Abstract
Transitional cell carcinoma (TCC), a urinary bladder tumor with high mortality, is encountered commonly in dogs. Whereas overexpression of epidermal growth factor receptor (EGFR) is associated with development of human urinary bladder cancer, information on EGFR expression in canine TCC is lacking. In this study, EGFR protein and mRNA expression in canine normal bladder (n=5), polypoid cystitis (n=5) and TCC (n=25) were examined by immunohistochemistry and real-time polymerase chain reaction. EGFR protein expression was significantly higher in TCC than that in normal healthy bladder (P<0.001) and polypoid cystitis (P<0.005). High EGFR protein expression was significantly (P<0.01) associated with TCC with a sensitivity of 72% and specificity of 100%. Comparative analysis of protein and mRNA expression levels in TCC showed significant positive correlation (r=0.88, P<0.05) between mRNA and protein expression. These findings suggest that intense expression of EGFR protein could be used as a marker to help canine TCC diagnosis.
Keywords: canine, epidermal growth factor receptor, transitional cell carcinoma, urinary bladder
Malignant urinary bladder tumors are classified as transitional cell carcinomas (TCC), adenocarcinomas or poorly differentiated carcinomas [21]. Among them, TCC accounts for 50% to 75% of reported cases of canine urinary bladder cancer [8]. Polypoid cystitis is a benign disease of the urinary bladder, characterized by inflammation of the bladder mucosa with polypoid mass [13]. Although the symptomatic and diagnostic features of these 2 distinct urinary diseases, TCC and polypoid cystitis, are similar, differential diagnosis of the malignant tumor from the benign disease is substantially crucial because the clinical outcome and relevant treatment for TCC, which has a poor prognosis even with intensive care using anticancer drugs, are completely different from those for polypoid cystitis, which has a good prognosis.
Because canine TCC shares many characteristics with human invasive urinary bladder TCC in terms of histopathology, biological behavior, response to medical therapy and prognostic factors, canine TCC is believed to be a model of human invasive TCC [3, 10]. Although many risk factors for canine TCC including topical insecticide exposure, obesity, cyclophosphamide administration, female gender and breed have been identified thus far [19], the detailed tumorigenic mechanism remains to be elucidated. A substantive comparative study on tumorigenic molecules, including growth factor receptors, expressed by canine TCC and human invasive TCC is anticipated as an important development toward understanding the mechanism.
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase in the ErbB family (EGFR, HER2, HER3 and HER4). In human medicine, EGFR has been reported to overexpress in many epithelial tumors, namely in non-small cell lung, colorectal, gastric, pancreatic, ovarian, breast and urinary bladder cancers [11, 22]. Extensive clinical studies of human urinary bladder cancer, including TCC, have shown that overexpression of EGFR is associated with more severe clinical behaviors and faster cancer progression [1, 12, 15]. Recently, based on the finding of a crucial role of EGFR in tumorigenesis [2], an attempt has been made to treat this cancer with the EGFR inhibitor gefitinib [4, 23]. In veterinary medicine, although overexpression of EGFR has been reported in mammary carcinoma, nasal tumor and osteosarcoma [6, 25, 26], information on EGFR expression in canine urinary bladder TCC is lacking. In this study, EGFR protein and mRNA expression in TCC were assessed by immunohistochemistry and quantitative real-time polymerase chain reaction (real-time PCR). By comparing these results with pathological and clinical outcomes, the feasibility of using EGFR expression as diagnostic and clinical markers for predicting prognosis of canine TCC was investigated retrospectively.
