Abstract
We investigated the in vitro differentiation of canine bone marrow stromal cells (BMSCs) into voltage- and glutamate-responsive neuron-like cells. BMSCs were obtained from the bone marrow of healthy beagle dogs. Canine BMSCs were incubated with the basal medium for neurons containing recombinant human basic fibroblast growth factor (bFGF; 100 ng/ml). The viability of the bFGF-treated cells was assessed by a trypan blue exclusion assay, and the morphology was monitored. Real-time RT-PCR was performed to evaluate mRNA expression of neuronal, neural stem cell and glial markers. Western blotting and immunocytochemical analysis for the neuronal markers were performed to evaluate the protein expression and localization. The Ca2+ mobilization of the cells was evaluated using the Ca2+ indicator Fluo3 to monitor Ca2+ influx. To investigate the mechanism of bFGF-induced neuronal differentiation, the fibroblast growth factor receptor inhibitor, the phosphoinositide 3-kinase inhibitor or the Akt inhibitor was tested. The bFGF treatment resulted in the maintenance of the viability of canine BMSCs for 10 days, in the expression of neuronal marker mRNAs and proteins and in the manifestation of neuron-like morphology. Furthermore, in the bFGF-treated BMSCs, a high concentration of KCl and L-glutamate induced an increase in intracellular Ca2+ levels. Each inhibitor significantly attenuated the bFGF-induced increase in neuronal marker mRNA expression. These results suggest that bFGF contributes to the differentiation of canine BMSCs into voltage- and glutamate-responsive neuron-like cells and may lead to the development of new cell-based treatments for neuronal diseases.
Keywords: basic fibroblast growth factor, bone marrow stromal cell, canine, neuron
Spinal cord injury is a disease that involves a loss of motor and sensory function and often results in disability, including paresis, paralysis, or urinary and/or fecal incontinence. When spinal cord injury occurs, the regeneration of spinal cord neurons is a difficult task [19]. Several therapies for spinal cord injury have been examined that promote conduction of electrical signals, replace lost tissue, induce neural regeneration and rescue tissue from secondary injury. Among the therapies, stem cell therapy has been expected to overcome incurable diseases, such as severe spinal cord injury. To date, induced pluriopotent stem cells, embryonic stem cells, neural progenitor cells, adipose-derived stem cells and bone marrow stromal cells (BMSCs) are expected to be potential stem cell sources for spinal cord regenerative therapy [8, 34, 38]. Among these stem cells, BMSCs have been the focus of research and clinical application for spinal cord regenerative therapy in dogs [8].
BMSCs are fibroblastic adherent cells and include a small number of mesenchymal stem cells that are capable of differentiation into several tissue-forming cell types, such as bone, cartilage, fat, muscle, heart and brain cells [27, 28, 34]. BMSCs are easily accessible through aspiration of the bone marrow, which allows researchers to avoid some ethical issues associated with embryonic stem cells. Therefore, transplantation of BMSCs is considered a clinically useful therapeutic approach for the treatment of spinal cord injury [35]. Transplantation of autologous and allogeneic BMSCs in dogs with spinal cord injury has been performed, and administration of BMSCs has been shown to significantly improve functional recovery of dogs with spinal cord injury compared with untreated spinal cord injury dogs [11]. However, the mechanism of spinal regeneration by canine BMSCs has been unclear. Two main aims of cell-based treatment for spinal cord injury are providing the cells with a microenvironment that supports or enhances the neuroprotective and regenerative ability of cells within the lesion, or replacing lost or injured cells, such as neurons. Many previous studies have shown that the trophic factors from transplanted BMSCs may play an important role for spinal regeneration [5, 9, 11, 22, 23, 25, 36]. In order to fully repair spinal injury with central necrosis, it is also necessary that transplanted BMSCs differentiate into functional neurons and replace the injured neurons. Another strategy to repair the lesion with central necrosis is transplantation of functional neurons derived from BMSCs.
In the previous studies on neuronal differentiation of canine BMSCs, it has been shown that canine BMSCs can form neurosphere-like clumps and differentiate into neuron-like cells expressing neuronal markers [6, 8, 12, 13, 20, 24]. However, to the best of our knowledge, the differentiation of canine BMSCs into functional neurons has not yet been proven.
In humans and mice, it has been reported that several growth factors, such as basic fibroblast growth factor (bFGF), nerve growth factor and neurotrophin-3, induced the differentiation of various types of stem cells into functional neurons [10, 14, 21, 26, 32, 33, 37]. To investigate the differentiation of canine BMSCs into functional neurons, we focused on bFGF among these growth factors in the present study. Basic fibroblast growth factor has a wide range of biological effects on cell growth, differentiation and survival [1]. It has been shown that bFGF is highly expressed in neuronal tissues and it performs an important function in neuronal regeneration and in recovery from spinal cord injury [4, 7, 15, 29]. Basic fibroblast growth factor has been demonstrated to induce differentiation of BMSCs into functional neurons in mice [33, 37].
To achieve further improve functional recovery and elucidate the mechanism that underlies the beneficial effects of BMSCs transplantation on dogs with spinal cord injury, the basic research on the differentiation of canine BMSCs into functional neurons is worthwhile. Thus, the purpose of the present study is to investigate differentiation of canine BMSCs into functional neurons.
MATERIALS AND METHODS
Isolation and culture of canine BMSCs: Three healthy beagle dogs (male, 3 years old) were used in the present study. This study was conducted under Nihon University Animal Care and Use Committee approval (AP12B015). All dogs were premedicated intravenously with midazolam hydrochloride (0.2 mg/kg; Astellas Pharma Inc., Tokyo, Japan) and butorphanol tartrate (0.2 mg/kg; Meiji Seika Pharma Co., Ltd., Tokyo, Japan). Anesthesia was induced with an intravenous injection of propofol (4.0 mg/kg; Intervet K.K., Osaka, Japan) and maintained with 1.5 to 2.0% isoflurane (Intervet K.K.) in 100% oxygen given in an endotracheal tube. Butorphanol tartrate (0.2 mg/kg) was again administered intravenously for pain relief before awakening. Canine BMSCs were isolated as described previously [8, 20]. Briefly, canine bone marrow was aspirated from the humerus, and mononuclear cells were separated by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich Inc., St. Louis, MO, U.S.A.). Following collection, the mononuclear cells were then transferred to a 75-cm2 plastic culture flask (Corning Inc. Life Sciences, Lowell, MA, U.S.A.) and static-cultured in an incubator at 5% CO2 and 37°C using α-modified Eagle minimum essential medium (Life Technologies Co., Carlsbad, CA, U.S.A.) with 10% fetal bovine serum (FBS; Life Technologies Co.). On the fourth day of culture, nonadherent cells were removed when the culture medium was replaced, thus isolating canine BMSCs. Canine BMSCs were harvested using 0.25% trypsin-ethylenediaminetetraacetic acid (Life Technologies Co.) once they reached approximately 90% confluence. Then, the collected cells were seeded at a density of 14,000 cells/cm2. The second-passage canine BMSCs were used for the following all experiments.
