Abstract
One hundred and twenty stranding events of Stejneger’s beaked whales were reported in Japan between 1999 and 2011. The purpose of this study is to introduce pathological data and to discuss probable causes of death for 44 Stejneger’s beaked whales among them. The significant pathological findings were the pulmonary edema, parasitic granulomatous nephritis, emaciation, amyloidosis, suppurative bronchopneumonia and so on. The probable causes of death were categorized as noninfectious in 43 of the cases, which included drowning, starvation and secondary amyloidosis. One individual was diagnosed with septicemia, which was the only example of an infectious disease. Because we could not always perform advanced analyses, such as microbiology tests, biotoxin examinations or contaminant analyses, the finality of our findings may be impaired. However, the present study has broad implications on the causes of death of Stejneger’s beaked whales of the seas around Japan, which are valuable for the future studies and for the detection of emerging diseases.
Keywords: Mesoplodon stejnegeri, pathology, septicemia, Stejneger’s beaked whale, stranding
The Stejneger’s beaked whale, Mesoplodon stejnegeri (True, 1885), belongs to the Order Cetartiodactyla, Suborder Odontoceti, Family Ziphiidae [43]. Most species of the genus Mesoplodon in the world are known to be very rare [28], whereas fairly large number of stranding events of M. stejnegeri have been reported in Japan [15]. According to the “Marine Mammals Stranding DataBase” for Japan accessible at the website of the National Museum of Nature and Science (http://svrsh1.kahaku.go.jp/marmam/) compiled jointly with the Institute of Cetacean Research and Shimonoseki Academy of Marine Science, 120 Stejneger’s beaked whales were reported to have been stranded along the coasts of Japan during the period of 1999 to 2011. Stranding information for this species has been mostly reported from coasts of the Sea of Japan, and some were from coastlines of Hokkaido along the Pacific Ocean and the Okhotsk Sea [15]. Although some publications on this species are available, most of them have mainly described on biological findings including reproductive status, age determinations and morphological description, such as skull shape [1, 26, 28, 33, 38, 43, 44], and one case report included a pathological description [37]. Among these 44 whales, 2 were diagnosed as having amyloidosis, and results of detailed pathological examinations were already published [41]. Here, we will take into consideration specifically the pathological findings for these 44 whales including these 2 individuals. As there are no reports on pathological studies of a substantial number of Stejneger’s beaked whales, the purpose of this study was to report significant pathological findings and to discuss probable causes of death based on the pathological examinations of the 44 cases.
MATERIALS AND METHODS
Among 120 stranded Stejneger’s beaked whales during the period of 1999 and 2011, complete necropsies were performed on 44 cases, which consisted of 2 neonates, 2 suckling individuals and 40 adults. They included 20 males, 23 females and an individual of unknown sex. The whales were found along the coasts of Hokkaido, Aomori, Akita, Yamagata, Niigata, Toyama, Ishikawa, Fukui, Kyoto, Hyogo, Tottori, Shimane, Yamaguchi and Fukuoka Prefectures in Japan (Fig. 1) [15].
Fig. 1.
Map of the stranding site. The whales were found along the coasts of Hokkaido, Aomori, Akita, Yamagata, Niigata, Toyama, Ishikawa, Fukui, Kyoto, Hyogo, Tottori, Shimane, Yamaguchi and Fukuoka Prefectures in Japan during the period 1999 to 2011.
External measurements were made according to Norris [34]. The mean value for body length was 467 cm for 18 of the adult males and 486 cm for 21 of the adult females. Total body weights were recorded for 5 adult males (the mean value was 886 kg) and 2 adult females (the mean value was 1,218 kg). The age class was determined based on the body color and shape as well as the body length [28]. The body lengths of the 2 neonates were 191 cm and 195 cm, and distinct fetal folds were recognized. The fetal folds appeared as 5 to 6 creases perpendicular to the body axis on both sides of the body. Until the juvenile period (or adolescence), the basic body color pattern is dorsally dark with an olive to dark gray color, contrasting with a lighter gray to white color in the ventral area. In the head, the dorsal dark pigmentation extends ventrally to surround the eye (Fig. 2). The total tone becomes darker as the animal grows, and they become completely black when they reach sexual maturity, at which point they reach 400 to 450 cm in body length. Neonate individuals tend to be found in early March to May in Japan (the website of the National Museum of Nature and Science, http://svrsh1.kahaku.go.jp/marmam/). Suckling individuals have many fringes (marginal papillae) in the tongue as in the case of most of the odontocetes known [38]. The fringes appear to fade gradually as the animal ages and weans. The species has a pair of teeth in the mandible, but they erupt only in males when they become sexually mature (Fig. 3). On the other hand, the teeth never erupt in females [28]. External observations were made to record signs of entanglement, shark bites and external parasites, which could be suggestive of causes of death.