MATERIALS AND METHODS
Dogs: Thirty dogs that were found by ultrasonographic examination to have massive lesions in the urinary bladder at Rakuno Gakuen University from 2004 to 2011 were enrolled in this study. Based on pathology after surgery or necropsy, 5 dogs were diagnosed with polypoid cystitis (2 males and 3 females) and 25 dogs with TCC (9 males and 16 females). The mean ages of the polypoid cystitis and TCC cases were 9.6 ± 3.7 years (range, 4–13 years) and 9.7 ± 2.4 years (range, 7–13 years), respectively. There was no significant difference in age between the 2 groups. The canine breeds most often encountered were Shi-Tzu (n=2, 40%) in polypoid cystitis cases and Shetland-sheepdogs (n=5, 20%) in TCC cases. All TCC cases were followed from the time of surgery for calculation of overall survival time. At the end of the follow-up period, 19 of the TCC dogs were confirmed to be dead of metastasis (n=5), secondary renal failure (n=2), infection (n=2), another tumor (n=1, lymphoma) and unknown reason (n=9). The mean follow-up period was 17 months (range, 1–60 months). Five normal beagles (2 males and 3 females) kept at the University of Rakuno Gakuen also were enrolled to obtain normal urinary bladder tissue via necropsy. The mean age of normal dogs at necropsy was 7.4 ± 0.5 years (range, 7–8 years). All experimental procedures in this study were carried out under approval (No.VH24A13) of the ethics committees of Rakuno Gakuen University.
Pathological examination: Samples of urinary bladder tissues fixed in 10% neutral buffered formalin were embedded in paraffin wax and sectioned at 3-µm thickness. Tissues were stained with hematoxylin and eosin (HE) and examined histopathologically. TCC was diagnosed according to the World Health Organization Histological Classification of Tumors of Domestic Animals [17].
Immunohistochemistry: Immunohistochemical staining was performed with a mouse monoclonal antibody raised against EGFR (Clone 31G7, 1:50, Zymed Laboratories, South San Francisco, CA, U.S.A.), which was already validated previously in another published work on canine tissues, based on an avidin-biotin-peroxidase complex (ABC) method [6]. Phosphate buffered saline (PBS) was used for dilution of the primary antibody. Antigen retrieval treatment was carried out by enzyme digestion: tissue sections were incubated with proteinase K (Dako, Tokyo, Japan) for 2 min at room temperature. Sectioned tissues were incubated overnight with EGFR antibody in a humid chamber at 4°C. Endogenous peroxidase activity was blocked by immersing tissues in 0.3% H2O2 in methanol for 10 min. Stain was developed in 0.05% 3, 3′-diaminobenzidine solution. Negative control data were obtained by using PBS that did not include the primary antibody.
Staining score (SS) was classified as the following: 0=no staining; 1=weak, incomplete membrane staining of any proportion of tumor cells; 2=complete membrane staining that is either non-uniform or weak in intensity, but with obvious circumferential distribution in >10% of cells, or intense, complete membrane staining of <30% of tumor cells; and 3=uniform, intense membrane staining of >30% of tumor cells [28]. All tissues were assessed and scored by K.H.
Real-time PCR: For real-time PCR, normal urinary bladder (n=3) and TCC (n=4) tissues were immersed in the RNAlater solution (Applied Biosystems, Foster City, CA, U.S.A.) overnight at 4°C and stored at −80°C after removal from the solution. Total RNA was extracted from tissues with the RNeasy Mini kit (Qiagen, Hilden, Germany). DNase digestion was performed with the RNase-Free DNase kit (Qiagen). cDNA was synthesized from 1 µg of total RNA with the ReverTra Ace reverse transcriptase kit (Toyobo, Osaka, Japan) and oligo dT primers (Toyobo) according to the manufacturer’s instructions. Primers 2328F (5′- CGAGCACAAGGACAACATCG −3′) and 2615R (5′- CTCCACACATCGCTTTGGTG −3′) were designed based on sequence information for canine EGFR mRNA (GenBank accession number XM_533073.3). All cDNA samples also were subjected to PCR amplification for a housekeeping gene, the canine ribosomal protein 19 gene (RP19). Primers 92F (5′-CCTTCCTCAAAAAGTCTGCG-3′) and 171R (5′-GTTCTCATCGTAGGGAGC-3′) were designed based on sequence information for canine ribosomal protein 19 mRNA (GenBank accession number XM538673). Real-time PCR was performed using the iQ5/MyiQ Single-Color thermal cycler (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Standard curves for quantitative estimation of EGFR and RP19 mRNA levels were drawn by amplification of control cDNA templates containing 103, 104, 106 and 108 copies. The reaction was performed in triplicate using the Quantitect SYBR Green PCR kit (Qiagen) according to the manufacturer’s instructions. The cDNA copy numbers for each gene were calculated from the standard curves, and relative expression levels (REL) of EGFR mRNA were obtained by normalizing the cDNA numbers of EGFR to those of RP19. The temperature cycling protocol consisted of 95°C for 1 min, followed by 35 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec. All data in this study are shown as mean ± standard deviation.