Flow cytometry: Cultured canine BMSCs were characterized by flow cytometry analysis based on the previous report [31]. The cells were placed in 5 ml round-bottom tubes (BD Biosciences, Tokyo, Japan) at 1 × 105 cells/tube with phosphate buffered saline (PBS; Sigma-Aldrich Inc.) containing 0.5% FBS and incubated with antibodies, including the anti-human CD29 mouse monoclonal antibody (eBioscience Inc., San Diego, CA, U.S.A.), the PE-conjugated anti-canine CD34 mouse monoclonal antibody (eBioscience Inc.), the anti-human/mouse CD44 rat monoclonal antibody (eBioscience Inc.) and the FITC-conjugated anti-canine CD45 rat monoclonal antibody (eBioscience Inc.) at 4°C for 45 min. Alexa fluor® 488-conjugated goat anti-mouse or rat IgG antibody (Life Technologies Co.) was used to label anti-CD29 and anti-CD44 antibodies, respectively, in darkness at 4°C for 30 min. To exclude dying cells, propidium iodide (Life Technologies Co.) was added at a final concentration of 2.5 µg/ml. An equal number of cells incubated with respective isotype control antibodies or only secondary antibodies were used as a control sample. The data were analyzed by recording 10,000 events on BD FACS Canto™ (BD Biosciences) by means of BD FACS Diva™ software (BD Biosciences) and FLOWJO software (Tree star Inc., Ashland, OR, U.S.A.).
Neuronal induction using bFGF: Canine BMSCs were placed in a 25-cm2 plastic culture flask (Corning Inc. Life Sciences) at a density of 4,000 cells/cm2. The neuronal induction using bFGF was conducted as described previously [10, 37]. Briefly, the medium was changed to Neurobasal-A medium (Life Technologies Co.) supplemented with 2% B-27 supplement (Life Technologies Co.) and 100 ng/ml recombinant human bFGF (Immunostep, Salamanca, Spain) at 24 hr of passage. Neurobasal-A medium supplemented with 2% B-27 supplement without bFGF was used as the medium in the control group. The neuronal induction medium was changed every 3 days. The cells were harvested using 0.25% trypsin-ethylenediaminetetraacetic acid at 0, 3, 5 and 10 days after the treatment, and their viability was assessed by means of a trypan blue exclusion assay (Wako Pure Chemical Industries Ltd., Osaka, Japan). The morphology of these cells was evaluated under an inverted microscope at indicated time points.
Real-time RT-PCR: Total RNAs were extracted from canine BMSCs before and after 3, 5, 10 days of the incubation with bFGF by using TRIzol® reagent (Life Technologies Co.). Canine BMSCs incubated in Neurobasal-A medium supplemented with 2% B-27 supplement without bFGF were used as a control group. The first-strand cDNA synthesis was carried out with 500 ng of total RNA using PrimeScript® RT Master Mix (TaKaRa Bio Inc., Otsu, Japan). Real-time RT-PCRs were performed with 2 µl of the first-strand cDNA in 25 µl (total reaction volume) with primers specific for canine neuronal (microtubule-associated protein 2 [MAP2], neurofilament light chain [NF-L] and neuron-specific enolase [NSE]), neural stem cells (nestin [NES]) and glial (glial fibrillary acidic protein [GFAP]) markers (Table 1) and SYBR® Premix Ex Taq™ II (TaKaRa Bio Inc.). The real-time RT-PCRs of no template controls were performed with 2 µl of RNase- and DNA-free water. In addition, real-time PCRs of no-reverse transcription controls were performed with 2 µl of each RNA sample. The PCRs were conducted using Thermal Cycler Dice® Real Time System II (TaKaRa Bio Inc.). The PCR reactions consisted of 1 cycle of denaturing at 95°C for 30 sec, 40 cycles of denaturing at 95°C for 5 sec and annealing and extension at 60°C for 30 sec. The specificity of each primer was verified using dissociation curve analysis and direct sequencing of each PCR product. The results were analyzed by means of the second derivative method and the comparative cycle threshold (ΔΔCt) method using TP900 DiceRealTime v4.02B (TaKaRa Bio Inc.). Amplification of β-glucuronidase [GUSB] from the same amount of cDNA was used as an endogenous control, and the amplification of the cDNA from non-treated canine BMSCs (0 day) was used as a calibrator standard.
Table 1. Primers for Real-time RT-PCR.
| Gene Name | Gene bank ID | Primer sequences |
|---|---|---|
| Microtubule-associated protein 2 (MAP2) | XM_845165.1 | F: 5′-AAGCATCAACCTGCTCGAATCC-3′ |
| R: 5′-GCTTAGCGAGTGCAGCAGTGAC-3′ | ||
| Neurofilament light chain (NF-L) | XM_534572.2 | F: 5′-TGAATATCATGGGCAGAAGTGGAA-3′ |
| R: 5′-GGTCAGGATTGCAGGCAACA-3′ | ||
| Neuron-specific enolase (NSE) | XM_534902.2 | F: 5′-GCATCCAGGCAGAGCAATCA-3′ |
| R: 5′-AATGGGTGGATGCAGCACAA-3′ | ||
| Nestin (NES) | XM_547531.2 | F: 5′-GGACGGGCTTGGTGTCAATAG-3′ |
| R: 5′-AGACTGCTGCAGCCCATTCA-3′ | ||
| Glial fibrillary acidic protein (GFAP) | XM_537614.2 | F: 5′-GCAGAAGTTCCAGGATGAAACCA-3′ |
| R: 5′-TCTCCAGATCCAGACGGGCTA-3′ | ||
| Glucuronidase β (GUSB) | NM_001003191.1 | F: 5′-ACATCGACGACATCACCGTCA-3′ |
| R: 5′-GGAAGTGTTCACTGCCCTGGA-3′ | ||
Western blotting: Canine BMSCs before and after 3, 5 and 10 days of the induction with or without bFGF were lysed with lysis buffer containing 100 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1 mM phenylmethanesulfonyl fluoride and complete mini EDTA-free protease inhibitor cocktail (Roche, Mannheim, Germany) at pH 7.4. Protein concentrations were adjusted using Bradford’s method [2]. Extracted proteins were boiled at 95°C for 5 min in sodium dodecyl sulfate buffer. Samples containing 10 µg of protein were loaded in each lane of 7.5% Mini-PROTEAN TGX gel (Bio-Rad, Hercules, CA, U.S.A.) and electrophoretically separated. Separated proteins were transferred to Immobilon-P Transfer Membranes (Merck Millipore, Billerica, MA, U.S.A.), treated with Block Ace (DS Pharma Biomedical, Osaka, Japan) for 50 min at room temperature and incubated for 120 min at room temperature with the primary antibodies: anti-human neurofilament light chain (NF-L) protein mouse monoclonal antibody (1:100; Thermo Fisher Scientific Inc., Rockford, IL, U.S.A.), anti-human neuron-specific enolase (NSE) mouse monoclonal antibody (1:200; DAKO North America Inc., Carpinteria, CA, U.S.A.) and anti-β-actin mouse monoclonal antibody (1:5,000; Sigma-Aldrich Inc.). After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse IgG (1:10,000; GE Healthcare, Piscataway, NJ, U.S.A.) for 90 min at room temperature. Immunoreactivity was detected using ECL Western blotting Analysis System (GE Healthcare). The chemiluminescent signals of the membranes were measured using ImageQuant LAS 4000 mini (GE Healthcare).