Fig. 2.

Neonate of M. stejnegeri. 191 cm. male. The basic body color pattern is dorsally dark with an olive to dark gray color, contrasting with a lighter gray to white color in the ventral area. In the head, the dorsal dark pigmentation extends ventrally to surround the eye until the juvenile period (or adolescence).
Fig. 3.

External appearances in an adult male of M. stejnegeri. An adult male had a pair of erupted tooth and parallel pairs linear scars in the body surface.
During the process of necropsy, gross pathological observations were made for all organs. Necessary tissues were sampled and fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin (HE, Hematoxylin 3G and Eosin, Sakura Finetek Japan, Tokyo, Japan) for examination by the light microscopy. The 2 individuals previously examined were additionally treated for special observations for amyloidosis. These included staining with toluidine blue, Alcian blue/periodic acid-Schiff and fluorochrome thioflavin T, as well as Congo red and examination by electron microscopy [41]. After summarizing significant pathologic findings of the 44 cases to discuss probable causes of death, the causes of death were categorized as infectious or noninfectious causes.
RESULTS
Complete necropsies were performed on 44 Stejneger’s beaked whales stranded in Japan in this study.
Significant external findings: None of the animals observed had white oval scars caused by the cookiecutter shark, Isistius brasiliensis [17, 44], or was infested with Pennella copepods. There was also no evidence suggesting entanglements or shark bite in any cases. Thirty-seven males had Conchoderma cirripedes attached to their tusks.
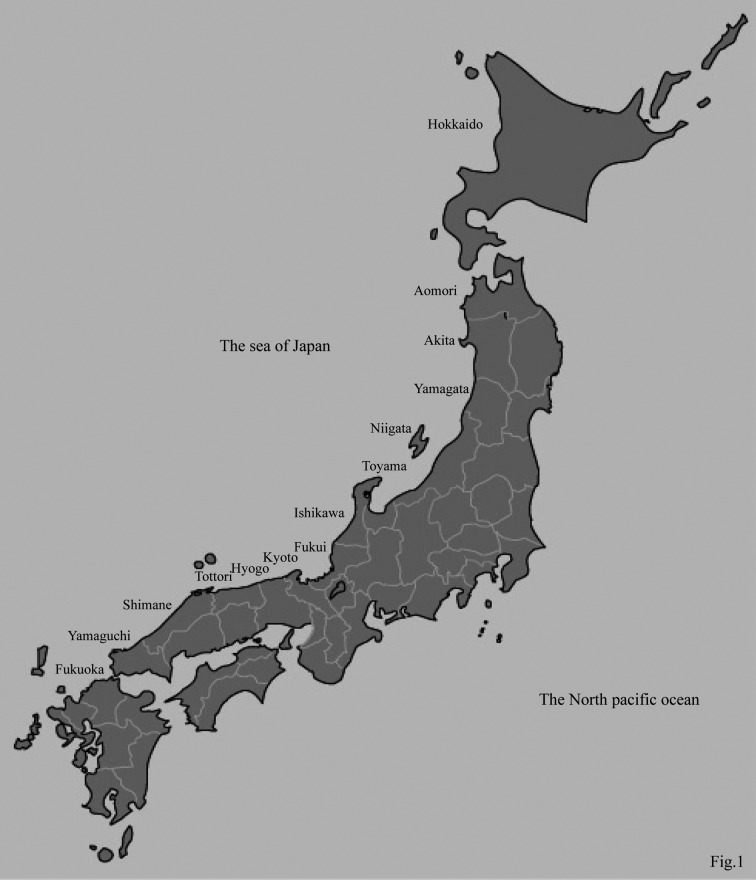
Significant pathological findings: Symptoms of pulmonary edema were observed in all 44 cases examined in this study and were frequently observed in both lungs (Fig. 4a). The lungs appeared dark and were wet and heavy in the gross examinations. Some red serous fluid and whitish foamy materials were also often observed in the trachea and bronchi (Fig. 4b). Microscopic examination revealed that the majority of alveoli were filled with a great deal of pinkish fluid containing some neutrophils, lymphocytes and macrophages, and there was diffuse congestion of the interalveolar septa, which indicated congestive edema.
Fig. 4.
Fig. 4a. Pulmonary edema was found in all of the 44 cases. The both lobes of the lung usually appeared dark, wet and heavy diffusely. Fig. 4b. In the case of pulmonary edema, some red serous fluid and whitish foamy material were often observed in the trachea and bronchi.