Statistical analysis: Statistical difference among normal urinary bladder tissue, polypoid cystitis and TCC groups, or statistical independence of variables was assessed using the analysis of variance (ANOVA) or the chi-square test, respectively. Comparison of EGFR mRNA expression between normal urinary bladder and TCC or correlation between EGFR mRNA and protein expression was performed with Mann-Whitney U test or Spearman’s rank correlation coefficient, respectively. Survival curves were calculated by the Kaplan-Meier method, and the difference among survival curves was analyzed by the Cox regression model. Differences were considered significant at P<0.05. Data analyses were carried out with Excel Toukei 2012 (SSRI, Tokyo, Japan).
RESULTS
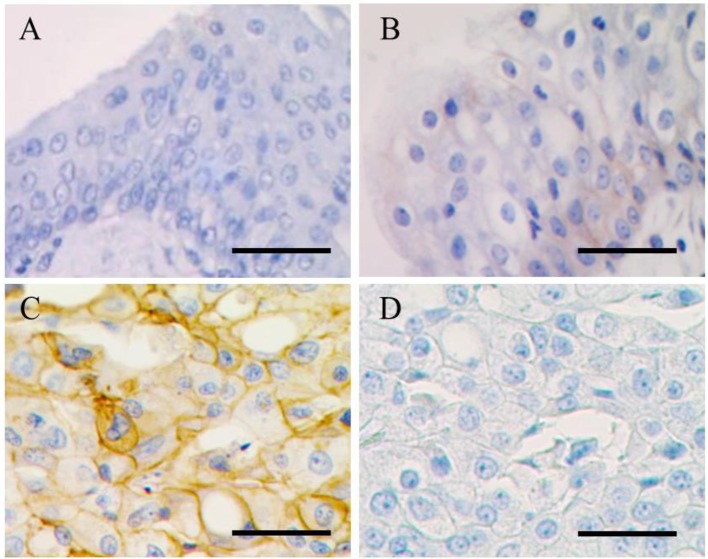
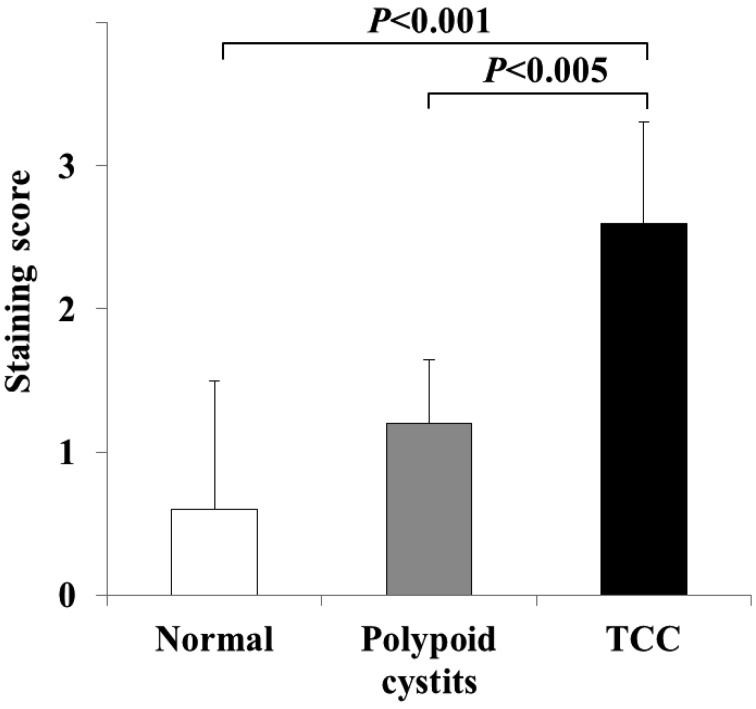
EGFR protein expression in normal urinary bladder, polypoid cystitis and TCC: Immunohistochemistry was performed to compare the distribution of EGFR protein expression among normal, polypoid cystitis and TCC dogs. A resultant immunohistochemistry image is shown in Fig. 1. Uniform, intense membrane staining was observed in TCC, whereas very weak, incomplete membrane staining was detected in polypoid cystitis. No significant immunohistochemical staining was observed in normal tissue. To assess statistically the difference in levels of EGFR protein expression, staining scores were assessed and compared among normal (n=5), polypoid cystitis (n=5) and TCC (n=25) dogs (Fig. 2). The staining score for TCC was significantly higher than scores for normal urinary bladder (P<0.001) and polypoid cystitis (P<0.005). No significant difference was found between normal urinary bladder and cystitis tissues.
Fig. 1.
Images of immunohistochemistry. Normal (A), polypoid cystitis (B) and TCC (C) epithelial cells show no, weak and intense immunohistochemical staining at the cell membrane, respectively. In a negative control study (D), no significant staining was observed at the neoplastic cell membrane in TCC. Bar=100 µm
Fig. 2.