Immunocytochemistry: Canine BMSCs were seeded on 35-mm glass base dish (Iwaki, Tokyo, Japan) and cultured for 24 hr. Before and after 10 days of the neuronal induction with or without bFGF, these cells were fixed in 4% paraformaldehyde (Nacalai Tesque Inc., Kyoto, Japan) for 15 min and processed for immunocytochemistry to examine the protein expression and the cellular localization of neuronal markers. The fixed cells were permeabilized by means of incubation in 0.2% Triton™ X-100 (Sigma-Aldrich Inc.) for 15 min at room temperature. Non-specific antibody reactions were blocked for 30 min with a serum-free blocking solution (DAKO North America Inc.). These cells were then incubated for 90 min at room temperature with primary antibodies: an anti-human NF-L protein mouse monoclonal antibody (Thermo Fisher Scientific Inc.) and an anti-human NSE mouse monoclonal antibody (DAKO North America Inc.). After a wash with PBS, these cells were incubated and visualized with Alexa fluor® 594-conjugated F (abʹ)2 fragments of goat anti-mouse IgG (H+L) (Life Technologies Co.), Alexa fluor® 488-conjugated phalloidin (Life Technologies Co.) and TO-PRO®-3-iodide (Life Technologies Co.) for 60 min in darkness at room temperature. The cells were also incubated with only secondary antibodies to control for nonspecific binding of the antibodies. Canine spinal cords were used as a positive control. These samples were washed 3 times with PBS, dried, mounted with ProLong® Gold Antifade Reagent (Life Technologies Co.) and observed with a confocal laser scanning microscope (LSM-510; Carl Zeiss AG, Oberkochen, Germany).
Ca2+ imaging: Canine BMSCs were seeded on 35-mm glass base dishes at a density of 4,000 cells/cm2. After 10 days of the neuronal induction with or without bFGF, the cells were incubated in 1 ml of Neurobasal-A medium containing 2% B-27 supplement and 4.0 µM Fluo3-AM (Dojindo Lab., Kumamoto, Japan) with or without 100 ng/ml bFGF for 30 min at 37°C in the dark. Following incubation, the cells were washed twice in PBS. After washing, the culture medium was changed to a Ca2+ imaging buffer (containing 120 mM NaCl, 5 mM KCl, 0.96 mM NaH2PO4, 1 mM MgCl2, 11.1 mM glucose, 1 mM CaCl2, 1 mg/ml bovine serum albumin and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; pH 7.4). The glass base dishes with the fluorescent dye-loaded cells were placed at room temperature on the stage of a confocal laser scanning microscope (LSM510). Fluorescence of the dye was produced using excitation from a 75-W xenon arc lamp with appropriate filter sets (excitation 488 nm and emission 527 nm). Frames in a time lapse sequence were captured every 2 sec. After baseline images were acquired, the cells were stimulated with 50 mM KCl (Wako Pure Chemical Industries Ltd.) or 100 µM L-glutamate (Wako Pure Chemical Industries Ltd.). The relative changes in intracellular Ca2+ concentrations over time were expressed as relative change in baseline fluorescence.
Inhibitor treatments: Canine BMSCs were placed in a 25-cm2 plastic culture flask at a density of 4,000 cells/cm2. The cells were pretreated with Neurobasal-A medium supplemented with 2% B-27 supplement containing the fibroblast growth factor receptor (FGFR) inhibitor SU5402 (25 µM; Sigma-Aldrich Inc.), the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 (50 µM; Cell Signaling Technology Japan K.K., Tokyo, Japan) or the Akt inhibitor MK2206 (1 µM; Selleck chemicals Llc., Houston, TX, U.S.A.) for 1 hr as previously reported methods with slight modifications [37], and then, neuronal induction using bFGF (100 ng/ml) was performed. After 3 days of the neuronal induction using bFGF, total RNAs were extracted from each sample, and then, real-time RT-PCRs were performed to evaluate the mRNA expression of MAP2 as described above.
Data analysis: The data for these experiments were calculated as mean ± standard error. Statistical analyses were performed using StatMate IV (ATMS, Tokyo, Japan). The comparison of the data between the bFGF group and the control group was analyzed by means of the unpaired t test. The data from the time course study by real-time RT-PCR were analyzed using two-way analysis of variance, and Tukey’s test was used as post hoc analysis. The data from the inhibitor study were analyzed using one-way analysis of variance, and Tukey’s test was used as post hoc analysis. The values of P less than 0.05 were considered significant.
