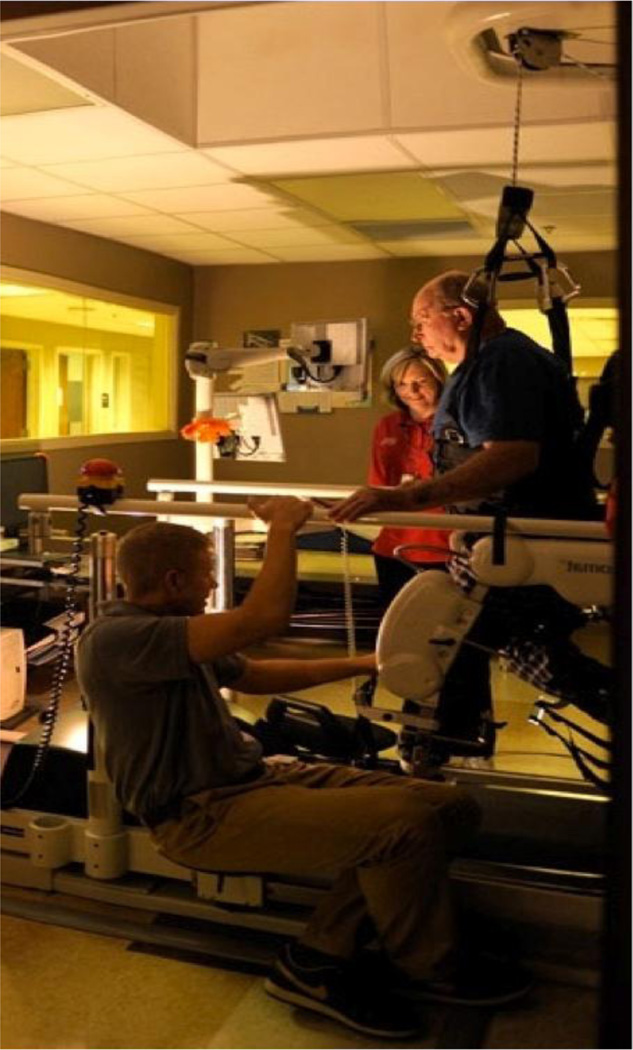
The Lokomat is an RGO that has been used with neurologically compromised individuals, including those with stroke (Figure 2) (Hesse, Schmidt, Werner, & Bardeleben, 2003; Mayr et al., 2007; Westlake & Patten, 2009; Winchester et al., 2005). The Lokomat exoskeleton facilitates a bilaterally symmetrical gait pattern as the subject attempts to walk on a treadmill (Hesse, et al., 2003; Hidler et al., 2009; Neckel, Wisman, & Hidler, 2006). This robotic mechanism can elicit a comparable-to-normal walking pattern with respect to gait cycle timing. Locomotor training with the use of the Lokomat has shown promise in small studies as a form of intensive, task-oriented, repetitive training for gait restoration in neurological populations (Hesse, et al., 2003; Husemann, Muller, Krewer, Heller, & Koenig, 2007; Mayr, et al., 2007; Westlake & Patten, 2009). However, recent, randomized controlled studies in stroke have shown that this intervention in its conventional form has no greater benefit than either intensive home health or standard physical therapy (Carda et al., 2012; Hidler, et al., 2009; Swinnen, Duerinck, Baeyens, Meeusen, & Kerckhofs, 2010; Vaney et al., 2012). Therefore, we developed a novel protocol that imposes progressive decrease of speed in conjunction with progressive decrease of guidance force to increase physical and mental demand as recovery progresses (Table 1). Additionally, this novel protocol requires attempted initiation of movement by the subject. The theoretical basis for our novel LT-RGO protocol is presented in the discussion section.
Fig. 2.
LT-RGO (Lokomat, Hocoma Inc, Switzerland). The harness system provides body weight support. The robotic exoskeleton facilitates a bilaterally symmetrical gait pattern as the user attempts to walk on the treadmill.
Table 1.