Staining scores from immunohistochemistry. Staining score in TCC was significantly higher than scores for normal urinary bladder (P<0.001) and polypoid cystitis (P<0.005).The scores (mean ± SD) of normal urinary bladder (n=5), polypoid cystitis (n=5) and TCC (n=25) are 0.6 ± 0.89, 1.2 ± 0.45 and 2.6 ± 0.71, respectively. Significance was analyzed by the Mann–Whitney U test.
Correlation between EGFR mRNA and protein expression: To examine expressional correlation between EGFR mRNA and protein levels, mRNA expression levels obtained by quantitative real-time PCR were compared with staining scores from immunohistochemistry.
EGFR mRNA expression level in TCC (REL: 0.27 ± 0.11 × 10−2) was significantly (P<0.05) higher than that in normal urinary bladder (REL: 0.06 ± 0.09 × 10−2). Significant positive correlation (r=0.88, P<0.05) was observed between mRNA and protein expression.
EGFR expression as a diagnostic marker for TCC: To study the feasibility of using EGFR protein expression as a diagnostic marker for TCC, dogs with polypoid cystitis and TCC enrolled in this study were divided into 2 groups depending on protein expression levels: a low-expression group (scores 0, 1 and 2) and a high-expression group (score 3). Incidence of TCC was compared between the 2 groups.
All dogs (100%) included in the high-expression group (n=18) and 7 dogs (41%) included in the low-expression group (n=17) were diagnosed with TCC based on pathology. The highest expression score (score 3) was significantly (P<0.001) associated with TCC, with a sensitivity of 72% (95% CI, 63–72%) and specificity of 100% (95% CI, 77–100%). These results suggest that high levels of EGFR expression could be used as a marker to aid in diagnosis of canine TCC in combination with malignant cytological features of TCC cells when immunostaining has been performed for biopsied samples.
Association between EGFR protein expression and TCC malignant behaviors: To study the association between EGFR protein expression and malignant tumor behaviors in TCC, we examined EGFR expression and its relationship with the presence of vessel invasion or lymph node metastasis. However, no significant association was found (Table 1).