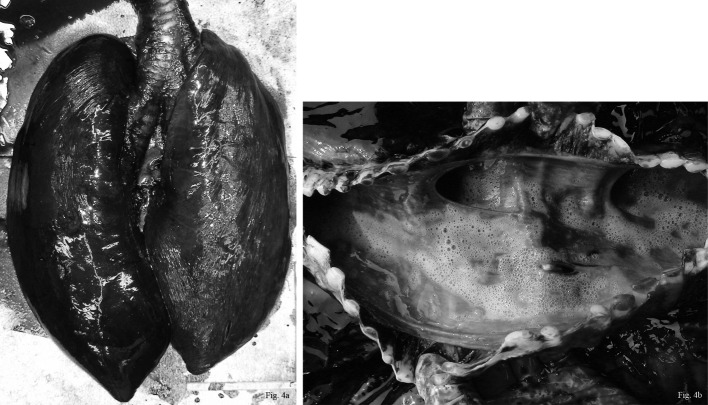
Parasitic granulomatous nephritis was observed in 37 cases with the kidneys being severely swollen and exhibiting many hard and white nodules in gross examinations of cross sections. Nodules excised from the renal lobe revealed the presence of Crassicauda nematodes (Fig. 5a). Some nematodes were also found in the ureters (Fig. 5b). Microscopic examination demonstrated that the lesions were composed of many multinucleated giant cells, lymphocytes, neutrophils, eosinophils and fibrous tissue containing some debris from parasites or calcified parasite remains. These nematodes were found only in the kidneys in all cases.
Fig. 5.
Fig. 5a. The kidneys showed swollen and exhibited many hard and white granulomatous nodules. Nodules excised from the renal lobe revealed the presence of Crassicauda nematodes. Fig. 5b. A cross-section of Crassicauda parasite was observed in the ureter. 2.5 magnifications. H. E.
Emaciation was observed in 17 cases, which showed a clearly visible “neck”, ribs and a row of spinous processes of vertebra clearly recognizable, because of the collapsed epaxial muscles on the both sides. Among these cases, one adult had a large ulcer in the esophagus, and another had some ulcers in the stomach and small intestine. Five adult whales had no stomach contents; however, there were no significant pathological changes in the organs. Two neonates and two sucklings were also severely emaciated, with no stomach contents and no significant pathological findings in the organs. The remaining 6 cases had no corresponding pathological findings that could have induced emaciation and no other lesions in gross and microscopic examinations.
Amyloidosis was diagnosed in 2 adult whales. These cases had large, pale and fragile livers with rounded edges. The heart had some white bands, and the pancreas was pale and fragile in one of these cases. Depositions of amyloid were mainly found in the liver, pancreas and kidney. In the spleen, amyloid was deposited in the germinal centers with diffuse atrophy of the splenic lymphoid follicles in the 2 whales. Parasitic granulomatous nephritis was also observed in the kidneys. The amyloid protein was typically stained red with Congo red staining. Weak reactions were observed when staining the amyloid with toluidine blue, periodic acid-Schiff-Alcian blue and thioflavin T. Positive deposition of the amyloid AA protein was detected through immunostaining in both cases. Amyloid fibers exhibited a typical framework of fine fibrils measuring about 11 nm in diameter under a transmitted electron microscope.
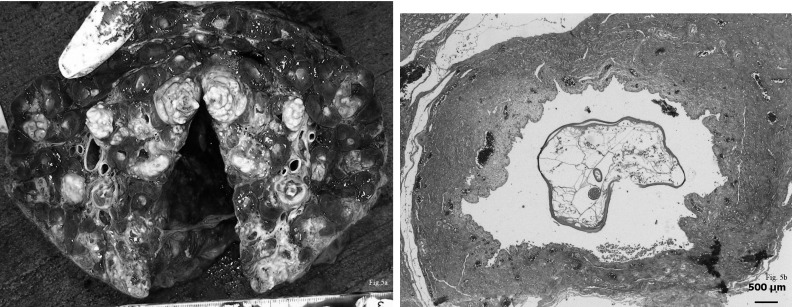
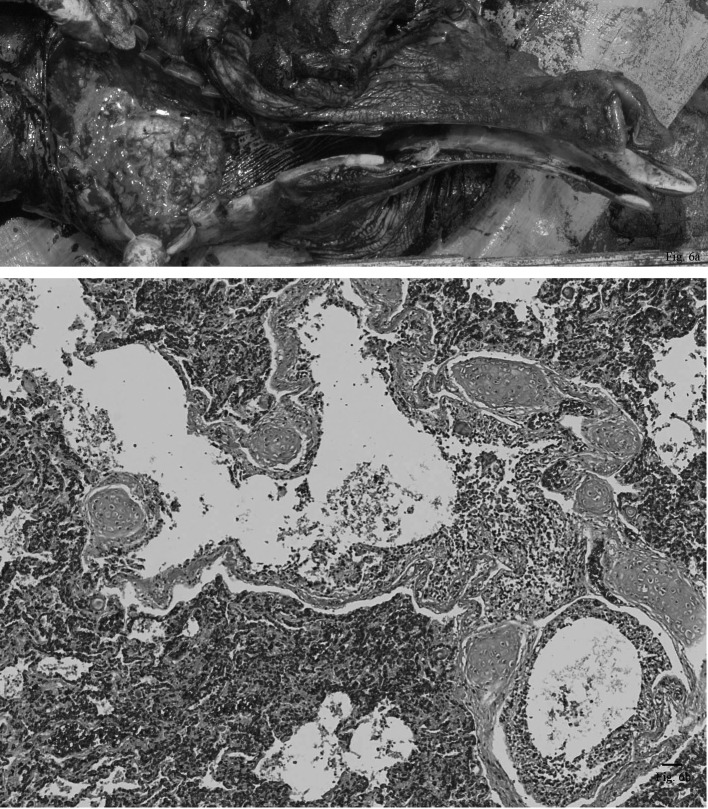
Suppurative bronchopneumonia was found in an adult male. Some abscesses were detected from the trachea to the bronchi (Fig. 6a). Abscesses of various sizes were also scattered diffusely throughout both lungs, and these were more severe in the right lung. A 2 cm vegetation was observed on the tricuspid valve of the heart. The heart looked normal in the size, texture and shape. All other organs appeared to be relatively normal. Microscopic observation of the abscesses in the trachea and bronchi demonstrated that they were filled with many detached epithelial cells, neutrophils, lymphocytes and macrophages. Inflammatory cells had also infiltrated, into the alveoli, causing hemorrhage and congestion of the interalveolar septa (Fig. 6b). The vegetation on the tricuspid valve of the heart consisted of fibrous materials and some inflammatory cell debris containing no microorganisms. The liver showed some inflammatory cells that had infiltrated to the portal triad. All other organs had no significant pathological changes in the microscopic examinations.