Novel protocol for LT-RGO with the Lokomat
| Weeks | Body Weight Support |
Time | Speed | Guidance Force* |
|---|---|---|---|---|
| 1 (sessions 1–3) | 40–50% | Targeted goal: 20–40 minutes per session | Initially at most comfortable speed, followed by progressive, rapid decrease to a speed slow enough to allow subject to initiate movement, thereby increasing engagement with the task and appropriate attentional demands. | Maximal decrease in percentage (so robotic exoskeleton provides least assistance possible) while maintaining a gait pattern within normal limits |
| 2 (sessions 4–6) | 40% | |||
| 3 (sessions 7–9) | 30% | |||
| 4 (sessions 10–12) | 20% |
Guidance force is defined as the torque produced by the hip and knee actuators of the Lokomat. The ability to adjust this torque allows the robotic assist legs to provide varied amounts of assistance, measured in percentages from 0–100% (from no assist to stiff servo system (high amount of holding torque to maintain positions accurately), respectively).
2.3 Outcome measures and analyses
Outcome measures were performed in a blinded fashion at baseline, at completion of the intervention, and at 1-month follow-up. The primary outcome measure was the 10 Meter Walk Test (10MWT) as walking speed is the best single variable to differentiate between household and community ambulatory function post-stroke (Mulroy et al., 2003; Perry, Garrett, Gronley, & Mulroy, 1995). It has excellent test-retest and intra-rater reliability for the chronic stroke population (Sullivan et al., 2011) and is considered more responsive than other functional scales to assess mobility changes post-stroke (Richards, Malouin, Dumas, & Tardif, 1995). Secondary outcome measures included the Timed Up and Go Test (TUG), the Berg Balance Scale (BBS), the Functional Ambulation Category (FAC), and the Stroke Impact Scale 16 (SIS-16). The TUG tests mobility, balance, and locomotor performance and has excellent test-retest reliability in stroke (Sullivan et al., 2011). The BBS assesses static balance and has excellent test-retest, inter-rater, and intra-rater reliability in chronic stroke (Sullivan et al., 2011). The FAC categorizes each subject using a 6-point scale from “0” (nonfunctional ambulatory) to “5” (ambulatory-independent), depending on how much assistance the subject requires from someone else when walking, regardless of assistive device (Sullivan et al., 2011; Teasell, Foley, & Salter, 2011). The FAC correlates significantly with temporal-distance measures (Sullivan et al., 2011). The SIS-16 is a self- or proxy-report with 16 items capturing daily activities (Sullivan et al., 2011). It encompasses 4 domains of the Stroke Impact Scale 2.0 (strength, hand function, mobility, and activities of daily living) with the subject rating each item (Likert scale from 1 (“could not do at all”) to 5 (“not difficult at all”)) (Sullivan et al., 2011).
Motor performance and participation evaluations took place at baseline, upon completion of the intervention period, and at 1-month follow-up. We compared baseline measures for the 2 groups to assess group differences prior to the intervention. Analysis of variance (ANOVA) model was fitted to each dependent variable to evaluate group (changes, i.e., post intervention compared to baseline; 1-month compared to baseline) main effects (active versus sham). Significance was accepted at α < 0.05. Statistics were calculated using StatView software.
2.4 Qualitative data collection and analyses
Semi-structured interviews were conducted with subjects and/or their caregivers regarding tDCS, LT-RGO, overall impressions of the study, and recommendations (see Appendix 1 for sample interview questions). Interviews took place in private settings at the study site at completion of the intervention period or at 1-month follow-up evaluation. The same researcher completed each interview. Interviews were recorded and transcribed verbatim. The interviewer checked transcriptions for accuracy and completed inductive thematic analysis (Braun & Clarke, 2006). The data were coded, and patterns of meaning were identified. For the qualitative results presented in this article, pseudonyms are used to conceal the identities of the participants.
3. Results
A total of 8 subjects (4 men) with chronic stroke, mean age of 67.8 years (range, 44–80 years), and average of 4 years since stroke onset (range, 1.1–11.6 years) completed the study (Table 2). Two subjects withdrew (1 after 2 sessions due to non-paretic arthritic knee pain unrelated to the study intervention; 1 prior to first attempt with LT-RGO due to contractures that developed between the initial screening and study onset). Six subjects lived with family, and 2 lived alone. All subjects completed every session of intervention with no adverse events. All subjects completed all evaluations except for 1 subject who did not complete 1-month follow-up due to Botox injections shortly after the completion evaluation. After the intervention period, all subjects and primary caregivers were questioned to determine interest in interviewing (qualitative portion of the study); and all consented except 2 study subjects who were unable to participate due to severe expressive aphasia. A total of 6 study participants and 4 caregivers completed interviews.