Table 1. Association between EGFR protein expression and TCC histological parameters.
| Histological parameters | n | EGFR | ||
|---|---|---|---|---|
| Low* | High* | |||
| Lymph node metastasis | ||||
| Absent | 22 | 6 (27%) | 16 (73%) | |
| Present | 3 | 1 (33%) | 2 (67%) | |
| P | 0.64 | |||
| Vessel invasion | ||||
| Absent | 8 | 4 (50%) | 4 (50%) | |
| Present | 17 | 3 (18%) | 14 (82%) | |
| P | 0.23 | |||
*:TCC patients were divided into 2 groups based on EGFR protein expression score obtained by immunohistochemistry: low- (scores 0, 1 and 2) and high- (scores 3) expression groups.
Statistical analysis was performed by the χ2 test.
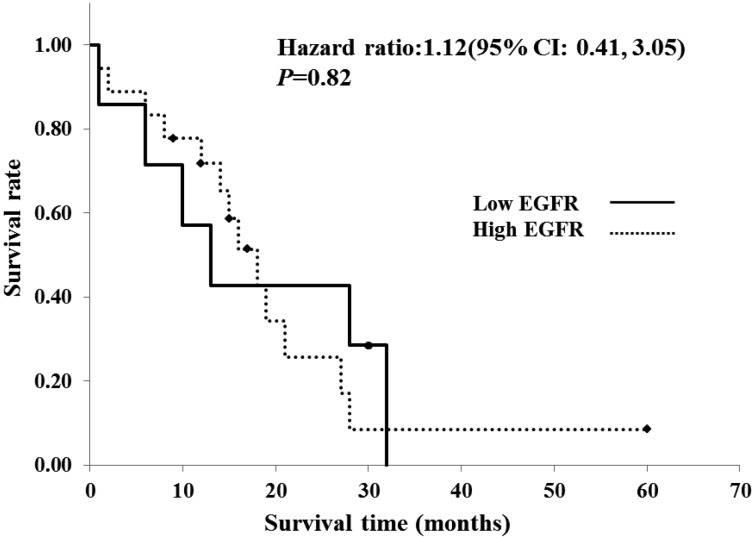
Association with clinical outcome in TCC: To investigate the association between protein expression and clinical outcome, survival times were compared between the low- and high-expression groups of TCC using Kaplan-Meier’s survival curves (Fig. 3). However, no significant association was observed between survival time and protein expression in TCC.
Fig. 3.
Twenty-five cases of canine TCC were divided into two groups, depending on the EGFR protein expression score obtained through immunohistochemistry: low- (scores 0, 1 and 2, n=7) and high- (scores 3, n=18) expression groups. Survival times were compared between the low- and high-expression groups using Kaplan-Meier’s survival curves. Significant correlation was not observed between survival times and EGFR expression levels in TCC (P=0.82) with a hazard ratio of 1.12 (95% confidence interval, 0.41–3.05) as a result of statistical analysis by the Cox regression model.
DISCUSSION
Overexpression of EGFR, which is involved in tumorigenesis in a variety of human cancers [2], is identified in human urinary bladder cancer [5, 20]. In accordance with this finding in human medicine, we demonstrated an increased expression of EGFR protein in canine TCC through scoring analysis by immunohistochemistry in this study. The underlying mechanisms of EGFR overexpression are not completely understood at present, although various mechanisms including gene amplification, activating mutation, increased transcription, loss of inhibitory signals and decreased protein recycling have been proposed [20]. However, overactivation of EGFR through the overexpression that promotes cellular growth and epithelial-mesenchymal transition (EMT) may be a crucial factor for tumorigenesis and metastasis [2, 14]. It has been reported in human medicine that EGFR overexpression activates intracellular signaling along the mitogen-activated protein kinase (MAPK) and phosphatidylinositide-3-kinase (PI3K) pathways [24]. Pathway activation leads to production of various proteins that promote cell cycle progression and cell survival [24]. It is also well known that many genes involved in EMT, which allows cells to reduce intercellular adhesion and increase cellular motility, are positioned downstream from the MAPK and PI3K pathways [7]
Although overexpression of EGFR also has been reported to be associated with pathological and clinical malignancy, including high histological grade, clinically advanced stage and poor prognosis in human bladder carcinomas [1, 12, 15], we could not demonstrate significant association between up-regulated EGFR expression and incidence of vessel invasion and lymph node metastasis or survival time in canine TCC.