Fig. 6.
Fig. 6a. Some abscesses were detected from the trachea to the bronchi in an adult male. Fig. 6b. Many neutrophils, lymphocytes and macrophages infiltrated into the alveoli, causing hemorrhage and congestion of the interalveolar septa. 5 magnifications. H. E.
Probable cause of the death: The 44 cases were categorized into noninfectious or infectious causes (Table 1). The 43 cases of noninfectious causes consisted of 24 cases of drowning, 6 cases of emaciation with no significant changes in gross and histopathological examinations, 2 cases of emaciation caused by some ulcers in the digestive tracts, 9 cases (5 adults, 2 neonates and 2 suckling individuals) of starvation and 2 cases of amyloidosis. Only one case was categorized as resulting from an infectious cause. This was a case of septicaemia caused by suppurative bronchopneumonia.
Table 1. It showed the details of noninfectious and infectious causes of the death in 44 cases.
| Noninfectious cause | the number of cases |
|---|---|
| Drowning of unknown cause | 24 |
| Emaciation of unknown cause | 6 |
| Emaciation caused by some ulcer in the digestive tracts | 2 |
| Starvation with emaciation and no stomach contents | 9 |
| Secondary amyloidosis | 2 |
| Total | 43 |
| Infectious cause | |
| Septicemia | 1 |
| Total | 1 |
| Total | 44 |
DISCUSSION
There are 14 known species in the world within the genus Mesoplodon (according to the List of Marine Mammal Species and Subspecies of the The Society for Marine Mammalogy). Although the number of stranding reports for the Stejneger’s beaked whale (M. stejnegeri) has been increasing in Japan [14], most previous publications have concerned basic biological data, such as reproductive function, physical maturity and so on (e. g., [11, 12]). Considering the difference in the frequency of cookiecutter shark bites and Pennella copepod infestation, Stejneger’s beaked whales in the seas around Japan are independent from the population of the same species found along the North American coasts and Aleutian Islands [44].
Of the significant pathological findings, pulmonary edema was observed in all cases examined. However, because their lungs are not lobulated [40], pulmonary edema tends to affect not partially, but diffusely and severely. It could cause death in whales due to acute right heart failure and/or drowning. Cowan et al. [5] reported that heart failure from hyperthermia and/or respiratory difficulties were sufficient to cause directly the death of dolphins stranded on the coast of the Gulf of Mexico. Right heart failure was not observed in any of the cases we examined, and hyperthermia was not confirmed in any cases. As no other significant changes that could directly induce the pulmonary edema and other significant changes were found in 24 of the cases examined, it appears that these whales eventually drowned and fell into acute respiratory failure in the process of dying.