RESULTS
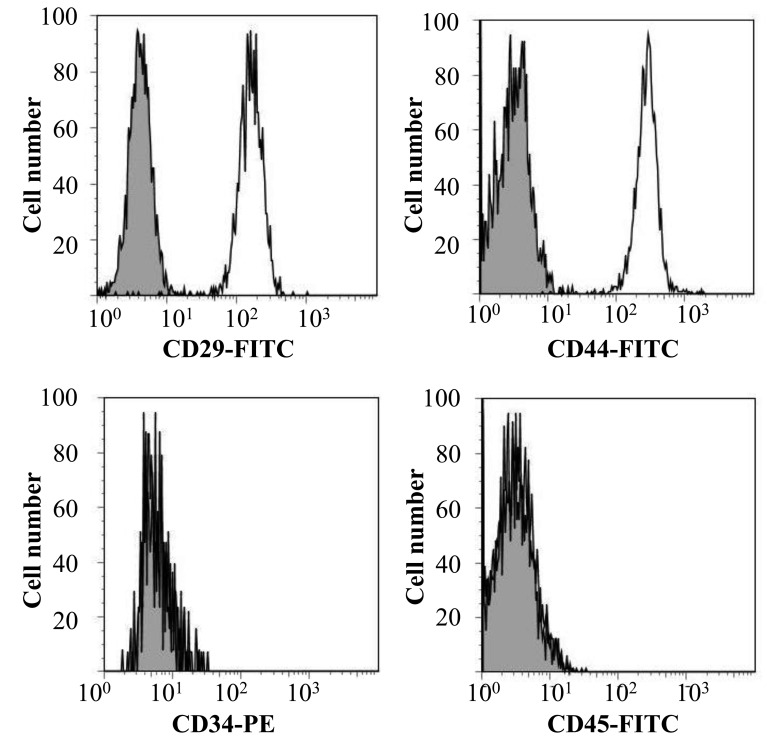
Characterization of canine BMSCs using flow cytometry: As shown in Fig. 1, the cells were strongly positive for the mesenchymal stem cell markers, CD29 (99.86 ± 0.14%) and CD44 (99.40 ± 0.05%). In contrast, the majority of the cells were negative for hematopoietic cell markers, CD34 (0.55 ± 0.01%) and CD45 (0.23 ± 0.05%).
Fig. 1.
Characterization of canine BMSCs by cell surface proteins. Solid and open histograms show non-specific and specific staining for the indicated marker, respectively.
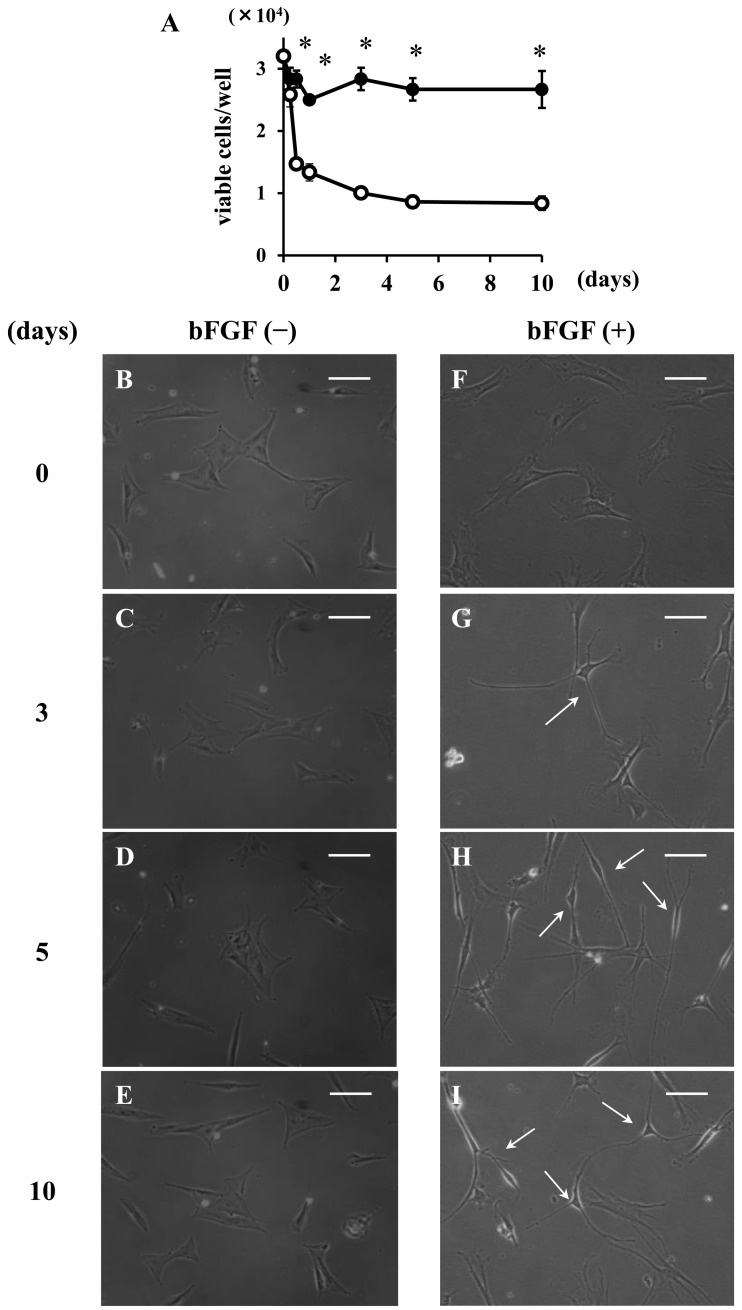
Effect of bFGF on cell viability: The viability of the cells incubated with bFGF (100 ng/ml) was maintained for at least 10 days, whereas without bFGF viability significantly decreased (Fig. 2A).
Fig. 2.
Effects of bFGF on cell viability (A) and morphology (B–I). The viability of canine BMSCs after incubation with (closed circles) and without (open circles) bFGF (100 ng/ml). Results are presented as mean ± SE from 3 independent experiments. *P<0.05, compared with no bFGF. The morphology of canine BMSCs before (B and F) and after 3 (C and G), 5 (D and H) and 10 days (E and I) of incubation with (F–I) or without (B–E) bFGF. The scale bar is 60 µm.
Differentiation of canine BMSCs into cells with a neuron-like shape after bFGF treatment: Morphologically, canine BMSCs were mostly of a flattened and fibroblast-like shape (Fig. 2B and 2F). The morphology of the cells remained fibroblast-like shape in the control group (Fig. 2C–2E). When the cells were incubated with bFGF, the cell shape started to change to neuron-like morphology, which was characterized by a small cell body and several long and sharp processes within 3 days and could be maintained for at least 10 days (Fig. 2G–2I). The percentage of canine BMSCs that changed to neuron-like morphology was 75.7 ± 4.3% at 10 days of neuronal induction using bFGF, whereas the percentage of the control group was 0.6 ± 0.7%.