However, given our result that high EGFR expression was significantly associated with TCC with a high specificity (100%), we could use EGFR expression as a sensitive diagnostic marker rather than a prognostic marker for canine TCC. Indeed, EGFR immunostaining is helpful for differential diagnosis with urine cytology in human medicine [9]. When prompt provisional diagnosis is required based only on cytological examination of urinary epithelial cells obtained by urethra catheterization, immunostaining for this protein may be helpful to improve the sensitivity of cytological diagnosis for TCC.
Through comparative analysis between protein and mRNA expression in TCC, we showed that EGFR mRNA levels were positively correlated with protein levels expressed in canine TCC tissues, in accordance with a previous study of human bladder cancers [27, 29]. Considering that real-time PCR is a more sensitive analytical method than immunohistochemistry and can analyze mRNA expression levels quantitatively with a very small amount of sample, it would be useful to develop an analytical method based on real-time PCR with an optical cut-off value of mRNA expression level for canine TCC diagnosis.
Evaluation of the expression of all Erb family proteins, EGFR, HER2, HER3 and HER4, has been proposed in order to assess prognosis in human bladder cancer, because not only EGFR but also HER2 overexpression has been suggested to be related to poor prognosis [18]. In another human study, it also has been shown that survival time is influenced by expression levels of HER3 and HER4 [16]. Even in canine malignant mammary tumor, EGFR expression by itself was not associated with survival time [6]. Based on these findings, expressional evaluation of HER2, HER3 and HER4 in addition to EGFR may be required for reliable prognosis in canine TCC.
In conclusion, although a further study through comprehensive analysis with more sensitive and objective methods is crucial to understanding the molecular mechanisms underlying differences in EGFR expression (high and low) and tumorigenesis observed in canine TCC, our results show that strong expression of EGFR protein could be used as a marker to help canine TCC diagnosis.
Acknowledgments
The authors thank Hiroko Mizooku, Kazuko Hirayama and Hiroyuki Taniyama (Department of Veterinary Pathology, Rakuno Gakuen University) for cooperation in histopathology.
REFERENCES
- 1.Chow N. H., Liu H. S., Lee E. I., Chang C. J., Chan S. H., Cheng H. L., Tzai T. S., Lin J. S.1997. Significance of urinary epidermal growth factor and its receptor expression in human bladder cancer. Anticancer Res. 17: 1293–1296. [PubMed] [Google Scholar]
- 2.De Luca A., Carotenuto A., Rachiglio A., Gallo M., Maiello M. R., Aldinucci D., Pinto A., Normanno N.2008. The role of the EGFR signaling in tumor microenvironment. J. Cell. Physiol. 214: 559–567. doi: 10.1002/jcp.21260 [DOI] [PubMed] [Google Scholar]
- 3.Dhawan D., Ramos-Vara J. A., Stewart J. C., Zheng R., Knapp D. W.2009. Canine invasive transitional cell carcinoma cell lines: in vitro tools to complement a relevant animal model of invasive urinary bladder cancer. Urol. Oncol. 27: 284–292. doi: 10.1016/j.urolonc.2008.02.015 [DOI] [PubMed] [Google Scholar]
- 4.Dominguez-Escrig J. L., Kelly J. D., Neal D. E., King S. M., Davies B. R.2004. Evaluation of the therapeutic potential of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in preclinical models of bladder cancer. Clin. Cancer Res. 10: 4874–4884. doi: 10.1158/1078-0432.CCR-04-0034 [DOI] [PubMed] [Google Scholar]