Table 2.
Participant Demographics
| Subject | Age | Group | Sex | Affected Hemisphere |
Stroke Type | Years Post- Stroke |
Baseline Walking Ability |
|---|---|---|---|---|---|---|---|
| 1 | 76 | Active | M | Left | Ischemic | 3 | Primarily wheelchair user; required assistive device, ankle-foot orthosis, and moderate physical assistance to walk |
| 2 | 64 | Active | M | Left | Hemorrhagic | 3 | Required walker, no physical assistance |
| 3 | 77 | Sham | F | Left | Ischemic | 1.9 | Primarily wheelchair user; required assistive device and moderate to maximal physical assistance to walk |
| 4 | 55 | Sham | F | Left | Ischemic | 2.7 | Required walker and supervision assistance |
| 5 | 44 | Active | M | Left | Hemorrhagic | 1.5 | Required cane and supervision assistance |
| 6 | 75 | Active | F | Left | Ischemic | 11.6 | Primarily wheelchair user; required a walker and supervision assistance to walk |
| 7 | 71 | Sham | F | Left | Ischemic | 1.1 | Primarily wheelchair user; required a quadricane and supervision assistance to walk |
| 8 | 80 | Sham | M | Left | Ischemic | 7.2 | Required a quadricane and supervision assistance |
Abbreviations: F, Female; M, Male
3.1 Quantitative results
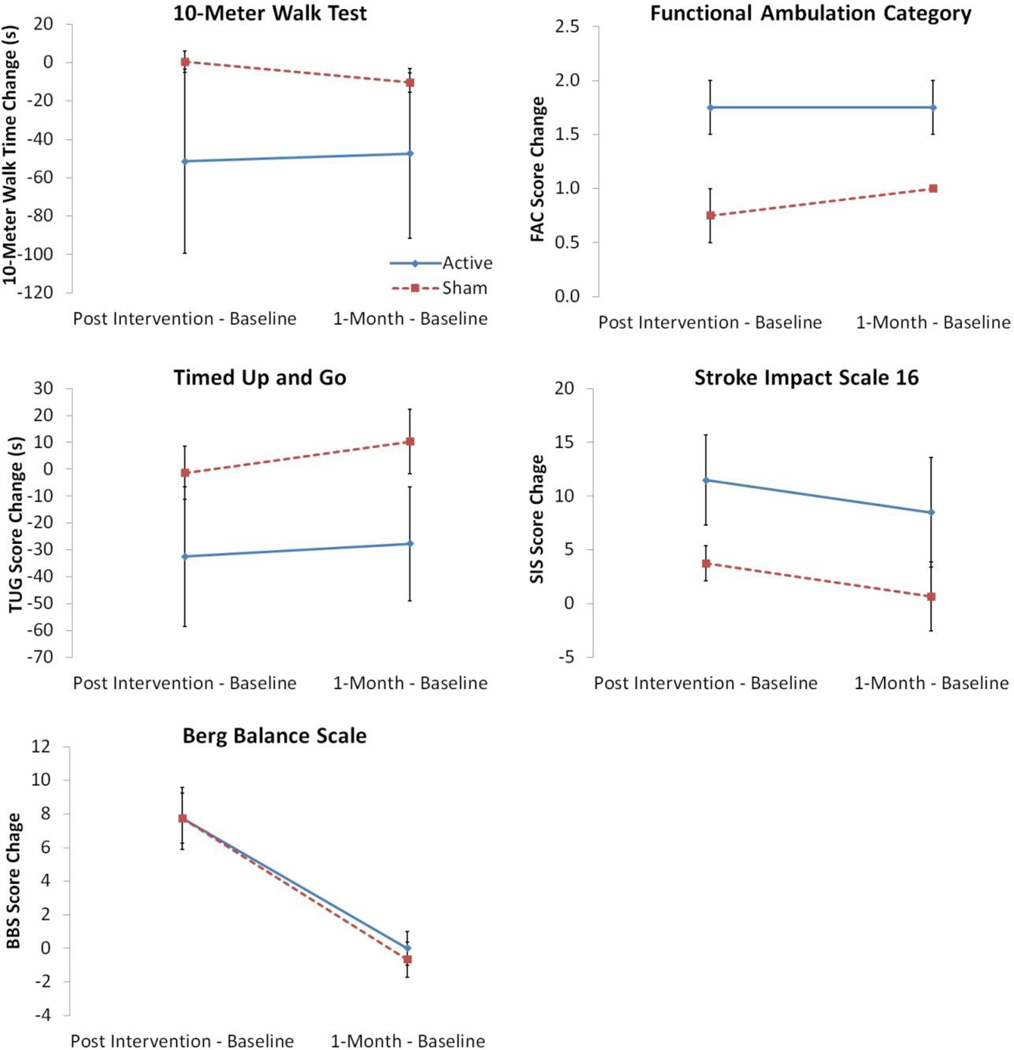
Baseline data shows no significant differences between the 2 groups (active and sham, Table 3). Data analysis demonstrates trends towards improvement for both groups, with the active tDCS group showing marked improvements compared with the sham tDCS group in all measures except BBS (p=0.919); see Figure 3 for group comparisons. The FAC results showed statistical significance in favor of the active tDCS group (p=0.028). The TUG and SIS-16 results favored the active tDCS group and approached significance (TUG p=0.066; SIS-16 p=0.062). The 10MWT results also favored the active tDCS group but were non-significant (p=0.19).
Table 3.
Group Baseline Data and Differences
| Outcome Measure | Active Group (mean±SE) |
Sham Group (mean±SE) |
P value |
|---|---|---|---|
| 10 Meter Walk Test (seconds) | 117.43 ± 83.89 | 65.55 ± 21.00 | 0.57 |
| Timed Up and Go (seconds) | 99.16 ± 60.27 | 59.81 ±19.99 | 0.56 |
| Stroke Impact Scale 16 | 54.25 ± 4.59 | 53.50 ± 4.98 | 0.92 |
| Functional Ambulation Category | 2.25 ± 0.48 | 2.75 ± 0.75 | 0.59 |