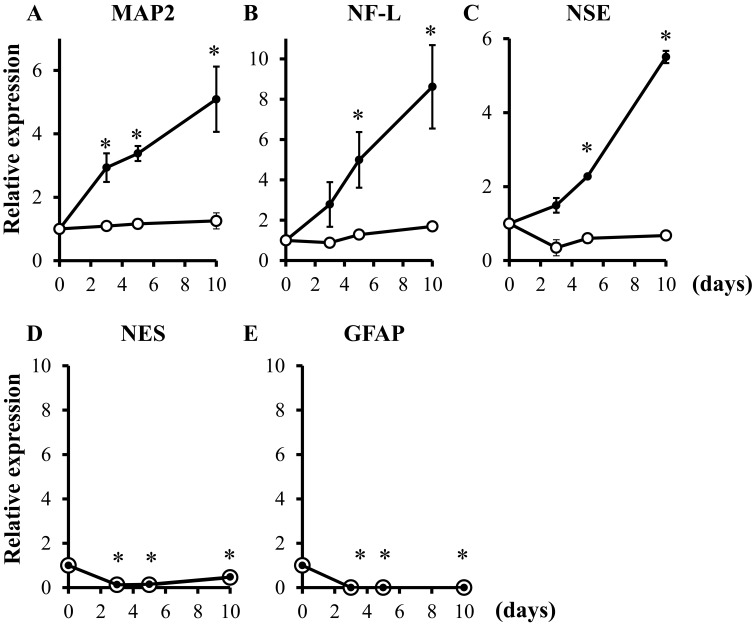
Quantitative analysis of bFGF-induced mRNA expression of neuronal, neural stem and glial markers in canine BMSCs: The mRNA expressions of neuronal markers were almost no change in the control group (Fig. 3A–3C). In bFGF-treated cells, expressions of mRNAs of neuronal markers significantly increased in a time-dependent manner (Fig. 3A–3C), whereas those of neural stem cell and glial markers clearly decreased (Fig. 3D and 3E).
Fig. 3.
Quantitative analysis of bFGF-induced mRNA expression of neuronal, neural stem cell and glial markers in canine BMSCs after incubation with (closed circles) or without (open circles) bFGF (100 ng/ml). Relative expression of MAP2 (A), NF-L (B), NSE (C), NES (D) and GFAP (E) mRNAs normalized to GUSB levels. The results are presented as mean ± SE from 3 independent experiments. *P<0.05, compared with day 0.
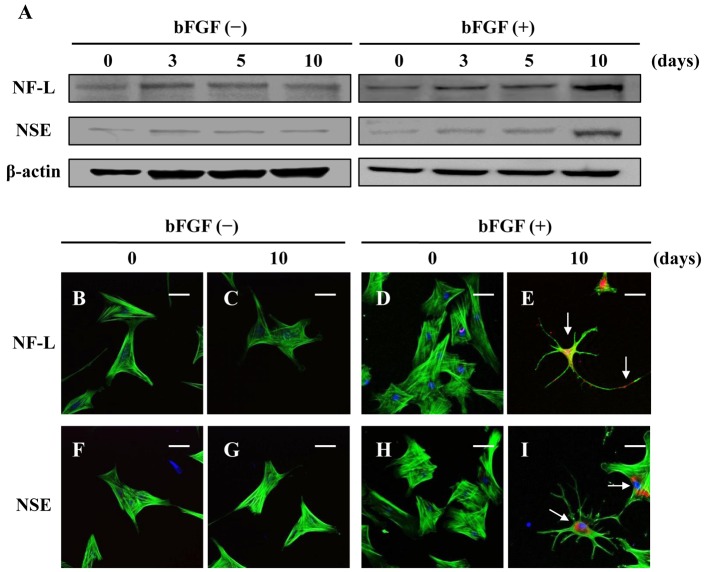
Protein expression and localization of neuronal markers in the neuron-like cells: The protein expressions of NF-L and NSE were almost no change in the control group (Fig. 4A). When canine BMSCs were stimulated by bFGF, the protein expressions of NF-L and NSE increased in a time dependent manner (Fig. 4A). In untreated canine BMSCs, NF-L expression was undetectable, but bFGF treatment resulted in the appearance of NF-L, which was localized in cell bodies and dendrites after 10 days (Fig. 4B–4E). Although NSE expression was less pronounced around the nucleus of untreated canine BMSCs, the expression was strongly enhanced by bFGF after 10 days of treatment (Fig. 4F–4I).
Fig. 4.
Expression and localization of neuronal markers in neuron-like cells differentiated from canine BMSCs. The protein expressions of NF-L (A; first row), NSE (A; second row) and β-actin (A; third row) were measured by Western blotting. The canine BMSCs were labeled with the fluorescent dye for nuclear staining (blue, nuclei), phalloidin (green, F-actin) and antibodies to NF-L (red, B–E) or NSE (red, F–I). The scale bar is 50 µm.
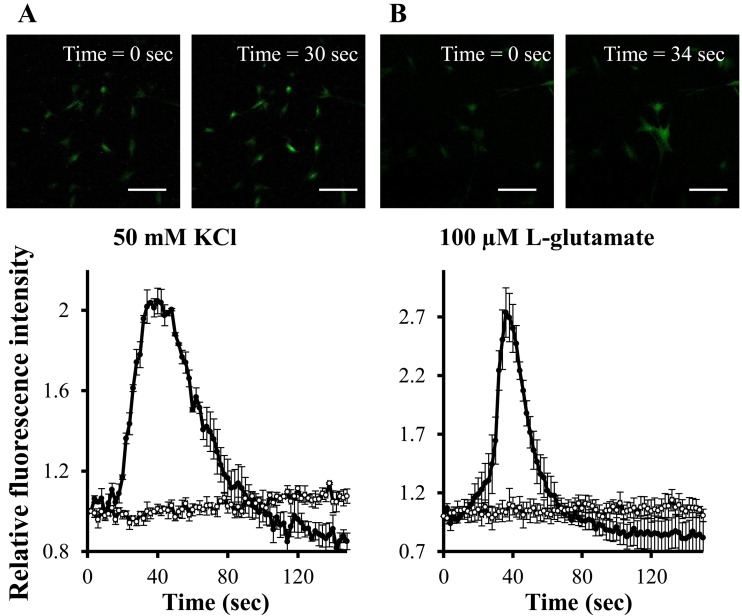
K+ and L-glutamate-induced Ca2+ mobilization in the neuron-like cells: As shown in Fig. 5A, 50 mM KCl induced an increase in intracellular Ca2+ concentrations of the bFGF-treated cells. We further examined the effect of the neurotransmitter L-glutamate on Ca2+ mobilization in the cells. L-glutamate (100 µM) also evoked a sharp rise in intracellular Ca2+ concentrations of the bFGF-treated cells (Fig. 5B). The increase in intracellular Ca2+ concentrations induced by KCl and L-glutamate was observed in 66.25 ± 0.08% and 54.14 ± 0.14% of the bFGF-treated cells, respectively. On the other hand, KCl and L-glutamate had almost no effect on the intracellular Ca2+ concentration of the control group.