- 5.el-Marjou A., Delouvée A., Thiery J. P., Radvanyi F.2000. Involvement of epidermal growth factor receptor in chemically induced mouse bladder tumour progression. Carcinogenesis 21: 2211–2218. doi: 10.1093/carcin/21.12.2211 [DOI] [PubMed] [Google Scholar]
- 6.Gama A., Gärtner F., Alves A., Schmitt F.2009. Immunohistochemical expression of Epidermal Growth Factor Receptor (EGFR) in canine mammary tissues. Res. Vet. Sci. 87: 432–437. doi: 10.1016/j.rvsc.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 7.Hardy K. M., Yatskievych T. A., Konieczka J., Bobbs A. S., Antin P. B.2011. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev. Biol. 11: 20. doi: 10.1186/1471-213X-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry C. J.2003. Management of transitional cell carcinoma. Vet. Clin. North Am. Small Anim. Pract. 33: 597–613. doi: 10.1016/S0195-5616(03)00032-9 [DOI] [PubMed] [Google Scholar]
- 9.Ikeda S., Funakoshi N., Suzuki K.2009. Usefulness of epidermal growth factor receptor and p53 cocktail immunostaining for differential diagnosis with urine cytology. Acta Cytol. 53: 29–35. doi: 10.1159/000325082 [DOI] [PubMed] [Google Scholar]
- 10.Knapp D. W., Glickman N. W., Denicola D. B., Bonney P. L., Lin T. L., Glickman L. T.2000. Naturally-occurring canine transitional cell carcinoma of the urinary bladder. A relevant model of human invasive bladder cancer. Urol. Oncol. 5: 47–59. doi: 10.1016/S1078-1439(99)00006-X [DOI] [PubMed] [Google Scholar]
- 11.Liang K., Ang K. K., Milas L., Hunter N., Fan Z.2003. The epidermal growth factor receptor mediates radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 57: 246–254. doi: 10.1016/S0360-3016(03)00511-X [DOI] [PubMed] [Google Scholar]
- 12.Lipponen P., Eskelinen M.1994. Expression of epidermal growth factor receptor in bladder cancer as related to established prognostic factors, oncoprotein (c-erbB-2, p53) expression and long-term prognosis. Br. J. Cancer 69: 1120–1125. doi: 10.1038/bjc.1994.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez I., Mattoon J. S., Eaton K. A., Chew D. J., DiBartola S. P.2003. Polypoid cystitis in 17 dogs (1978–2001). J. Vet. Intern. Med. 17: 499–509. [DOI] [PubMed] [Google Scholar]
- 14.McConkey D. J., Choi W., Marquis L., Martin F., Williams M. B., Shah J., Svatek R., Das A., Adam L., Kamat A., Siefker-Radtke A., Dinney C.2009. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 28: 335–344. doi: 10.1007/s10555-009-9194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellon K., Wright C., Kelly P., Horne C. H., Neal D. E.1995. Long-term outcome related to epidermal growth factor receptor status in bladder cancer. J. Urol. 153: 919–925. doi: 10.1016/S0022-5347(01)67604-3 [DOI] [PubMed] [Google Scholar]
- 16.Memon A. A., Sorensen B. S., Meldgaard P., Fokdal L., Thykjaer T., Nexo E.2006. The relation between survival and expression of HER1 and HER2 depends on the expression of HER3 and HER4: a study in bladder cancer patients. Br. J. Cancer 94: 1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meuten D. J., Everitt J., Inskeep W., Jacobs R. M., Peleteiro M, Thompson K. G.2004. Urinary bladder tumors. pp. 26–37. In: WHO Histological Classification of Tumors of the Urinary Bladder System of Domestic Animals, 2nd ed. (Schulman, F. Y. ed.), Armed Forces Institute of Pathology, Washington, D.C. [Google Scholar]