| Berg Balance Scale | 32.50 ± 4.63 | 32.25 ± 6.89 | 0.98 |
Fig. 3.
Results comparing group data (active versus sham tDCS) for the 10 Meter Walk Test, Timed Up and Go, Functional Ambulation Category, and Berg Balance Score including change from baseline to post-intervention (4 weeks) and change from baseline to 1-month follow-up.
3.2 Qualitative findings
We identified 5 overarching themes that are labeled in the following subheadings and subsequently described.
3.2.1. Hope for recovery
Participants expressed a variety of reasons for joining the study, all of which indicated a hope for recovery. As one participant stated, “Cause, God, if I could just walk again… You don’t know… how much it would mean to me” (Xavier). Participants also described a high level of motivation and belief in the concept of doing whatever it might take to support their recovery; they recognized the role of volunteering for research studies as one mechanism to support recovery.
3.2.2. Desire for innovation
This theme emerged from discussion about perceptions regarding the brain stimulation and the LT-RGO. Participants perceived these interventions as innovative means of facilitating a return of function. One participant described this by saying, “I’m always interested in things that are outside the box. To me, that’s innovative thinking… I think it’s better to find the root of the problem and try to fix it instead of takin’ a pill to just decrease the symptoms” (Joanna).
3.2.3. Team support
Working with a hopeful, encouraging research team was important to participants. A sense of support and optimism from the team was valued and motivating. Trust in the researchers was also important to the subjects with stroke and their caregivers: “My mom does not work with you if she doesn’t trust you” (Belinda, caregiver).
3.2.4. Improved participation
Participants expressed a sense of improved ability to participate in daily life within the home, community, or both, compared with how they felt before the study. Categories within this theme included better locomotor performance, improved balance and related confidence, and enhanced communication and cognition. Subjects in both groups contributed to all categories within this theme except that only subjects receiving the active tDCS contributed to “enhanced communication and cognition.”