Fig. 5.
K+- and L-glutamate-induced mobilization of Ca2+ in neuron-like cells derived from canine BMSCs after incubation with (closed circles) and without (open circles) bFGF (100 ng/ml). The cells were stimulated with either 50 mM KCl (A) or 100 µM L-glutamate (B). Images of Ca2+ response to KCl or L-glutamate in the fluorescent dye-loaded cells treated with bFGF were displayed in the upper panel. Green fluorescence shows the changes in intracellular Ca2+ concentration, indicating neuronal activation. Changes in intracellular Ca2+ concentration are displayed in the bottom panel. The scale bar is 200 µm.
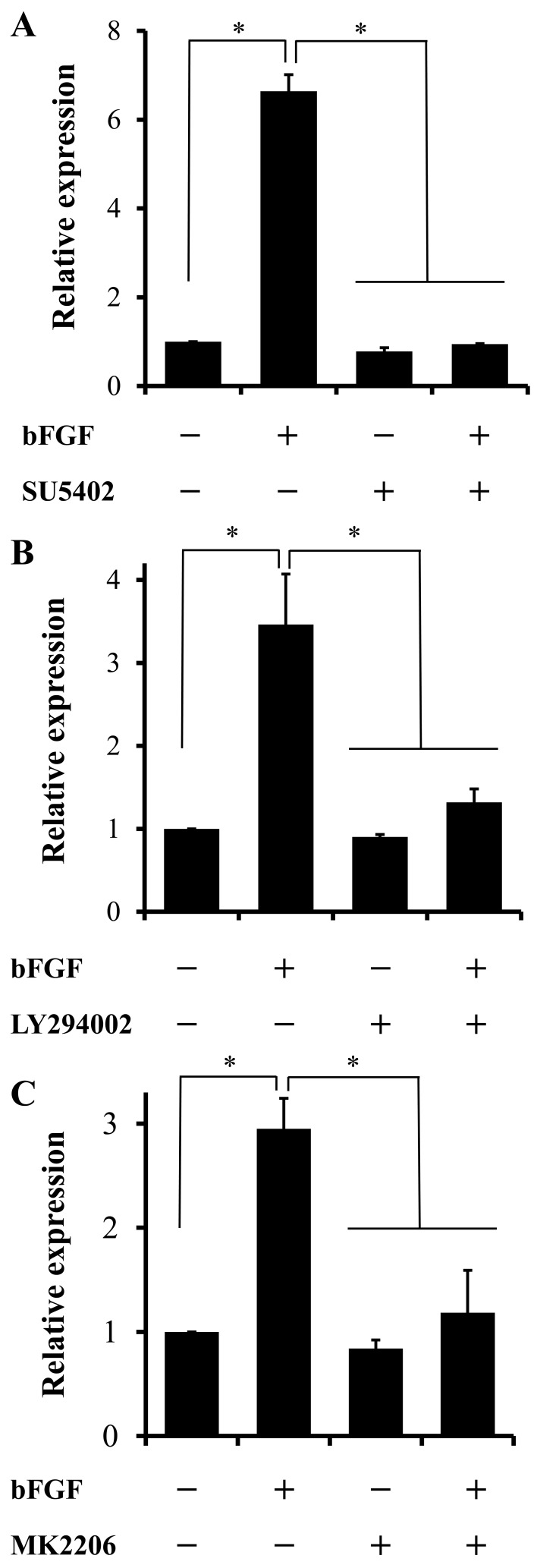
Involvement of FGFR, PI3K and Akt to the bFGF-induced neuronal differentiation of canine BMSCs: The bFGF-induced increase in MAP2 mRNA expression was significantly attenuated by the inhibitor of SU5402, LY294002 or MK2206 (Fig. 6). These inhibitors at experimental dose have no influence to the viability of the cells by means of trypan blue exclusion assay.
Fig. 6.
Contribution of FGFR, PI3K and Akt to bFGF-induced MAP2 mRNA expression. Cells were incubated with or without bFGF (100 ng/ml) in the absence or presence of the FGFR inhibitor SU5402 (A; 25 µM), the PI3K inhibitor LY294002 (B; 50 µM) or the Akt inhibitor MK2206 (C; 1 µM) for 1 hr. *P<0.05, compared with the other groups.
DISCUSSION
Canine BMSCs were well characterized by the expression profile of cell surface markers. We observed that the cells isolated from bone marrow were positive for the mesenchymal stem cell markers and negative for the hematopoietic cell markers. These results are consistent with previous reports [12, 23, 31], indicating that the cells were BMSCs.
In the previous study, dibutyryl cyclic AMP and methylisobutylxanthine have been reported to induce neuron-like morphology in canine BMSCs, but the cell viability was uncertain [12]. We reported that β-mercaptoethanol and butylated hydroxyanisole induced the expression of the neuronal markers and neuronal morphology in canine BMSCs, however, a large number of the cells detached from the culture flask during the neuronal induction [8, 20]. We previously suggested that longer durations of cell viability would be necessary to confirm whether canine BMSCs could differentiate into functional neurons [20]. In our present study, incubation with bFGF resulted in the maintenance of the viability of canine BMSCs for significant periods in the expression of neuronal markers. Previously, it was reported that many types of mouse, human and canine cells were cultured for long time in the media containing bFGF, indicating that bFGF was involved in the maintenance of viability of the mouse, human and canine cells [1, 13, 24, 33]. Therefore, bFGF might contribute to maintenance of the cell viability during the neuronal differentiation of canine BMSCs. On the basis of the previous and our preliminary studies, we employed 100 ng/ml bFGF in the present study. These results suggest that 100 ng/ml bFGF is possibly appropriate for the neuronal differentiation of canine BMSCs [10].
To investigate whether bFGF induced canine BMSCs into neuronal lineage, we observed the mRNA and protein expression of neuronal markers. In this study, bFGF induced the expression of neuronal marker mRNAs (MAP2, NF-L and NSE) and proteins (NF-L and NSE) and the manifestation of neuron-like morphology. On the other hand, the results of real-time RT-PCR amplification demonstrated that mRNA expressions of neural stem cell (NES) and glial (GFAP) markers clearly decreased. These findings suggested that bFGF induced differentiation of canine BMSCs into neuron-like cells expressing neuronal markers and astrocytic differentiation of these cells was definitively ruled out.