- 18.Miyamoto H., Kubota Y., Noguchi S., Takase K., Matsuzaki J., Moriyama M., Takebayashi S., Kitamura H., Hosaka M.2000. C-ERBB-2 gene amplification as a prognostic marker in human bladder cancer. Urology 55: 679–683. doi: 10.1016/S0090-4295(99)00604-4 [DOI] [PubMed] [Google Scholar]
- 19.Mutsaers A. J., Widmer W. R., Knapp D. W.2003. Canine transitional cell carcinoma. J. Vet. Intern. Med. 17: 136–144. doi: 10.1111/j.1939-1676.2003.tb02424.x [DOI] [PubMed] [Google Scholar]
- 20.Neal D. E., Marsh C., Bennett M. K., Abel P. D., Hall R. R., Sainsbury J. R., Harris A. L.1985. Epidermal-growth factor receptors in human bladder cancer: comparison of invasive and superficial tumours. Lancet 1: 366–368. doi: 10.1016/S0140-6736(85)91386-8 [DOI] [PubMed] [Google Scholar]
- 21.Norris A. M., Laing E. J., Valli V. E., Withrow S. J., Macy D. W., Ogilvie G. K., Tomlinson J., McCaw D., Pidgeon G., Jacobs R. M.1992. Canine bladder and urethral tumors: A retrospective study of 115 cases (1980–1985). J. Vet. Intern. Med. 6: 145–153. doi: 10.1111/j.1939-1676.1992.tb00330.x [DOI] [PubMed] [Google Scholar]
- 22.Nutt J. E., Mellon J. K., Qureshi K., Lunec J.1998. Matrix metalloproteinase-1 is induced by epidermal growth factor in human bladder tumour cell lines and is detectable in the urine of patients with bladder tumours. Br. J. Cancer 78: 215–220. doi: 10.1038/bjc.1998.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono M., Kuwano M.2006. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin. Cancer Res. 12: 7242–7251. doi: 10.1158/1078-0432.CCR-06-0646 [DOI] [PubMed] [Google Scholar]
- 24.Rowinsky E. K.2004. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu. Rev. Med. 55: 433–457. doi: 10.1146/annurev.med.55.091902.104433 [DOI] [PubMed] [Google Scholar]
- 25.Selvarajah G. T., Verheije M. H., Kik M., Slob A., Rottier P. J., Mol J. A., Kirpensteijn J.2012. Expression of epidermal growth factor receptor in canine osteosarcoma: association with clinicopathological parameters and prognosis. Vet. J. 193: 412–419. doi: 10.1016/j.tvjl.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 26.Shiomitsu K., Johnson C. L., Malarkey D. E., Pruitt A. F., Thrall D. E.2009. Expression of epidermal growth factor receptor and vascular endothelial growth factor in malignant canine epithelial nasal tumours. Vet. Comp. Oncol. 7: 106–114. doi: 10.1111/j.1476-5829.2009.00178.x [DOI] [PubMed] [Google Scholar]
- 27.Thøgersen V. B., Jørgensen P. E., Sørensen B. S., Bross P., Orntoft T., Wolf H., Nexø E.1999. Expression of transforming growth factor alpha and epidermal growth factor receptor in human bladder cancer. Scand. J. Clin. Lab. Invest. 59: 267–277. doi: 10.1080/00365519950185634 [DOI] [PubMed] [Google Scholar]
- 28.Wolff A. C., Hammond M. E., Schwartz J. N., Hagerty K. L., Allred D. C., Cote R. J., Mitchell D., Fitzgibbons P. L., Hanna W. M., Langer A., McShane L. M., Paik S., Pegram M. D., Perez E. A., Press M. F., Rhodes A., Sturgeon C., Taube S. E., Tubbs R., Vance G. H., van de Vijver M., Wheeler T. M., Hayes D. F.2007. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 131: 18–43. [DOI] [PubMed] [Google Scholar]
- 29.Wood D. P., Jr, Fair W. R., Chaganti R. S.1992. Evaluation of epidermal growth factor receptor DNA amplification and mRNA expression in bladder cancer. J. Urol. 147: 274–277. [DOI] [PubMed] [Google Scholar]