Parasitic granulomatous nephritis caused by Crassicauda nematodes was found in 37 cases. The life cycle of these nematodes is not yet known; however, some of them had eggs, suggesting that the Stejneger’s beaked whale is one of their definitive hosts. Three other species of Mesoplodon are known to live in Japanese waters: they are the ginkgo-toothed beaked whale (M. ginkgodens), Blainville’s beaked whale (M. densirostris) and Hubbs’ beaked whale (M. carlhubbsi). Crassicauda nematode infections were observed in all these species (our findings). Crassidauda nematodes were reported in the kidney of a Mesoplodon individual from the Pacific coast [14] and in the kidneys and ureters of a Cuvier’s beaked whale (Ziphius cavirostris) [19]. Araki et al. collected a nematode species from the ureter of Baird’s beaked whales captured in Japanese waters and identified it as C. giliakiana [2]. Kagei et al. reported C. grampicola infections in the Risso’s dolphin (Grampus griseus) in Japan [18]; however, the site of infection differs from those seen in Stejneger’s beaked whales or other Ziphiid species. They were found in the pterygoid sinus, causing purulent exudate and necrosis of the mucosa [9]. Crassicauda nematodes were also observed in the caudal muscle of the bottlenose dolphin (Tursiops truncatus) [32] and in the mammary gland of a long-beaked common dolphin (Delphinus capensis) (our findings). Araki et al. found C. boopis in the blubber of the Dall’s porpoise, Phocoenoides dalli [2]. The site of infection of these Crassicauda nematodes also differs from those seen in Stejneger’s beaked whales. Severe infection of Crassicauda nematodes was reported in larger baleen whales, and a possible significant threat was suggested [22]. Infestation with Crassicauda nematodes is known to occur in various species of cetaceans including the Stejneger’s beaked whale [14, 18, 19]. More attention should be paid to the pathological influence of this infestation.
Emaciation was observed in 17 cases including 13 adults, 2 neonates and 2 sucklings. Six of the 13 adults did not show any corresponding pathological findings that could induce emaciation. As another 5 adults did not have any stomach contents and significant changes related to emaciation were confirmed, we concluded that the cause of death for these animals was merely starvation. In bottlenose dolphins in South Carolina, the most prevalent noninfectious cause of death was reported to be emaciation with an unapparent underlying cause similar to that in our cases [25]. As mentioned in this reference, presumably the lack of prey or inability to catch prey for undetected reasons was the underlying problem. Two other adult whales showed some ulcers in the digestive tracts, and we concluded that this condition possibly induced emaciation and caused them to die. In additions, 2 neonates and 2 sucklings were also severely emaciated and had no stomach contents; however, there were no corresponding pathological changes that could induce emaciation. This suggested that they were merely separated from their dam and were not able to survive by themselves, as in the case of bottlenose dolphins in South Carolina [26].
Secondary amyloidosis was caused by parasitic granulomatous nephritis, which is categorized as a chronic infection, and this was similar to the cases of secondary amyloidosis seen in domestic animals [39]. Amyloids are pathological fibers composed of several types of pre-proteins and a β-structure fibrous protein. Amyloidosis is well recognized in humans [29, 30] and a variety of domestic and wild animals [20, 23, 27, 39] including birds [6, 31] and reptiles [7]. However, there have been few reports of amyloidosis in cetaceans. Howard identified one older specimen of common dolphin with amyloid deposition in the germinal centers of the spleen [13]. Cowan described four specimens of bottlenose dolphins with systemic amyloid deposits mainly in the kidneys, salivary glands and lungs [4]. Shindo and Yamato reported the deposition of amyloid in a Stejneger’s beaked whale stranded along the coast of Niigata, Japan [37], with deposition of amyloid found in the liver and kidney, which was the same as in our cases. Despite its morphologic uniformity, two types of amyloid have been identified generally [27]. One is referred to as amyloid light chain (AL) and is produced from immunoglobulin light chains. AL is associated with B-lymphocyte abnormalities, including myeloma. The other amyloid consists of a non-immunoglobulin amyloid-associated protein (AA). AA is derived from serum amyloid A protein, an acute-phase immunoreactant that is produced in excess as a result of chronic antigenic stimulation. Most cases of amyloidosis in domestic animals are idiopathic and appear to be of the AA type [27]. A comparison of amyloidosis in the Stejneger’s beaked whale and that of cattle revealed similar findings as well as findings based on molecular [36] and morphological [42] likenesses. Although parasitic infections have been frequently reported in other cetaceans including Phocoenid species finless porpoise, Neophocaena phocaenoides [21, 35] and Dall’s porpoise, Phocoenoides dalli [3, 8, 24], and Delphinidae species common dolphin, Delphinus delphis and striped dolphin, Stenella coeruleoalba [16], there are no previous reports of the relationship between parasitic infections and amyloidosis. A further study is needed to determine whether chronic inflammatory lesions including parasitic infections can lead to amyloidosis in cetaceans.