In the bFGF-treated BMSCs, a high concentration of KCl and L-glutamate induced an increase in intracellular Ca2+ concentration. Because it is well known that a high concentration of KCl and neurotransmitters, such as L-glutamate, stimulate Ca2+ influx via activation of voltage-dependent Ca2+ channels in neurons [32, 33], it is likely that the increase in intracellular Ca2+ concentration in bFGF-treated cells is caused by Ca2+ influx. Taken together, these results suggest that bFGF induced differentiation of canine BMSCs into voltage- and glutamate-responsive neuron-like cells. To confirm whether the bFGF-induced cells have fully neuronal function, detailed investigation about the electrophysiological function using patch clamp technique and the function of neurotransmitter release will be necessary.
It has been reported that bFGF stimulates FGFRs and subsequently activates the downstream molecules, such as mitogen-activated protein kinases, phospholipase C-γ and PI3K [16, 30, 37]. Basic fibroblast growth factor has been reported to activate the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway and consequently induces neuronal differentiation in rat pheochromocytoma cells, mouse neural stem cells, and mouse and human BMSCs [3, 14, 17, 18, 37]. However, to the best of our knowledge, no study has reported that the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway is involved in the neuronal differentiation in dogs. In the recent study, in dogs, the PI3K/Akt signaling pathway was shown to be involved in neuronal differentiation of adipose tissue-derived stem cells, although neuronal function of the cells was obscure [26]. In our study, in the presence of an inhibitor of FGFR, of PI3K or of Akt, bFGF failed to induce neuronal differentiation of BMSCs. Therefore, in dogs, it seems probable that the FGFR and the PI3K/Akt signaling pathway is involved in bFGF-induced neuronal differentiation of BMSCs.
In conclusion, bFGF contributes to maintenance of viability of canine BMSCs for long time and induces the differentiation of canine BMSCs into voltage- and glutamate-responsive neuron-like cells. Our results may lead to the development of new cell-based treatments for neuronal diseases, especially severe spinal cord injury.
Acknowledgments
This work was supported in part by a Nihon University College of Bioresource Sciences Research Fund for 2012 and 2013 and a Grant-in-Aid for Scientific Research (#24580465 and #24580433) from the Ministry of Education, Science, Sports and Culture of Japan. We thank Dr. Sachiko Matsuura for many helpful discussions and technical assistance with the immunocytochemical experiment.
REFERENCES
- 1.Bikfalvi A., Klein S., Pintucci G., Rifkin D. B.1997. Biological roles of fibroblast growth factor-2. Endocr. Rev. 18: 26–45. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M. M.1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 3.Chan W. S., Sideris A., Sutachan J. J., Montoya G. J. V., Blanck T. J., Recio-Pinto E.2013. Differential regulation of proliferation and neuronal differentiation in adult rat spinal cord neural stem/progenitors by ERK1/2, Akt, and PLCγ. Front Mol. Neurosci. 6: doi:10.3389/fnmol.2013.00023 . doi: 10.3389/fnmol.2013.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiba S., Kurokawa M. S., Yoshikawa H., Ikeda R., Takeno M., Tadokoro M., Sekino H., Hashimoto T., Suzuki N.2005. Noggin and basic FGF were implicated in forebrain fate and caudal fate, respectively, of the neural tube-like structures emerging in mouse ES cell culture. Exp. Brain Res. 163: 86–99. doi: 10.1007/s00221-004-2148-y [DOI] [PubMed] [Google Scholar]
- 5.Chiba Y., Kuroda S., Maruichi K., Osanai T., Hokari M., Yano S., Shichinohe H., Hida K., Iwasaki Y.2009. Transplanted bone marrow stromal cells promote axonal regeneration and improve motor function in a rat spinal cord injury model. Neurosurgery 64: 991–999. doi: 10.1227/01.NEU.0000341905.57162.1D [DOI] [PubMed] [Google Scholar]
- 6.Chung C. S., Fujita N., Kawahara N., Yui S., Nam E., Nishimura R.2013. A comparison of neurosphere differentiation potential of canine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells. J. Vet. Med. Sci. 75: 879–886. doi: 10.1292/jvms.12-0470 [DOI] [PubMed] [Google Scholar]
- 7.Doniach T.1995. Basic FGF as an inducer of anteroposterior neural pattern. Cell 83: 1067–1070. doi: 10.1016/0092-8674(95)90133-7 [DOI] [PubMed] [Google Scholar]
- 8.Edamura K., Kuriyama K., Kato K., Nakano R., Teshima K., Asano K., Sato T., Tanaka S.2012. Proliferation capacity, neuronal differentiation potency and microstructures after the differentiation of canine bone marrow stromal cells into neurons. J. Vet. Med. Sci. 74: 923–927. doi: 10.1292/jvms.11-0388 [DOI] [PubMed] [Google Scholar]
- 9.Ide C., Nakai Y., Nakano N., Seo T. B., Yamada Y., Endo K., Noda T., Saito F., Suzuki Y., Fukushima M., Nakatani T.2010. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 1332: 32–47. doi: 10.1016/j.brainres.2010.03.043 [DOI] [PubMed] [Google Scholar]
- 10.Jang S., Cho H. H., Cho Y. B., Park J. S., Jeong H. S.2010. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 11: 25 doi:10.1186/1471-2121-11-25 . doi: 10.1186/1471-2121-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung D. I., Ha J., Kang B. T., Kim J. W., Quan F. S., Lee J. H., Woo E. J., Park H. M.2009. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J. Neurol. Sci. 285: 67–77. doi: 10.1016/j.jns.2009.05.027 [DOI] [PubMed] [Google Scholar]
- 12.Kamishina H., Deng J., Oji T., Cheeseman J. A., Clemmons R. M.2006. Expression of neural markers on bone marrow-derived canine mesenchymal stem cells. Am. J. Vet. Res. 67: 1921–1928. doi: 10.2460/ajvr.67.11.1921 [DOI] [PubMed] [Google Scholar]
- 13.Kamishina H., Cheeseman J. A., Clemmons R. M.2008. Nestin-positive spheres derived from canine bone marrow stromal cells generate cells with early neuronal and glial phenotypic characteristics. In Vitro Cell. Dev. Biol. Anim. 44: 140–144. doi: 10.1007/s11626-008-9089-x [DOI] [PubMed] [Google Scholar]