For locomotor performance, participants described improvements in over-ground gait speed and pattern. As examples, 1 caregiver reported his wife’s foot drag ceased. Another caregiver reported her husband traversed from the car to the house in 7 fewer steps and was more willing to wear shoes during household ambulation. Two participants reported they were able to walk without their cane at home for the first time since stroke: “I can walk further, faster, and I’ve even got so I can walk by myself… now that I took the Lokomat… I even walked 2 blocks the other day” (Xavier). One participant in the active tDCS group reported enhanced ability to participate in household activities of daily living (eg, cleaning, putting away dishes). This same participant also described the ability to descend a flight of stairs in his home for the first time since the stroke: “[Stairs are] easier… a long flight of stairs to get to the basement. And I made the steps the other day… this is the first time coming down the stairs. I would go, before, up the stairs, no problem. But down the stairs was a problem until two weeks ago,” (Jackson).
In describing improved balance ability and confidence, participants described feeling more steady with activities and having less fear of falling, which increased willingness to attempt activities. For example, 1 subject was previously fearful of falling and did not trust his wife to walk with him; but by the end of the study, his wife reported that this fear had profoundly diminished. Two participants noted improvements in steadiness with car transfers. Another participant reported increased confidence to negotiate challenging surfaces, specifically walking over snow and ice.
For enhanced communication and cognition, participants noted improvements in willingness to attempt communication, improved reading comprehension, word-finding ability, improved articulation, and/or improved voice quality (eg, loudness). Jackson commented on this, stating: “I recognized early on that speech came on stronger… I was talking louder.”
3.2.5 Enhanced engagement with the locomotor training
Five subjects had previously participated in conventional LT-RGO with the Lokomat in standard outpatient physical therapy. This prior experience enabled them to compare conventional LT-RGO with the novel LT-RGO used in this study. While one subject was able to share feedback due to expressive aphasia, the other four subjects reported that compared with conventional LT-RGO, the novel LT-RGO required more concentration, focus, and facilitated more engagement with the activity. For example, carrying on a conversation during novel LT-RGO was difficult without the deterioration of their gait pattern, whereas subjects could converse easily while performing conventional LT-RGO. One subject described how the novel LT-RGO enhanced her engagement and ability to participate as an active learner:
The goal of walking on the Lokomat 2 years ago was to be able to walk as quickly as possible and… move as much as I could, and this time, it was a matter of refinement, and really focused on perfecting the move rather than the sheer quantity of movement… I was able to concentrate. It was actually difficult… because as she [the therapist] slows the machine down more and more, and she uses the body support less and less, more of the self control has to come from me… so the walking and the refinement of my gait has to come more from me, from my concentration, from my mental and physical ability. –Joanna
4. Discussion
Our study was the first to evaluate the effects of non-invasive brain stimulation combined with motor training to promote gait recovery in chronic stroke subjects with low ambulatory status. Marked improvements were noted in locomotor function for both groups, however greater improvement was observed in the active tDCS group on all measures except BBS. While only FAC showed significant difference between the active and sham groups, it is conceivable that a larger sample size would have yielded significance on additional measures. These results show that active tDCS paired with our novel LT-RGO yields substantial improvement in gait in subjects with chronic stroke.
The BBS showed similar, remarkable improvements immediately after the training in both groups. The improvement, however, was not evident at the 1-month follow-up. This may suggest that tDCS has no enduring effect on balance as measured by the BBS. Investigating alternate balance outcome measures (eg, Activities-Specific Balance Confidence Scale; the Postural Assessment Scale for Stroke Patients) may be worthwhile in future trials.
We combined non-invasive brain stimulation with a novel form of LT-RGO that aligns more closely and comprehensively with basic principles of cortical plasticity than conventional LT-RGO. There was no scientific justification to pair cortical stimulation (tDCS) with locomotor training that did not specifically target cortical plasticity (i.e., conventional LT-RGO, which may target spinal excitability). Conventional LT-RGO uses progressively faster treadmill speeds over the course of intervention. This approach supports locomotion driven by or leading to activation of spinal mechanisms and other, non-cortical influences on gait function (eg, spinal reflexes, central pattern generators, lower extremity electromyographic activity, and cardiovascular conditioning) (Chen & Patten, 2006; Knikou, 2010; Koenig et al., 2009; Krewer et al., 2007). Although these non-cortical mechanisms do have influence on functional return, the importance of cortical mechanisms may supersede non-cortical influences on initial motor learning and enduring performance change after stroke. The conceptual basis for our novel approach to LT-RGO is to target cortical plasticity via use of progressively decreasing speed in conjunction with progressively decreasing guidance force. This novel approach increases physical and mental demand as time progresses. The increase in cortical demand results from a direct proportional decrease in momentum (a passive physical assist) as velocity is decreased (momentum = mass * velocity).