A septicemia caused by suppurative bronchopneumonia is a case of infectious cause of death in Stejneger’s beaked whale examined in the present study. Generally, the pathogens responsible for causing suppurative bronchopneumonia and septicemia in cetaceans are similar to those of domestic animals including Streptococcus sp., Staphylococcus sp., Salmonella sp., Pasteurella sp., Klebsiella pneumonia and others [10]. Although the absence of bacterial analysis decreases the conclusiveness of the findings, the pattern of the inflammatory reaction led to a diagnosis of suppurative bronchopneumonia in this study. According to the data on bottlenose dolphins stranded in North Carolina, the probable causes of death of 39.5% of 97 dolphins were determined to be infectious diseases, including bacterial and/or parasitic pneumonia and bacterial peritonitis [25].
The present study reported significant pathological findings and discussed probable causes of death for 44 Stejneger’s beaked whales stranded and investigated in Japan from 1999 to 2011. We diagnosed 43 noninfectious cases and 1 infectious case with respect to the probable causes of death. Although the absence of consistent use of microbiology examinations, biotoxin tests and contaminant analyses reduces the finality of the findings, the present study had broad implications regarding the baseline data for the causes of the death of Stejneger’s beaked whales for future studies and for the detection of emerging diseases.
Acknowledgments
We really appreciate the support of all collaborators and local stranding networks in our stranding survey. We would like to express our gratitude to Dr. Toshiaki Kuramoti for providing us with information about the parasitology of marine mammals and Dr. Hajime Ishikawa for organizing the information for most of the stranding events. We would like to express our great gratitude to the Stranding Network of Hokkaido and the Nippon Cetology Research Group for giving us large amounts of stranding information, measured values and help with this study. We are greatly thankful to countless people including Drs. Masao Amano, Tsuneo Kakuda, Kazumi Arai and Akiko Yatabe, Messrs. Osamu Shibata, Hidemi Kudo, Masayuki Ishii and Satoshi Yamamoto and Mses. Kana Shibayama, Rie Imai, Ayako Umetani and Yuki Suzuki for their strenuous efforts with regard to the stranding survey in Japan.
REFERENCES
- 1.Arai K., Yamada T. K., Takano Y.2004. Age estimation of male Stejneger’s beaked whales (Mesoplodon stejnegeri) based on counting of growth layers in tooth cementum. Mammal Study 29: 125–136. doi: 10.3106/mammalstudy.29.125 [DOI] [Google Scholar]
- 2.Araki J., Machida M., Kuramochi T.1994. Three species of Crassicauda (Nematoda, Spirurida) from cetaceans in Japanese and adjacent water. Bull. Natl. Sci. Mus. Tokyo Ser. A 20: 59–65. [Google Scholar]
- 3.Conlogue G. J., Ogden J. J. A., Foreyt W. J.1985. Parasites of the Dall’s porpoise (Phocoenoides dalli). J. Wildl. Dis. 21: 160–166. doi: 10.7589/0090-3558-21.2.160 [DOI] [PubMed] [Google Scholar]
- 4.Cowan D. F.1995. Amyloidosis in the bottlenose dolphin, Tursiops truncatus. Vet. Pathol. 32: 311–314. doi: 10.1177/030098589503200314 [DOI] [PubMed] [Google Scholar]
- 5.Cowan D. F., Walker W. A., Brownell R. L., Jr1986. Pathology of small cetaceans stranded along southern California beaches. pp. 323–367. In: Research on Dolphins (Bryden, M. M. and Harrison, R. J. eds.), Oxford University Press, Oxford. [Google Scholar]
- 6.Cowan D. F.1968a. Avian amyloidosis. Pathol Vet.. 5: 51–58. [PubMed] [Google Scholar]
- 7.Cowan D. F.1968b. Diseases of captive reptiles. J. Am. Vet. Med. Assoc. 153: 848–859. [PubMed] [Google Scholar]
- 8.Dailey M. D.1971. Distribution of helminthes in Dall’s porpoise (Phocoenoides dalli True). J. Parasitol. 57: 1348. doi: 10.2307/3277996 [DOI] [PubMed] [Google Scholar]