- 14.Lim M. S., Nam S. H., Kim S. J., Kang S. Y., Lee Y. S., Kang K. S.2007. Signaling pathways of the early differentiation of neural stem cells by neurotrophin-3. Biochem. Biophys. Res. Commun. 357: 903–909. doi: 10.1016/j.bbrc.2007.04.045 [DOI] [PubMed] [Google Scholar]
- 15.Liu W. G., Wang Z. Y., Huang Z. S.2011. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol. Res. 33: 686–693. doi: 10.1179/1743132810Y.0000000031 [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J.1997. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276: 955–960. doi: 10.1126/science.276.5314.955 [DOI] [PubMed] [Google Scholar]
- 17.Mruthyunjaya S., Manchanda R., Godbole R., Pujari R., Shiras A., Shastry P.2010. Laminin-1 induces neurite outgrowth in human mesenchymal stem cells in serum/differentiation factors-free conditions through activation of FAK-MEK/ERK signaling pathways. Biochem. Biophys. Res. Commun. 391: 43–48. doi: 10.1016/j.bbrc.2009.10.158 [DOI] [PubMed] [Google Scholar]
- 18.Mullenbrock S., Shah J., Cooper G. M.2011. Global expression analysis identified a preferentially nerve growth factor-induced transcriptional program regulated by sustained mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) and AP-1 protein activation during PC12 cell differentiation. J. Biol. Chem. 286: 45131–45145. doi: 10.1074/jbc.M111.274076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura M., Okano H.2013. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 23: 70–80. doi: 10.1038/cr.2012.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano R., Edamura K., Sugiya H., Narita T., Okabayashi K., Moritomo T., Teshima K., Asano K., Nakayama T.2013. Evaluation of mRNA expression levels and electrophysiological function of neuron-like cells derived from canine bone marrow stromal cells. Am. J. Vet. Res. 74: 1311–1320. doi: 10.2460/ajvr.74.10.1311 [DOI] [PubMed] [Google Scholar]
- 21.Neuhuber B., Gallo G., Howard L., Kostura L., Mackay A., Fischer I.2004. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J. Neurosci. Res. 77: 192–204. doi: 10.1002/jnr.20147 [DOI] [PubMed] [Google Scholar]
- 22.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K., Suzuki Y., Ide C., Inaba T.2011. Evaluation of transplantation of autologous bone marrow stromal cells into the cerebrospinal fluid for treatment of chronic spinal cord injury in dogs. Am. J. Vet. Res. 72: 1118–1123. doi: 10.2460/ajvr.72.8.1118 [DOI] [PubMed] [Google Scholar]
- 23.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K., Harada Y., Suzuki Y., Ide C., Inaba T.2012. Safety of autologous bone marrow stromal cell transplantation in dogs with acute spinal cord injury. Vet. Surg. 41: 437–442. doi: 10.1111/j.1532-950X.2011.00959.x [DOI] [PubMed] [Google Scholar]
- 24.Oda Y., Tani K., Kanei T., Haraguchi T., Itamoto K., Nakazawa H., Taura Y.2013. Characterization of neuron-like cells derived from canine bone marrow stromal cells. Vet. Res. Commun. 37: 133–138. doi: 10.1007/s11259-013-9555-0 [DOI] [PubMed] [Google Scholar]
- 25.Ohta M., Suzuki Y., Noda T., Ejiri Y., Dezawa M., Kataoka K., Chou H., Ishikawa N., Matsumoto N., Iwashita Y., Mizuta E., Kuno S., Ide C.2004. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp. Neurol. 187: 266–278. doi: 10.1016/j.expneurol.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 26.Park S. S., Lee Y. J., Han H. J., Kweon O. K.2011. Role of laminin-111 in neurotrophin-3 production of canine adipose-derived stem cells: Involvement of Akt, mTOR, and p70S6K. J. Cell. Physiol. 226: 3251–3260. doi: 10.1002/jcp.22686 [DOI] [PubMed] [Google Scholar]
- 27.Poulsom R., Alison M.2002. Adult stem cell plasticity. J. Pathol. 197: 441–456. doi: 10.1002/path.1176 [DOI] [PubMed] [Google Scholar]
- 28.Prockop D. J.1997. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71–74. doi: 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- 29.Reynolds B. A., Weiss S.1992. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710. doi: 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- 30.Schlessinger J.2000. Cell signaling by receptor tyrosine kinases. Cell 103: 211–225. doi: 10.1016/S0092-8674(00)00114-8 [DOI] [PubMed] [Google Scholar]
- 31.Takemitsu H., Zhao D., Yamamoto I., Harada Y., Michishita M., Arai T.2012. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet. Res. 8: 150–158. doi: 10.1186/1746-6148-8-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tondreau T., Dejeneffe M., Meuleman N., Stamatopoulos B., Delforge A., Martiat P., Bron D., Lagneaux L.2008. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics 9: 166. doi: 10.1186/1471-2164-9-166.doi: 10.1186/1471-2164-9-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tropel P., Platet N., Platel J. C., Noël D., Albrieux M., Benabid A. L., Berger F.2006. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 24: 2868–2876. doi: 10.1634/stemcells.2005-0636 [DOI] [PubMed] [Google Scholar]
- 34.Vats A., Bielby R. C., Tolley N. S., Nerem R., Polak J. M.2005. Stem cells. Lancet 366: 592–602. doi: 10.1016/S0140-6736(05)66879-1 [DOI] [PubMed] [Google Scholar]
- 35.Wright K. T., El Masri W., Osman A., Chowdhury J., Johnson W. E.2011. Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells 29: 169–178. doi: 10.1002/stem.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S., Suzuki Y., Ejiri Y., Noda T., Bai H., Kitada M., Kataoka K., Ohta M., Chou H., Ide C.2003. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 72: 343–351. doi: 10.1002/jnr.10587 [DOI] [PubMed] [Google Scholar]
- 37.Yang H., Xia Y., Lu S. Q., Soong T. W., Feng Z. W.2008. Basic fibroblast growth factor-induced neuronal differentiation of mouse bone marrow stromal cells requires FGFR-1, MAPK/ERK, and transcription factor AP-1. J. Biol. Chem. 283: 5287–5295. doi: 10.1074/jbc.M706917200 [DOI] [PubMed] [Google Scholar]
- 38.Zietlow R., Lane E. L., Dunnett S. B., Rosser A. E.2008. Human stem cells for CNS repair. Cell Tissue Res. 331: 301–322. doi: 10.1007/s00441-007-0488-1 [DOI] [PubMed] [Google Scholar]