A recent study in subjects with stroke showed that tDCS paired with another form of locomotor training, Gait Trainer GT1 (Reha-Stim, Berlin, Germany) did not lead to more improved functional outcomes than control conditions (Geroin, et al., 2011). Investigators in this study applied 1.5 mA during locomotor training for 7 minutes. In our study we delivered 2.0 mA for 20 minutes before the training. It is possible that the intensity, duration and timing of their tDCS protocol were not optimal for gait rehabilitation in stroke.
Qualitative findings indicate participants in both groups experienced improved participation in the home and/or community (eg, ability to clean the kitchen, complete car transfers, ascend/descend stairs, walk, and maintain energy levels). This finding is consistent with the observed improvement in SIS-16 scores. Only participants who received active tDCS described enhanced communication and cognition. Finally, based on anecdotal observations and the qualitative data, the novel LT-RGO protocol in this study increased demands for attention and concentration. Subjects who had previously used the conventional LT-RGO during the outpatient physical therapy reported that our novel LT-RGO was physically and mentally more challenging.
The qualitative themes that emerged may aid the design of future studies. More specifically, the qualitative findings support the potential use of additional or alternate outcome measures pertaining to speech, communication, cognition, kinematic gait and balance assessments, clinical balance (eg, Activities-specific Balance Confidence (ABC) Scale; Falls Efficacy Scale) (Sullivan et al., 2011), stair navigation (eg, Functional Gait Assessment (Wrisley, Marchetti, Kuharsky, & Whitney, 2004), and home/community participation (eg, Stroke Impact Scale 3.0 (Sullivan et al., 2011), Patient Specific Functional Scale (Horn, et al., 2012). Additional recommendations for future studies include investigation of the locomotor training approach in this study compared to conventional protocols and investigation of the optimal timing of tDCS application (eg, prior to versus simultaneous application with locomotor training).
The main limitation of this study is the small sample size. Also, while we attempted to initiate locomotor training as soon as possible following tDCS stimulation, we need to more closely monitor the time between stimulation and training. Given that increased corticospinal excitability exists for 60 minutes post-anodal stimulation (Jeffery, et al., 2007), we recommend initiating gait training as quickly as possible post-tDCS and recording the time between each. While we encouraged subjects to use the hand support rails as little as possible, we did not strictly control for upper extremity positioning. This may have been important as tibialis anterior and quadriceps muscles activation during swing phase increases with unsupported arm movements (Stephenson, De Serres, & Lamontagne, 2010). Transferability of the qualitative data to future subjects’ experiences may also be construed as a limitation.
5. Conclusion
This study supports the feasibility of using tDCS in conjunction with our novel LT-RGO to optimize gait recovery in subjects with chronic stroke and low ambulatory status. The participants’ insight gleaned from qualitative data provides guidance in designing future trials. Given the promising results even with a small sample size, a full-scale investigation based on this feasibility study is warranted with expanded outcome measures (eg, measures of neuroplasticity such as transcranial magnetic stimulation) and longer-term follow-ups.
Acknowledgments
We thank the study participants and caregivers for their time, efforts, and insight. Special thanks to support personnel, Daniel Aken, Lisa Bailey, and Lowell Napier, for their assistance. We also extend appreciation to Cheryl Carrico, MS, OT/L for editing and to Dr. David Jackson for referring the subjects.
This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1RR033173. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Appendix 1
Examples of interview guide questions*
| Tell me your thoughts and impressions of participating in this study. (probes: experience with the brain stimulation, experience with the walking intervention, experiences with the research team) |
| Why did you want to participate in this study? |
| What changes have you noticed in yourself or your abilities, if any, either during or after the study ended? |
| How does a difficulty with moving or walking impact life at home and in the community? |
| What would it mean for you to be able to move better? |
Questions were adjusted as needed to address either the person with stroke or his/her caregiver. Interviews conducted either immediately post-intervention or at one-month follow-up based on subject availability.