- 9.Dailey M., Stroud R.1978. Parasites and associated pathology observed in cetacean stranded along the Oregon coast. J. Wildl. Dis. 14: 503–511. doi: 10.7589/0090-3558-14.4.503 [DOI] [PubMed] [Google Scholar]
- 10.Dunn J. L., Buck J. D., Robeck T. R.2001. 16. The bacterial diseases of cetaceans and pinnipeds. pp. 309–336. In: Marine Mammal Medicine 2nd ed. (Dierauf, L. A. and Gulland, F. M. D. eds.), CRC Press, Boca Raton. [Google Scholar]
- 11.Honma Y.1994. Histological studies on the ovaries of beaked whales, Mesoplodon stejnegeri, stranded on the coast of Niigata district, Sea of Japan. Rep. Sado Marine Biol. Station. Niigata Uni. 24: 1–10. [Google Scholar]
- 12.Honma Y., Yamada T. K.1995. Further notes on ovarian histology of the Stejneger’s beaked whale, Mesoplodon stejnegeri, from a recent stranding on the coast of Niigata district, Sea of Japan. Bull. Natl. Sci. Mus. Tokyo Ser. A 21: 109–118. [Google Scholar]
- 13.Howard B. E.1983. Miscellaneous diseases. pp. 163–225. In: Pathobiology of Marine Mammal Diseases, vol.1 (Howard, B. E. ed.), CRC Press, Boca Raton. [Google Scholar]
- 14.Ichihara A., Nagasaki T.1975. Crassicauda sp. from Mesoplodon sp. caught at the pacific coast of Japan. Kisechugaku Zasshi 24: 86. [Google Scholar]
- 15.Ishikawa H., Goto M., Mogoe T.2013. Stranding record in Japan 1901–2012. Shimonoseki Marine Sci. Rep. 1: 1–314. [Google Scholar]
- 16.Jaber J. R., Pérez J., Arbelo M., Zafra R., Fernández A.2006. Pathological and immunohistochemical study of gastrointestinal lesions in dolphins stranded in the Canary Islands. Vet. Rec. 159: 410–414. doi: 10.1136/vr.159.13.410 [DOI] [PubMed] [Google Scholar]
- 17.Jones E. C.1971. Isistius brasiliensis, a squaloid shark, the probable cause of crater wounds on fishes and cetaceans. Fish Bull. 69: 791–798. [Google Scholar]
- 18.Kagei N., Morimitsu T.1992. Crassicauda grampicola parasiting in the cranial sinus of Risso’s dolphin. Kisechugaku Zasshi 41: 84. [Google Scholar]
- 19.Kikuchi S., Kazuno Y., Kiryu M., Nakajima M.1995. Morphology of Crassicauda giliakiana (Nematoda; Spirurida) from a Cuvier’s beaked whale Ziphius carvirostris. Kisechugaku Zasshi 43: 228–237. [Google Scholar]
- 20.Kim D. Y., Taylor H. W., Eades S. C., Cho D.Y.2005. Systemic AL amyloidosis associated with multiple myeloma in a horse. Vet. Pathol. 42: 81–84. doi: 10.1354/vp.42-1-81 [DOI] [PubMed] [Google Scholar]
- 21.Kuramochi T., Kikuchi T., Okamura H., Tatsukawa T., Doi H., Nakamura K., Yamada T. K., Koda Y., Yoshida Y., Matsuura M., Sakakibara S.2000. Parasitic helminth and epizoit fauna of finless porpoise in the Inland Sea of Japan and the western north Pacific with a preliminary note on faunal difference by host’s local population. Mem. Natl. Sci. Mus. Tokyo 33: 83–96. [Google Scholar]
- 22.Lambertsen R. H.1992. Crassicaudosis: a parasitic disease threatening the health and population recovery of large baleen whales. Rev. Sci. Tech. 11: 1131–1141. [PubMed] [Google Scholar]
- 23.Ludlage E., Murphy C. L., Davern S. M., Solomon A., Weiss D. T., Glenn-Smith D., Dworkin S., Mansfield K. G.2005. Systemic AA amyloidosis in the common marmoset. Vet. Pathol. 42: 117–124. doi: 10.1354/vp.42-2-117 [DOI] [PubMed] [Google Scholar]
- 24.Machida M.1974. Helminth parasites of the True’s porpoise, Phocoenoides truei Andrews. Bull Natl. Sci. Mus., Tokyo 17: 221–226. [Google Scholar]
- 25.McFee W. E., Lipscomb T. P.2009. Major pathologic findings and probable causes of mortality in bottlenose dolphins stranded in South Carolina from 1993 to 2006. J. Wildl. Dis. 45: 575–593. doi: 10.7589/0090-3558-45.3.575 [DOI] [PubMed] [Google Scholar]
- 26.MacLeod C. D.2006. How big is a beaked whale? A review of body length and sexual size dimorphism in the family Ziphiidae. J. Cetacean Res. Manag. 7: 301–308. [Google Scholar]