Footnotes
Declaration of Interest: The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Contributor Information
Megan M. Danzl, University of Kentucky, Department of Rehabilitation Sciences, 900 South Limestone, CTW 210, Lexington, KY, 40536-0200.
Kenneth C. Chelette, University of Kentucky, Department of Physical Medicine and Rehabilitation, Cardinal Hill Rehabilitation Hospital, 2050 Versailles Road, Lexington, KY, 40504.
Kara Lee, Cardinal Hill Rehabilitation Hospital, 2050 Versailles Road, Lexington, KY, 40504.
Dana Lykins, Cardinal Hill Rehabilitation Hospital, 2050 Versailles Road, Lexington, KY, 40504.
Lumy Sawaki, Cardinal Hill Endowed Research Scholar in Stroke and Spinal Cord Injury, University of Kentucky, Department of Physical Medicine and Rehabilitation, Cardinal Hill Rehabilitation Hospital, 2050 Versailles Road, Lexington, KY, 40504.
References
- Been G, Ngo TT, Miller SM, Fitzgerald PB. The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res Rev. 2007;56(2):346–361. doi: 10.1016/j.brainresrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25(2):123–129. [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS) Neuropsychologia. 2009;47(1):212–217. doi: 10.1016/j.neuropsychologia.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Boros K, Poreisz C, Munchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci. 2008;27(5):1292–1300. doi: 10.1111/j.1460-9568.2008.06090.x. [DOI] [PubMed] [Google Scholar]
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3:77–101. [Google Scholar]
- Carda S, Invernizzi M, Baricich A, Comi C, Croquelois A, Cisari C. Robotic Gait Training Is not Superior to Conventional Treadmill Training in Parkinson Disease: A Single-Blind Randomized Controlled Trial. Neurorehabilitation and neural repair. 2012;26(9):1027–1034. doi: 10.1177/1545968312446753. [DOI] [PubMed] [Google Scholar]
- Chen G, Patten C. Treadmill training with harness support: selection of parameters for individuals with poststroke hemiparesis. J Rehabil Res Dev. 2006;43(4):485–498. doi: 10.1682/jrrd.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25(44):10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Tsuji T, Honaga K, Hase K, Ushiba J, Liu M. Transcranial direct current stimulation modulates the spinal plasticity induced with patterned electrical stimulation. Clin Neurophysiol. doi: 10.1016/j.clinph.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, Smania N. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabil. 2011;25(6):537–548. doi: 10.1177/0269215510389497. [DOI] [PubMed] [Google Scholar]
- Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol. 2003;16(6):705–710. doi: 10.1097/01.wco.0000102630.16692.38. [DOI] [PubMed] [Google Scholar]
- Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23(1):5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- Horn KK, Jennings S, Richardson G, Vliet DV, Hefford C, Abbott JH. The patient-specific functional scale: psychometrics, clinimetrics, and application as a clinical outcome measure. J Orthop Sports Phys Ther. 2012;42(1):30–42. doi: 10.2519/jospt.2012.3727. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19(1):14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Husemann B, Muller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke. 2007;38(2):349–354. doi: 10.1161/01.STR.0000254607.48765.cb. [DOI] [PubMed] [Google Scholar]
- Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res. 2007;182(2):281–287. doi: 10.1007/s00221-007-1093-y. [DOI] [PubMed] [Google Scholar]
- Jo JM, Kim YH, Ko MH, Ohn SH, Joen B, Lee KH. Enhancing the working memory of stroke patients using tDCS. Am J Phys Med Rehabil. 2009;88(5):404–409. doi: 10.1097/PHM.0b013e3181a0e4cb. [DOI] [PubMed] [Google Scholar]
- Johansson BB. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke. 2000;31(1):223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89(11):879–886. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- Knikou M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin Neurophysiol. 2010;121(10):1655–1668. doi: 10.1016/j.clinph.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Koenig AC, Somaini L, Pulfer M, Holenstein T, Omlin X, Wieser M, et al. Model-based Heart rate prediction during Lokomat walking. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1758–1761. doi: 10.1109/IEMBS.2009.5333096. [DOI] [PubMed] [Google Scholar]