- 27.Maxie M. G.1991. The Urinary System. pp. 447–522. In: Pathology of Domestic Animals. 4th ed., vol.2 (Jubb, K. V. F., Kennedy, P. C. and Palmer, N. eds.), Academic Press, London. [Google Scholar]
- 28.Mead J. G.1989. Beaked whales of the genus Mesoplodon. pp. 349–430. In: Handbook of Marine Mammals, vol. 4 (Ridgway, S. H. and Harrison, R. J. eds.), Academic Press, London. [Google Scholar]
- 29.Merlini G., van der Hilst J. C., Simon A., Drenth J. P.2003. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 349: 583–596. doi: 10.1056/NEJMra023144 [DOI] [PubMed] [Google Scholar]
- 30.Merlini G., Westermark P.2004. The systemic amyloidosis: clearer understanding of the molecular mechanisms offers hope for more effective therapies. J. Intern. Med. 255: 159–178. doi: 10.1046/j.1365-2796.2003.01262.x [DOI] [PubMed] [Google Scholar]
- 31.Meyerholz D. K., Vanloubbeeck Y. E., Hostetter S. J., Jordan D. M., Fales-Williams A. J.2005. Surveillance of amyloidosis and other diseases at necropsy in captive trumpeter swans (Cygnus buccinator). J. Vet. Diagn. Invest. 17: 295–298. doi: 10.1177/104063870501700318 [DOI] [PubMed] [Google Scholar]
- 32.Nishimura S., Araga C., Noda R.1961. Endo-parasites of bottlenose dolphins. J. Jpn. Assoc. Zool. Gardens and Aquarium 3: 33–36. [Google Scholar]
- 33.Nishiwaki M.1962. Mesoplodon bowdoini stranded at Akita Beach, Sea of Japan. Sci. Rep. Whales Res. Inst. 16: 61–77. [Google Scholar]
- 34.Norris K. S.1961. Standardized methods for measuring and recording data on the smaller cetaceans. J. Mammal. 42: 471–476. doi: 10.2307/1377364 [DOI] [Google Scholar]
- 35.Ozaki Y.1935. Trematode parasites of Indian porpoise Neophocaena phocaenoides, Gray. J. Sci. Hiroshima Univ. Ser. B. Div. l. 3: 115–138. [Google Scholar]
- 36.Shimamura M., Yasue H., Ohshima K., Abe H., Kato H., Kishiro T., Goto M., Munechika I., Okada N.1997. Molecular evidence from retroposons that whales form a clade within even-toed ungulates. Nature 388: 666–670. doi: 10.1038/41759 [DOI] [PubMed] [Google Scholar]
- 37.Shindo J., Yamato A.1995. Pathology of Stejnegeri beaked whale Mesoplodon stejnegeri stranded at Joetsu-city. Nihonkai Cetology 5: 27–29. [Google Scholar]
- 38.Shindo J., Yamada T. K., Yoshimura K., Kageyama I.2008. Morphology of the tongue in a newborn Stejneger’s beaked whale (Mesoplodon stejnegeri). Okajimas Folia Anat. Jpn. 84: 121–124. doi: 10.2535/ofaj.84.121 [DOI] [PubMed] [Google Scholar]
- 39.Shtrasburg S., Gal R., Gruys E., Perl S., Martin B. M., Kaplan B., Koren R., Nyska A., Pras M., Livneh A.2005. An ancillary tool for the diagnosis of amyloid A amyloidosis in a variety of domestic and wild animals. Vet. Pathol. 42: 132–139. doi: 10.1354/vp.42-2-132 [DOI] [PubMed] [Google Scholar]
- 40.Sliper E. J.1962. pp.1–493. Whales (Translated by Pomerans, A. J.). Hutchinson & Co., Ltd., London. [Google Scholar]
- 41.Tajima Y., Shimada A., Yamada T. K., Cowan D. F.2007. Amyloidosis in two Stejneger’s beaked whales (Mesoplodon stejnegeri) stranded at the Sea of Japan. J. Zoo Wildl. Med. 38: 108–113. doi: 10.1638/05-108.1 [DOI] [PubMed] [Google Scholar]
- 42.Thewissen J. G., Madar S. I.1999. Ankle morphology of the earliest cetaceans and its implications for the phylogenetic relations among ungulates. Syst. Biol. 48: 21–30. doi: 10.1080/106351599260418 [DOI] [PubMed] [Google Scholar]
- 43.True F. W.1885. Contributions to the history of the Commander Islands. No. 5- Description of a new species of Mesoplodon, M. Stejnegeri, obtained by Dr. Leonard Stejneger, in Bering Island. Proc. United States Natl. Mus. 8: 584–585. doi: 10.5479/si.00963801.8-540.584 [DOI] [Google Scholar]
- 44.Walker W. A., Hansen M. B.1999. Biological observations on Stejneger’s beaked whale, Mesoplodon stejnegeri, from strandings on Adak, Alaska. Mar. Mamm. Sci. 15: 1314–1329. doi: 10.1111/j.1748-7692.1999.tb00893.x [DOI] [Google Scholar]