- Kopp B, Kunkel A, Muhlnickel W, Villringer K, Taub E, Flor H. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. Neuroreport. 1999;10(4):807–810. doi: 10.1097/00001756-199903170-00026. [DOI] [PubMed] [Google Scholar]
- Krewer C, Muller F, Husemann B, Heller S, Quintern J, Koenig E. The influence of different Lokomat walking conditions on the energy expenditure of hemiparetic patients and healthy subjects. Gait Posture. 2007;26(3):372–377. doi: 10.1016/j.gaitpost.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Chadaide Z, Boros K, Nitsche MA, Rothwell JC, et al. Bidirectional modulation of primary visual cortex excitability: a combined tDCS and rTMS study. Invest Ophthalmol Vis Sci. 2007;48(12):5782–5787. doi: 10.1167/iovs.07-0706. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Weber K, Stinear J. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res. 2011;209(1):9–17. doi: 10.1007/s00221-010-2511-0. [DOI] [PubMed] [Google Scholar]
- Mayr A, Kofler M, Quirbach E, Matzak H, Frohlich K, Saltuari L. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. Neurorehabil Neural Repair. 2007;21(4):307–314. doi: 10.1177/1545968307300697. [DOI] [PubMed] [Google Scholar]
- Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait and Posture. 2003;18(114):125. doi: 10.1016/s0966-6362(02)00165-0. [DOI] [PubMed] [Google Scholar]
- Neckel N, Wisman W, Hidler J. Limb alignment and kinematics inside a Lokomat robotic orthosis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2698–2701. doi: 10.1109/IEMBS.2006.259970. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114(11):2220–2222. doi: 10.1016/s1388-2457(03)00235-9. author reply 2222–2223. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, et al. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci. 2007;27(14):3807–3812. doi: 10.1523/JNEUROSCI.5348-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24(8):1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tarazona F, Catala MD. Applications of transcranial magnetic stimulation in studies on motor learning. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:157–161. [PubMed] [Google Scholar]
- Paulus W. Transcranial direct current stimulation (tDCS) Suppl Clin Neurophysiol. 2003;56:249–254. doi: 10.1016/s1567-424x(09)70229-6. [DOI] [PubMed] [Google Scholar]
- Perry J, Garrett M, Gronley J, Mulroy S. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- Richards C, Malouin F, Dumas F, Tardif D. Gait velocity as an outcome measure of locomotor recovery after stroke. St. Louis, MO: Mosby; 1995. [Google Scholar]
- Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert Rev Med Devices. 2008;5(6):759–768. doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008;65(12):1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson JL, De Serres SJ, Lamontagne A. The effect of arm movements on the lower limb during gait after a stroke. Gait Posture. 2010;31(1):109–115. doi: 10.1016/j.gaitpost.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan J, Pinto-Zipp G, Crowner B, Kluding P, Nichols D, Rose D, Yoshida R. American Physical Therapy Association StrokEDGE task force recommendations. [Retrieved July 11, 2012];Neurology Section StrokEDGE Documents. 2011 doi: 10.2522/ptj.20120492. from http://www.neuropt.org/go/healthcare-professionals/neurology-section-outcome-measures-recommendations/stroke. [DOI] [PubMed] [Google Scholar]
- Swinnen E, Duerinck S, Baeyens JP, Meeusen R, Kerckhofs E. Effectiveness of robot-assisted gait training in persons with spinal cord injury: a systematic review. J Rehabil Med. 2010;42(6):520–526. doi: 10.2340/16501977-0538. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res. 2009;196(3):459–465. doi: 10.1007/s00221-009-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasell R, Foley N, Salter K. EBRSR: Evidence-based Review of Stroke Rehabilitation. 13th edition ed. London: EBRSR; 2011. [DOI] [PubMed] [Google Scholar]
- Vaney C, Gattlen B, Lugon-Moulin V, Meichtry A, Hausammann R, Foinant D, et al. Robotic-assisted step training (lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabilitation and neural repair. 2012;26(3):212–221. doi: 10.1177/1545968311425923. [Randomized Controlled Trial Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil. 2009;6:18. doi: 10.1186/1743-0003-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester P, McColl R, Querry R, Foreman N, Mosby J, Tansey K, et al. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair. 2005;19(4):313–324. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–918. [PubMed] [Google Scholar]